Synalpheus anasimus Chace, 1972
|
publication ID |
https://doi.org/10.11646/zootaxa.3598.1.1 |
|
publication LSID |
lsid:zoobank.org:pub:74562879-7AB4-42D7-B894-09BFA4885324 |
|
persistent identifier |
https://treatment.plazi.org/id/041D87E9-971A-FFB3-FF7C-5D26FDC4FDF5 |
|
treatment provided by |
Felipe (2021-08-24 19:43:40, last updated by Plazi 2023-11-04 17:27:33) |
|
scientific name |
Synalpheus anasimus Chace, 1972 |
| status |
|
Synalpheus anasimus Chace, 1972 View in CoL View at ENA
(Figs 7, 8)
Synalpheus anasimus Chace 1972: 82 View in CoL , figs 25–28; Dardeau 1984: 17; Duffy 1992: 131; Martínez-Iglesias et al. 1996: 35; Morrison et al. 2004: 12 (Appendix A); McClure 2005: 168, fig. 33.
? Synalpheus aff. anasimus View in CoL — Hernández Aguilera et al. 1996: 35.
Synalpheus anasimanus (lap. cal.)— Banner & Banner 1975: 274.
Zuzalpheus anasimus — Ríos & Duffy 2007: 79.
Material examined. St. Martin: 1 male, FLMNH UF 31975 , Réserve Naturelle de Saint-Martin, sta. 28, Pinel Island , in crevices of coral rubble, 0.5–1 m, coll. A. Anker [fcn BSTM-0493*] .
Description. For detailed description and illustrations see Chace (1972); diagnostic features of the St. Martin specimen are illustrated in Fig. 7.
Size range. Male from St. Martin cl 4.4 mm; male holotype, cl 2.2 mm; ovigerous females, cl 3.2 mm ( Chace 1972) .
Colour in life. Mostly semitransparent, major chela amber-orange distally (Fig. 8).
Type locality. Bahía de la Ascensión, Mexico .
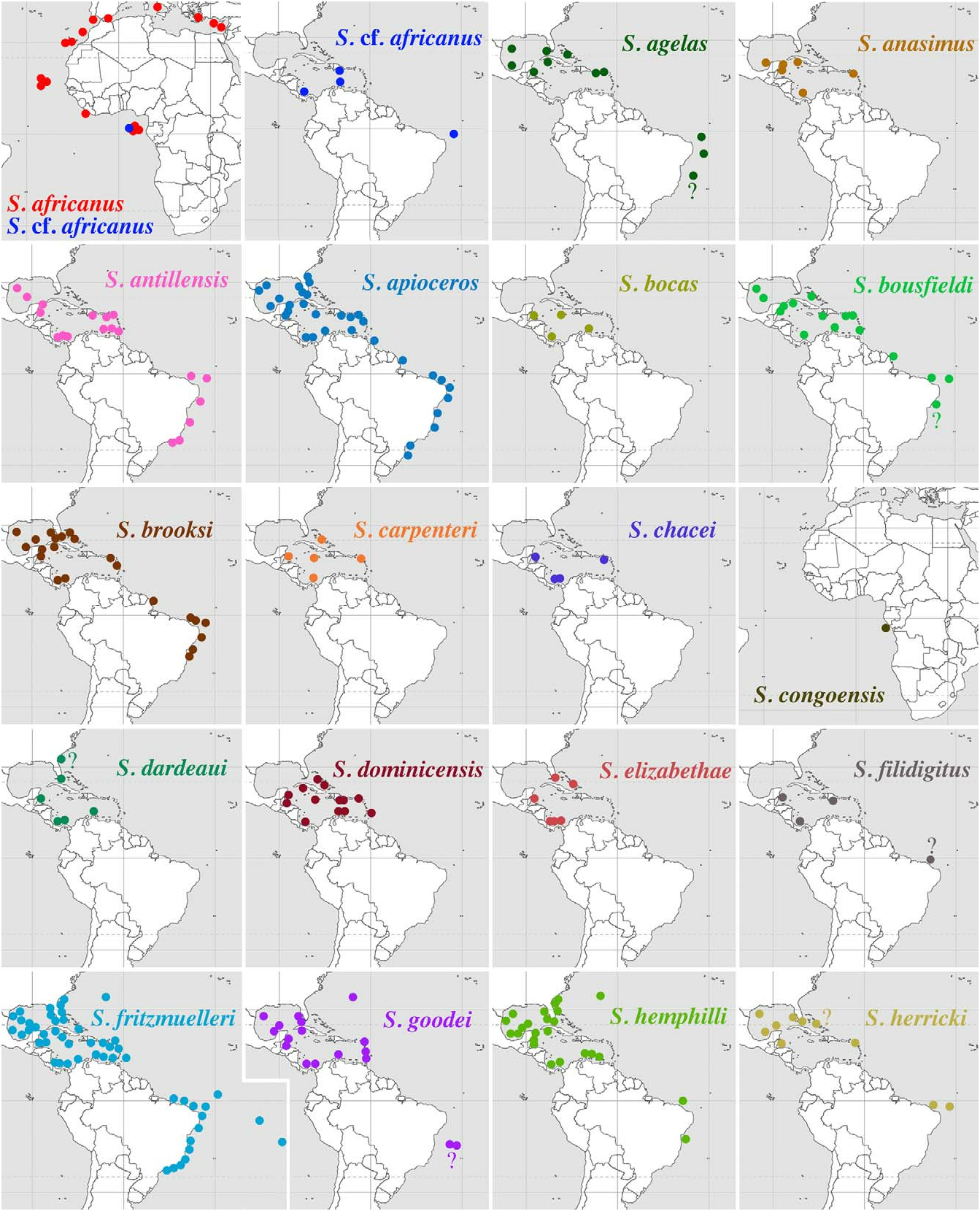
Distribution. Western Atlantic: Gulf of Mexico: Mexico [Puerto Morelo]; Caribbean Sea: Mexico [Bahía de la Ascensión, Bahía del Espíritu Santo], Cuba, Panama, St. Martin ( Chace 1972; Duffy 1992; Martínez-Iglesias et al. 1996; McClure 2005; present study; see map in Fig. 51 View FIGURE 51 ).
Ecology. The St. Martin specimen was found in crevices of coral rubble in very shallow water (less than 1 m). Some type specimens (females) came from the “upper portions of much eroded coral standing in 10 feet [~ 3 m] of water” ( Chace 1972) .
Remarks. Chace (1972) noticed some morphological variation in the Yucatan material of S. anasimus . The non-type ovigerous females differ from the male holotype in the presence of five (instead of four) articles in the carpus of the second pereiopods, longer antennular peduncles, and a longer and somewhat differently shaped scaphocerite. The male holotype also has a distinctly more appressed stylocerite, which is distinctly separated from the lateral margin of the first article in females. These differences led Chace (1972) to report the two ovigerous females as “ Synalpheus anasimus ?”
In the single male from St. Martin, the stylocerite surpasses the distal margin of the first peduncular article (Fig. 7a); the carpus of the second pereiopod has five articles (Fig. 7c, as in the ovigerous female from Yucatan); the rostrum is as wide as and slightly longer than the orbital teeth (Fig. 7a); the minor chela carpus is slightly longer than in the holotype or the ovigerous female and with the fingertips strongly bidentate; the scaphocerite blade is slender and reaching half-length of the scaphocerite (Fig. 7a, more like in the ovigerous female); the telson has four dorsal spiniform setae both situated in its anterior half (Fig. 7d), and the uropodal exopod has two distolateral teeth (Fig. 7e) vs. one tooth in the holotype and one or two teeth in the ovigerous female. The major cheliped of the St. Martin male (Fig. 7b) is remarkably similar in general shape and proportions to that of the holotype of S. anasimus . Thus, the St. Martin specimen clearly falls within the range of variation reported for S. anasimus by Chace (1972), extending its geographic range to the extreme northeastern Caribbean Sea. However, it remains unclear whether S. anasimus is a single, morphologically variable species (the 2.2 mm cl holotype may be an immature male) or comprises more than one taxon. More material from throughout its range is needed to investigate this problem both morphologically and genetically.
Banner, D. M. & Banner, A. H. (1975) The alpheid shrimp of Australia. Part 2: the genus Synalpheus. Records of the Australian Museum, 29, 267 - 389.
Chace, F. A., Jr. (1972) The shrimps of the Smithsonian-Bredin Caribbean expeditions with a summary of West Indian shallowwater species (Crustacea: Decapoda: Natantia). Smithsonian Contributions to Zoology, 98, 1 - 179.
Dardeau, M. R. (1984) Synalpheus shrimps (Crustacea: Decapoda: Alpheidae). I. The Gambarelloides group, with a description of a new species. Memoirs of the Hourglass Cruises, 7, 1 - 125.
Duffy, J. E. (1992) Host use patterns and demography in a guild of tropical sponge-dwelling shrimps. Marine Ecology Progress Series, 90, 127 - 138.
Hernandez Aguilera, J. L., Toral Almazan, R. E. & Ruiz Nuno, J. A. (1996) Especies catalogadas de crusteceos estomatopodos y decapodos para el Golfo de Mexico, Rio Bravo, Tamps. A Puerto Progreso, Yuc. Secretaria de Marina y Comision Nacional para el Conocimiento y Uso de la Biodiversidad, Direccion de Oceanografia, Secretaria de Marina, Mexico, i - xiii + 1 - 132.
Martinez-Iglesias, J. C., Carvacho, A. & Rios, R. (1996) Catalogo de los carideos marinos (Crustacea, Decapoda, Caridea) de las aguas someras de Cuba. Avicennia, 4 / 5, 27 - 40.
McClure, M. (2005) Alpheidae. In Hernandez Aguilera, J. L., Ruiz Nuno, J. A., Toral Almazan, R. E. & Arenas Fuentes, V. (eds), Camarones, langostas y cangrejos de la costa este de Mexico. Volume 1, pp. 119 - 202. Estudio y Conservacion de la Naturaleza, A. C. y CONABIO, Mexico.
Morrison, C. L., Rios, R. & Duffy, J. E. (2004) Phylogenetic evidence for an ancient rapid radiation of Caribbean spongedwelling snapping shrimps (Synalpheus). Molecular Phylogenetics & Evolution, 30, 563 - 581.
Rios, R. & Duffy, J. E. (2007) A review of the sponge-dwelling snapping shrimp from Carrie Bow Cay, Belize, with description of Zuzalpheus, new genus, and six new species (Crustacea: Decapoda: Alpheidae). Zootaxa, 1602, 3 - 89.
FIGURE 51. Presently known geographic ranges of Synalpheus africanus Crosnier & Forest, 1965; S. cf. africanus (eastern and western Atlantic); S. agelas Pequegnat & Heard, 1979; S. anasimus Chace, 1972; S. antillensis Coutière, 1909; S. apioceros Coutière, 1909; S. bocas Anker & Tóth, 2008; S. bousfieldi Chace, 1972; S. brooksi Coutière, 1909; S. carpenteri Macdonald & Duffy, 2006; S. chacei Duffy, 1998; S. congoensis Crosnier & Forest, 1965; S. dardeaui (Ríos & Duffy, 2007); S. dominicensis Armstrong, 1949; S. elizabethae (Ríos & Duffy, 2007); S. filidigitus Armstrong, 1949; S. fritzmuelleri Coutière, 1909; S. goodei
| FLMNH |
Florida Museum of Natural History |
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |
Synalpheus anasimus Chace, 1972
| Anker, Arthur, Pachelle, Paulo P. G., Grave, Sammy De & Hultgren, Kristin M. 2012 |
Zuzalpheus anasimus
| Rios, R. & Duffy, J. E. 2007: 79 |
Synalpheus aff. anasimus
| Hernandez Aguilera, J. L. & Toral Almazan, R. E. & Ruiz Nuno, J. A. 1996: 35 |
Synalpheus anasimanus
| Banner, D. M. & Banner, A. H. 1975: 274 |
Synalpheus anasimus
| McClure, M. 2005: 168 |
| Morrison, C. L. & Rios, R. & Duffy, J. E. 2004: 12 |
| Martinez-Iglesias, J. C. & Carvacho, A. & Rios, R. 1996: 35 |
| Duffy, J. E. 1992: 131 |
| Dardeau, M. R. 1984: 17 |
| Chace, F. A., Jr. 1972: 82 |