Stromateiformes
|
publication ID |
https://doi.org/10.1093/zoolinnean/zlab058 |
|
DOI |
https://doi.org/10.5281/zenodo.6771661 |
|
persistent identifier |
https://treatment.plazi.org/id/0E16878B-FFF9-FFCD-FF71-FBCCFBE9F89F |
|
treatment provided by |
Plazi (2022-06-27 06:56:26, last updated 2024-11-27 09:30:39) |
|
scientific name |
Stromateiformes |
| status |
|
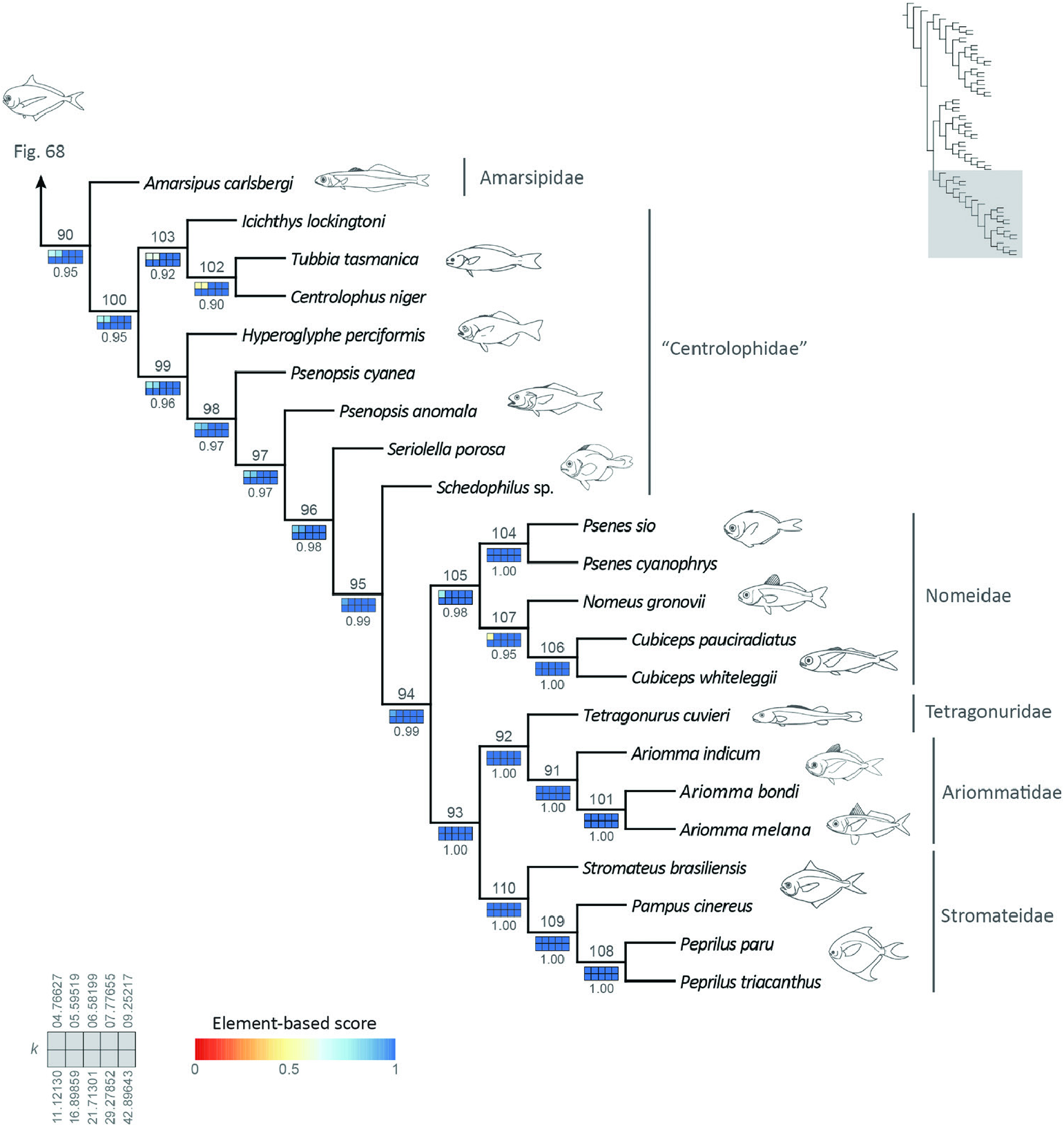
Node 90 = Order Stromateiformes
Amarsipus carlsbergi , Icichthys lockingtoni , Tubbia tasmanica , Centrolophus niger , Hyperoglyphe perciformis , Psenopsis cyanea , Psenopsis anomala , Seriolella porosa , Schedophilus sp. , Psenes sio , Psenes cyanophrys , Nomeus gronovii , Cubiceps whiteleggii , Cubiceps pauciradiatus , Tetragonurus cuvieri , Ariomma indicum , Ariomma bondi , Ariomma melana , Stromateus brasiliensis , Pampus cinereus , Peprilus triacanthus and Peprilus paru .
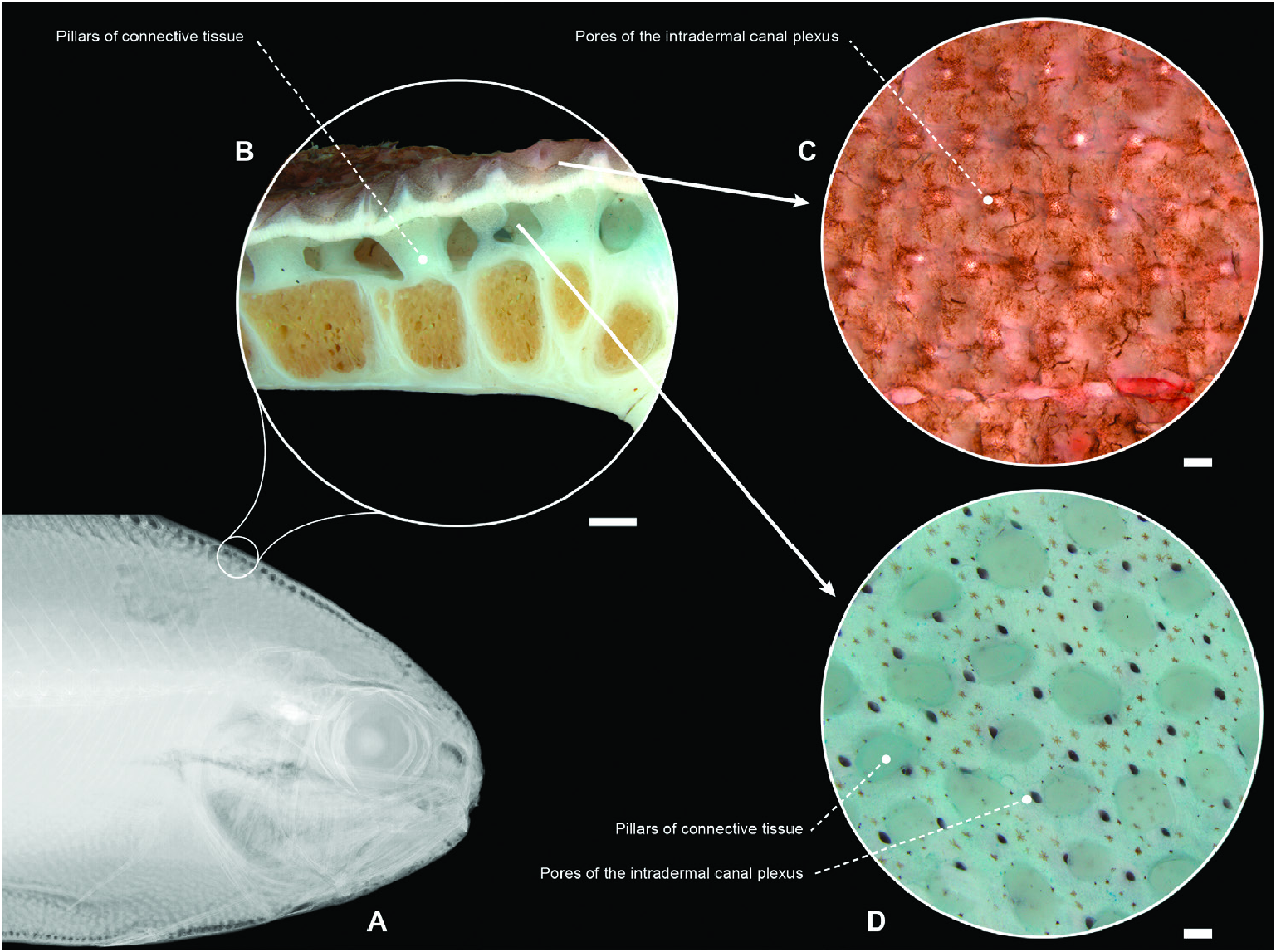
Unambiguous synapomorphies: Character 6 (22> 24): number of anal-fin soft rays increased to 24; character 11 (9> 10–12): number of ventral procurrent caudalfin rays increased to 10–12; character 36 (0> 1): medial bony suture between dorsal and ventral hypohyals absent; character 40 (1> 0): premaxillary teeth uniserial; character 42 (0> 1): palatine teeth absent; character 94 (0> 1): adductor mandibulae pars malaris subdivided into retromalaris and promalaris subsections; character 150 (0> 1): anteroventral section of epaxialis present; character 182 (0> 1): intradermal canal plexus present.
Support: Relative Bremer = 90%.
Remarks: The order Stromateiformes ( Fig. 69 View Figure 69 : node 90) encompasses six families: Amarsipidae , Centrolophidae , Nomeidae , Tetragonuridae , Ariommatidae and Stromateidae . It is supported by eight unambiguous synapomorphies ( Fig. 70 View Figure 70 ). The two most obvious synapomorphies for the order are the presence of uniserial teeth in the jaws (character 40) and an intradermal canal plexus on the trunk (= part of the subdermal canal system of Haedrich, 1967; Fig. 63 View Figure 63 ; character 182). Within the analysed taxa, homoplastic occurrences of uniserial teeth appear only in the icosteid Icosteus aenigmaticus , the scombrid Scomber sp. (Scombridae) and the mugilid Mugil curema , and the intradermal canal plexus is uniquely present in Stromateiformes , with a single reversal in Tetragonuridae . The other five synapomorphies retrieved by this analysis involve characters that are variously homoplastic within the order or among Percomorphacea, namely branched anal-fin rays 24 or more (character 6), ventral procurrent caudal-fin rays ten or more, absence of a bony suture between dorsal and ventral hypohyals (character 36, state 1), absence of palatine teeth (character 42, state 1), pars malaris of the adductor mandibulae divided into retromalaris and promalaris (character 94, state 1) and presence of an anteroventral subsection of the epaxialis (character 150).
The allocation of Amarsipus to Stromateiformes and, consequently, the diagnosis of the order, has been the subject of recurrent debates. Haedrich (1969) described Amarsipidae as a stromateiform based on five characteristics: greatly expanded lachrymal, protruding top of the head, bony bridge over the anterior vertical canal of the inner ear (= pons moultoni sensu Haedrich, 1971), jaws with uniserial teeth and an extensive intradermal canal system over the body (head and trunk). Although not discussed explicitly by the author, these characters constituted a novel and expanded diagnosis for Stromateiformes , because its previous definition listed only the pharyngeal sac and uniserial jaw teeth ( Haedrich, 1967: p. 45). We have included in our matrix two of the five characters listed by Haedrich (1969): the uniserial jaw teeth and the intradermal canal system over the body (head and trunk). Although our analysis corroborates the first as a synapomorphy for Stromateiformes (our character 40), the latter is revealed to be a compound character that we treat as two independent characters: an extreme condition of the dendritic pore-opening pattern of the cephalic laterosensory system (our character 189; Figs 14 View Figure 14 , 15 View Figure 15 , 29 View Figure 29 ) and a specialization of the trunk skin into an intradermal plexus of canals (our character 182; Fig. 63 View Figure 63 ). According to our phylogeny, only the trunk intradermal canals are recovered herein as a stromateiform synapomorphy, because the dendritic pattern of the cephalic laterosensory system is highly homoplastic across percomorphs. Haedrich’s (1969) remaining synapomorphies for the order were not coded herein for different reasons. The protruding top of the head is expressed in a morphological cline across percomorphs, which hampers the delimitation of unambiguous character states. Regarding the lachrymals of Amarsipus carlsbergi , our observations did not notice any extraordinary expansions of this bone, a fact that Haedrich (1969: p. 10) himself also mentioned in the original description of this taxon. The third of Haedrich’s (1969) characters, the pons moultoni, was not included in our analysis because it would demand destructive dissections that would hamper the observations of other osteological or myological characters contained in the same area.
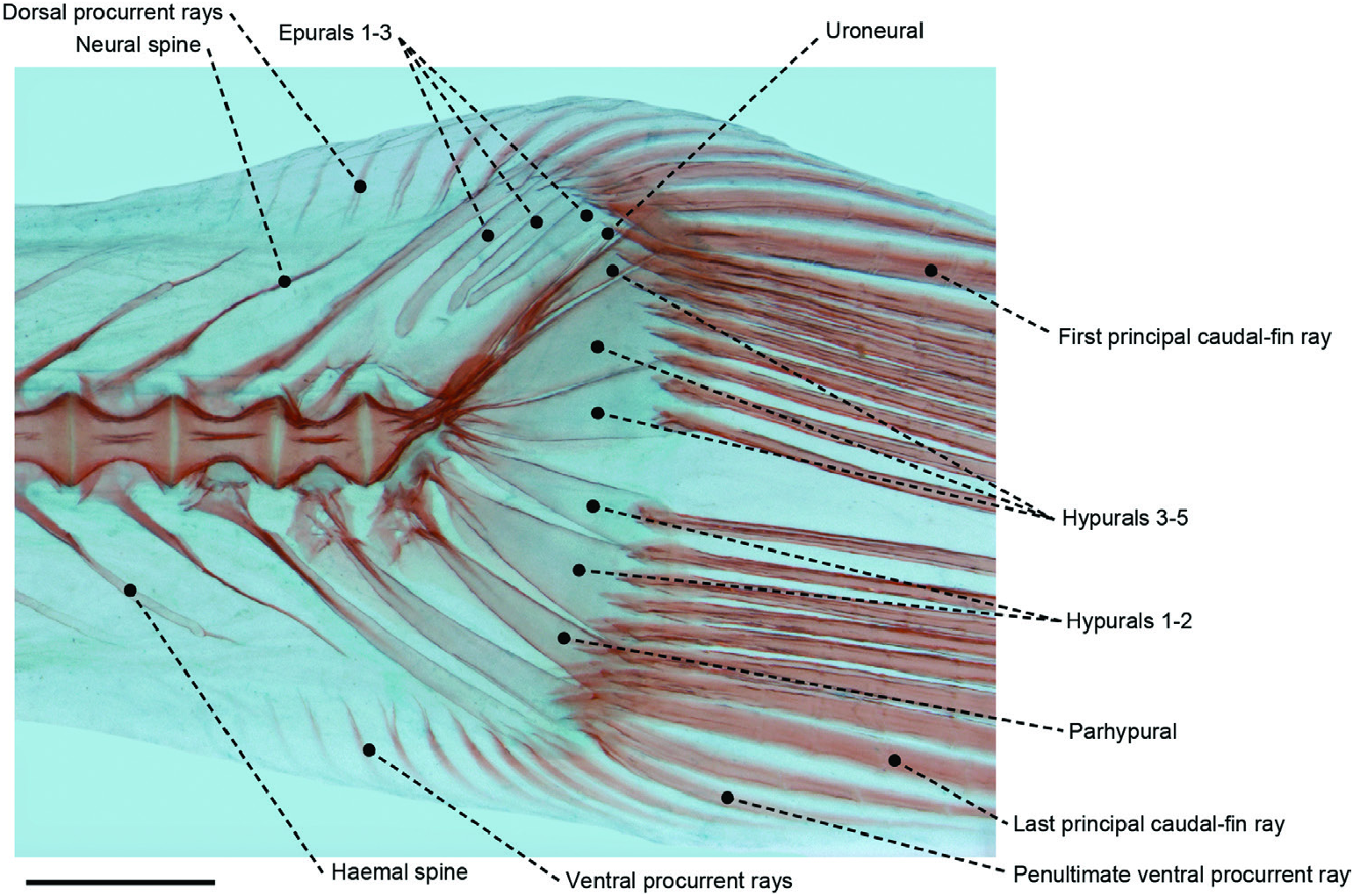
Our final cladogram is in agreement with Horn’s (1984) placement of Amarsipidae as the sister group of the remaining Stromateiformes . However, we reject all Horn’s stromateiform synapomorphies, i.e. cycloid scales, a scaled preopercle and six hypurals ( Horn, 1984: characters 14, 16 and 23). Horn’s (1984) erroneous high hypural count for Amarsipus was based on a misidentification of the parhypural as the first hypural ( Fig. 11 View Figure 11 ), an error inherited from previous studies on the group ( Haedrich, 1967: figs 10, 12, 17, 24, 33, 36, 42, 47; Haedrich, 1969: fig. 5). The optimization of cycloid scales as a synapomorphy of the Stromateiformes was probably attributable to the limited outgroup comparison used in his analysis, which included only one species of Scorpididae , Girellidae and Kyphosidae . Our analysis shows that the distribution of cycloid scales (character 199) is much broader and that it is present primitively in a larger clade (node 77; Fig. 67 View Figure 67 ), including not only Stromateiformes , but several other percomorph lineages. The remainder of Horn’s stromateiform synapomorphies described scales on the preopercle (comparable to our character 192, scales on the lateral portion of the head, a necessary modification of Horn’s character 16). Yet, this character is resolved as plesiomorphically present in percomorphs and does not result in a stromateiform synapomorphy.
Doiuchi et al. (2004) offered a different hypothesis of stromateiform intrarelationships and, consequently, a modification of the characters supporting monophyly of the order. In their study, the centrolophids formed a basal stromateiform polytomy and Amarsipidae was deeply nested within Stromateiformes as the sister group of the clade comprising Ariommatidae , Nomeidae , Tetragonuridae and Stromateidae ( Doiuchi et al., 2004: fig. 12). According to these authors, the pharyngeal sac was present primitively in Stromateiformes ( Doiuchi et al., 2004: character 21), constituting a synapomorphy for the order that was reversed secondarily in Amarsipus carlsbergi . Other stromateiform synapomorphies proposed by Doiuchi et al. (2004) included palatine toothless, dorsal and ventral hypohyal articulation lacking interdigitating sutures medially, ceratobranchial 5 forked posteriorly, anterior ventral prezygapophyses present on posteriormost abdominal vertebra and on caudal vertebrae, dorsal end of extrascapula not articulating with other skull bones, scales cycloid and presence of intradermal canal plexus on body ( Doiuchi et al., 2004: characters 7, 11, 20, 21, 22, 31, 40 and 43, respectively). We included five of the eight characters of Doiuchi et al. (2004) in our matrix (our characters 36, 42, 157, 182 and 199). Of these, three (i.e. toothless palatine, absence of a bony suture between dorsal and ventral hypohyals and intradermal canal plexus) are confirmed as valid stromateiform synapomorphies. The remainder of the characters of Doiuchi et al. (2004) showed ambiguous states when sampled on a broader percomorph outgroup and, consequently, were not included in our matrix.
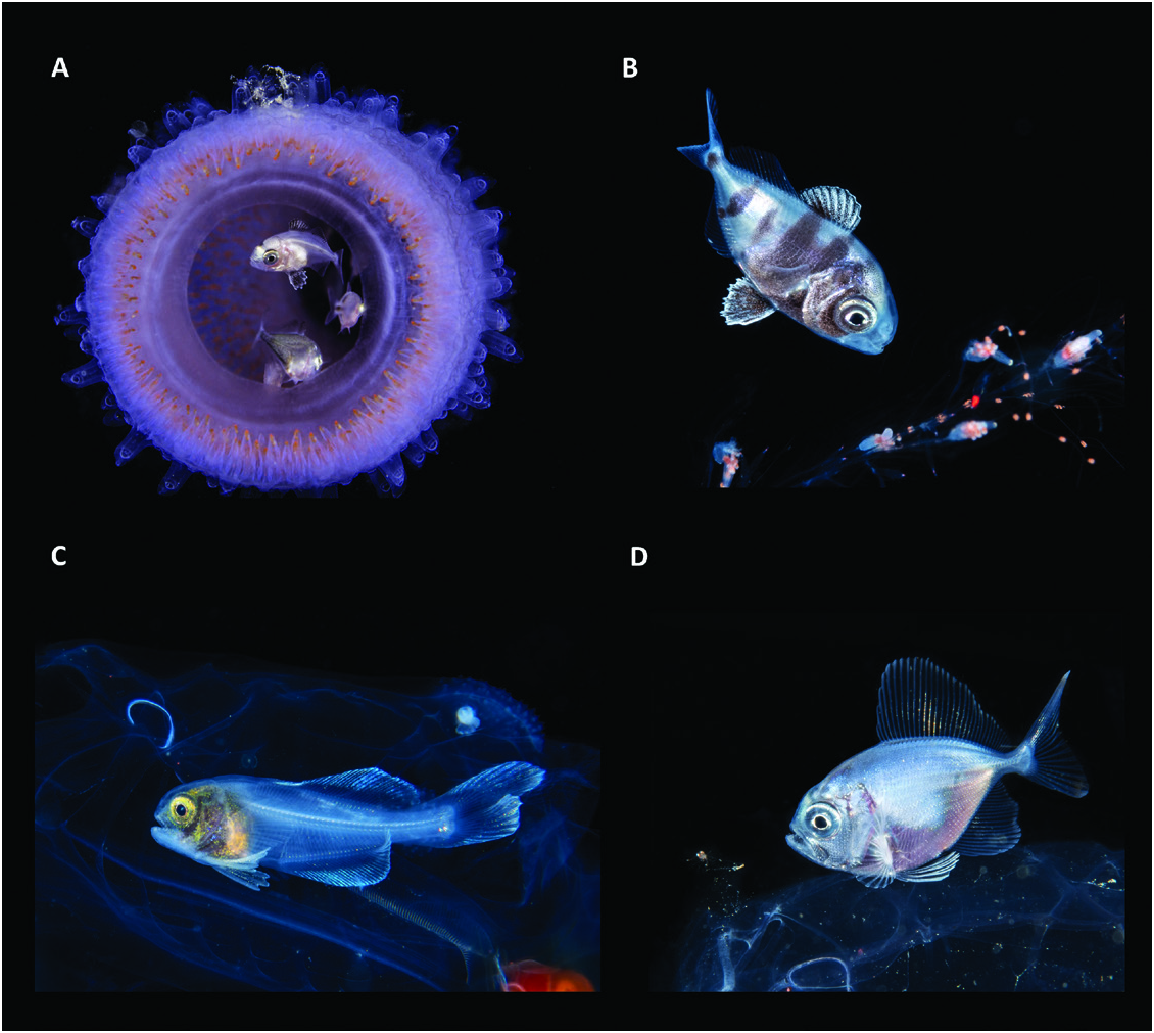
The conflicting positioning of the Amarsipidae within Stromateiformes is partly because Amarsipus has a mosaic of primitive and derived stromateiform features. Characters agreeing with its basal placement within the order involve the absence of the pharyngeal sac, unfused hypurals, three epurals ( Fig. 11 View Figure 11 ) and an epaxial musculature not covering the skull roof. Nonetheless, Amarsipus has some derived stromateiform characteristics, such as an elevated vertebral count (47), otherwise found in only a few taxa (e.g. Stromateus , 47; Tetragonurus , 53; Icichthys , 60), and a reduced number of branchiostegals (six). Moreover, contrary to previous assumptions (e.g. Horn, 1984: character 27), young amarsipids do associate with gelata, more specifically with salps and pyrosomes ( Fig. 65C View Figure 65 ; Janssen & Harbison, 1981; Harbison, 1993). In contrast to the more widespread stromateiform association with medusae, symbioses with salps and pyrosomes are rare among members of the order and reported elsewhere only for some Cubiceps and Tetragonurus ( Janssen & Harbison, 1981) . Amarsipidae and Tetragonuridae seem to be particularly specialized in living inside and feeding on salps ( Harbison, 1993), a behaviour that might be secondary to the pre-existing association with medusae widespread among stromateoids and also present in some of its immediate outgroup. The relatively long and compressed bodies of Amarsipus and Tetragonurus might be an adaptation for life in such confined spaces ( Figs 65C View Figure 65 , 71 View Figure 71 ; Janssen & Harbison, 1981). A sister-group relationship between Amarsipidae and Tetragonuridae was recently proposed by the molecular analysis by Campbell et al. (2018). However, according to the authors, this clade was resolved as more closely related to the Scombridae , while the remaining Stromateiformes were grouped with Bramidae , Icosteidae and Caristiidae . A Tetragonuridae + Amarsipidae clade was not supported by our analysis in any scenario, and the placement of Tetragonurus in a clade containing Ariommatidae and Stromateidae is strongly supported by a series of morphological characters (see discussion below: nodes 93 and 92).
Campbell MA, Sado T, Shinzato C, Koyanagi R, Okamoto M, Miya M. 2018. Multilocus phylogenetic analysis of the first molecular data from the rare and monotypic Amarsipidae places the family within the Pelagia and highlights limitations of existing data sets in resolving pelagian interrelationships. Molecular Phylogenetics and Evolution 124: 172 - 180.
Doiuchi R, Sato T, Nakabo T. 2004. Phylogenetic relationships of the stromateoid fishes (Perciformes). Ichthyological Research 51: 202 - 212.
Haedrich RL. 1967. The stromateoid fishes: systematics and a classification. Bulletin of the Museum of Comparative Zoology 135: 310 - 139.
Haedrich RL. 1969. A new family of aberrant stromateoid fishes from the equatorial Indo-Pacific. Dana Reports 76: 1 - 14.
Haedrich RL. 1971. The pons moultoni, a significant character. Copeia 1971: 167 - 169.
Harbison GR. 1993. The potential of fishes for the control of gelatinous zooplankton. International Council for the Exploration of the Sea (ICES) 74: 1 - 10.
Horn MH. 1984. Stromateoidei: development and relationships. In: Moser HG, Richards WJ, Cohen DM, Fahay MP, Kendall AW Jr, Richardson SL, eds. Ontogeny and systematics of fishes. Based on an International Symposium dedicated to the memory of Elbert Halvor Ahlstrom. New York: American Society of Ichthyologists and Herpetologists, 620 - 636.
Janssen J, Harbison GR. 1981. Fish in salps: the association of squaretails (Tetragonurus spp.) with pelagic tunicates. Journal of the Marine Biological Association of the United Kingdom 61: 917 - 927.
Figure 11. Caudal skeleton of a cleared and stained specimen of the amarsipid Amarsipus carlsbergi (SIO 75-122) in left lateral view. Scale bar: 2 mm.
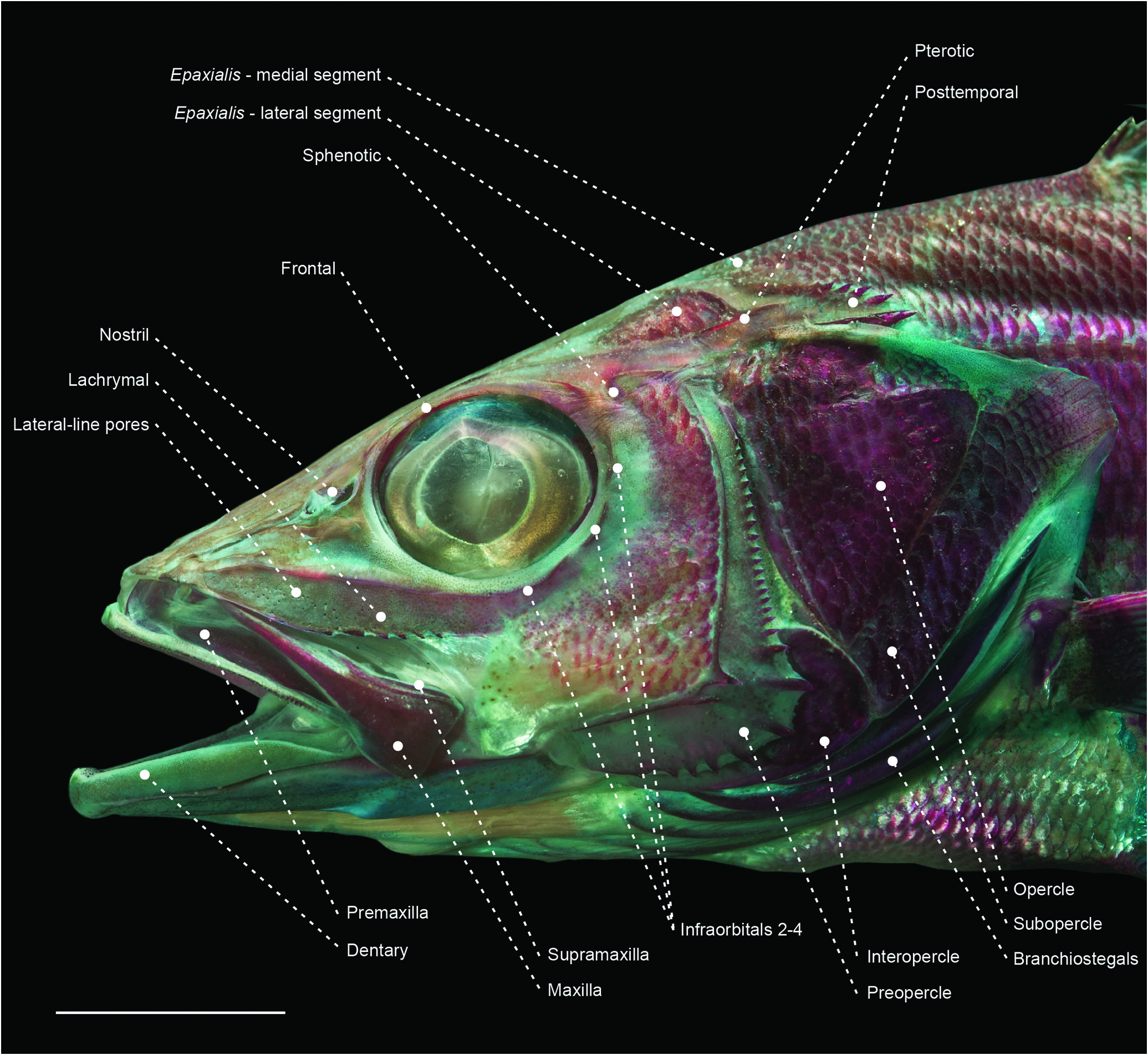
Figure 14. Head of the centropomid Centropomus parallelus (MZUSP 108244) in left lateral view. Scale bar: 10 mm.
Figure 15. Head of the arripid Arripis georgianus (MZUSP 119735) in left lateral view. Scale bar: 10 mm.
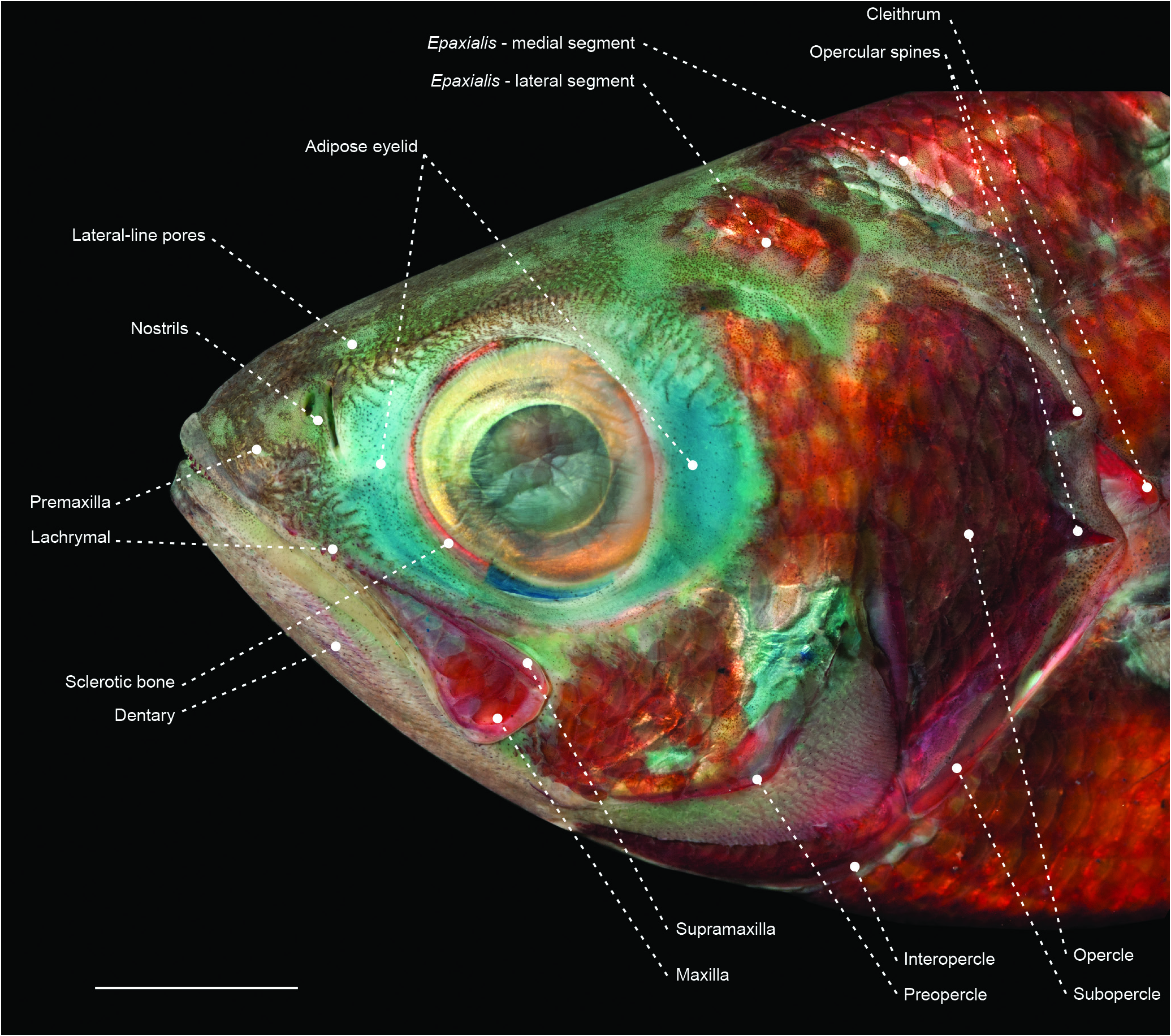
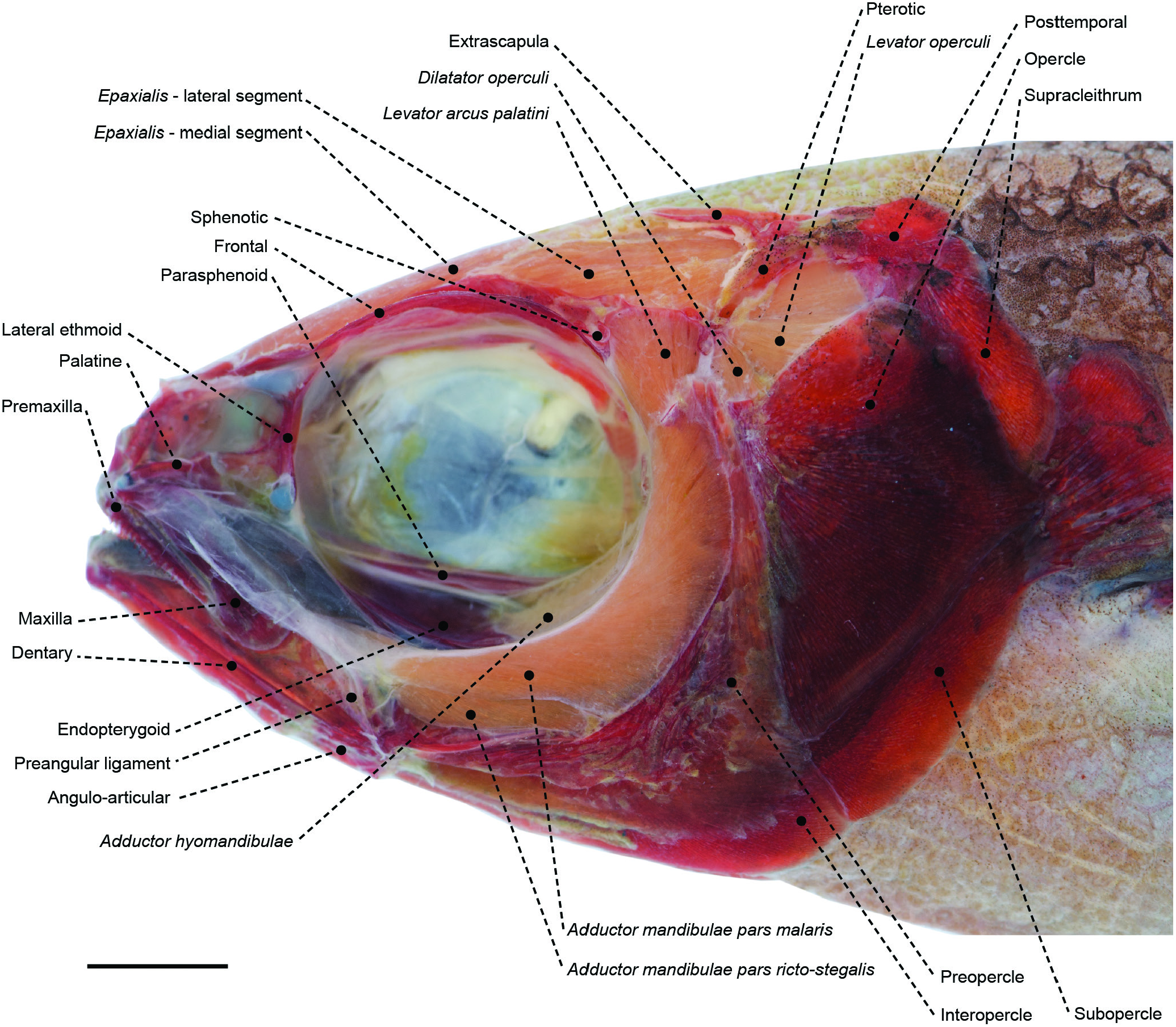
Figure 29. Superficial cranial musculoskeletal system of the nomeid Cubiceps pauciradiatus (MZUSP 80701) in left lateral view. Eye and infraorbital series removed. Scale bar: 5 mm.
Figure 63. Intradermal canal plexus of the centrolophid Tubbia tasmanica (CSIRO H 6979-03, 325.2 mm standard length). A, intradermal canals in a radiographed specimen. B, transverse section of the superficial layers of skin and axial musculature. C, high magnification of external appearance of skin surface. D, internal surface of the same piece. Visible pores connect the external surface to the interdermal space (C, D). Scale bars: 1 mm (in B–D).
Figure 65. Examples of the association between juvenile fishes and pelagic, gelatinous invertebrates. A, B, the nomeid Nomeus gronovii sheltering inside a pyrosome (A) and swimming around the tentacles of a pelagic siphonophore (B). C, the amarsipid Amarsipus carlsbergi hiding in the abdominal cavity of a pelagic salp. D, a bramid Brama sp. resting on the top of a jellyfish. Images from Suzan Meldonian (A) and Linda Ianniello (B–D).
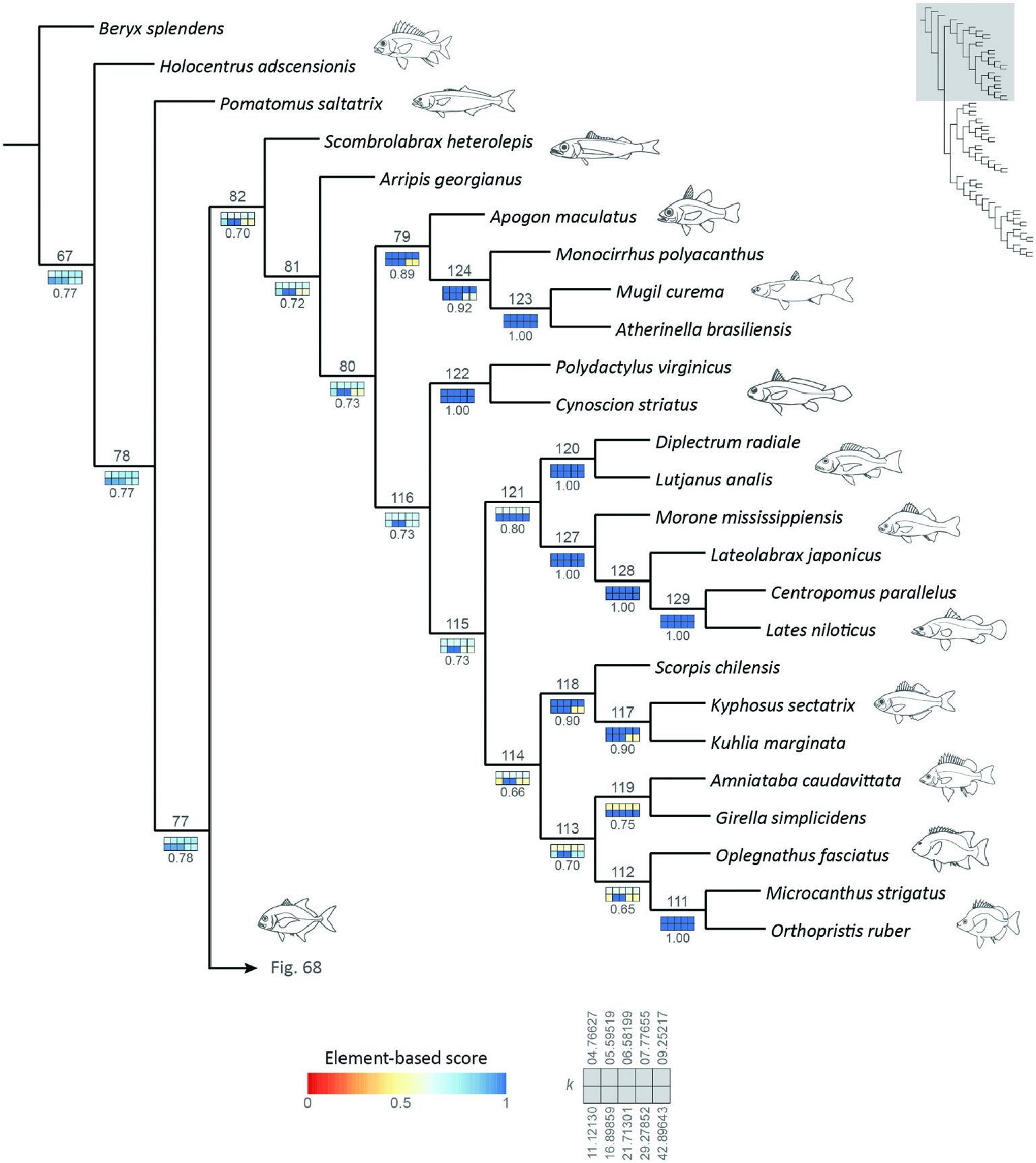
Figure 67. Reference tree obtained under implied-weights parsimony (k = 13.56554), with sensitivity analysis of alternative weighting schemes, highlighting the interrelationships of node 82. Squares representing each value of k are coloured according to their respective element-based scores. Numbers below sensitivity plots indicate the average element-based comparison score for the clade. Node numbers referenced in the text displayed above each clade.
Figure 69. Reference tree obtained under implied-weights parsimony (k = 13.56554), with sensitivity analysis of alternative weighting schemes, highlighting the interrelationships of Stromateiformes (node 90). Squares representing each value of k are coloured according to their respective element-based scores. Numbers below sensitivity plots indicate the average element-based comparison score for the clade. Node numbers referenced in the text displayed above each clade.
Figure 70. Unambiguous phenotypic synapomorphies for Stromateiformes superimposed on the reference tree of the order obtained under implied-weights parsimony (k = 13.56554).
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Order |