Kylinxia zhangi ZENG,ZHAO AND HUANG
|
publication ID |
https://DOi.ORg/10.1038/s41586-020-2883-7 |
|
DOI |
https://doi.org/10.5281/zenodo.4717879 |
|
persistent identifier |
https://treatment.plazi.org/id/03F987E3-3E16-FFC6-FF63-1270176457BC |
|
treatment provided by |
Valdenar (2021-04-24 19:12:58, last updated 2023-11-02 15:06:20) |
|
scientific name |
Kylinxia zhangi ZENG,ZHAO AND HUANG |
| status |
GEN.ETSP.NOV. |
Kylinxia zhangi ZENG,ZHAO AND HUANG , GEN.ETSP.NOV.
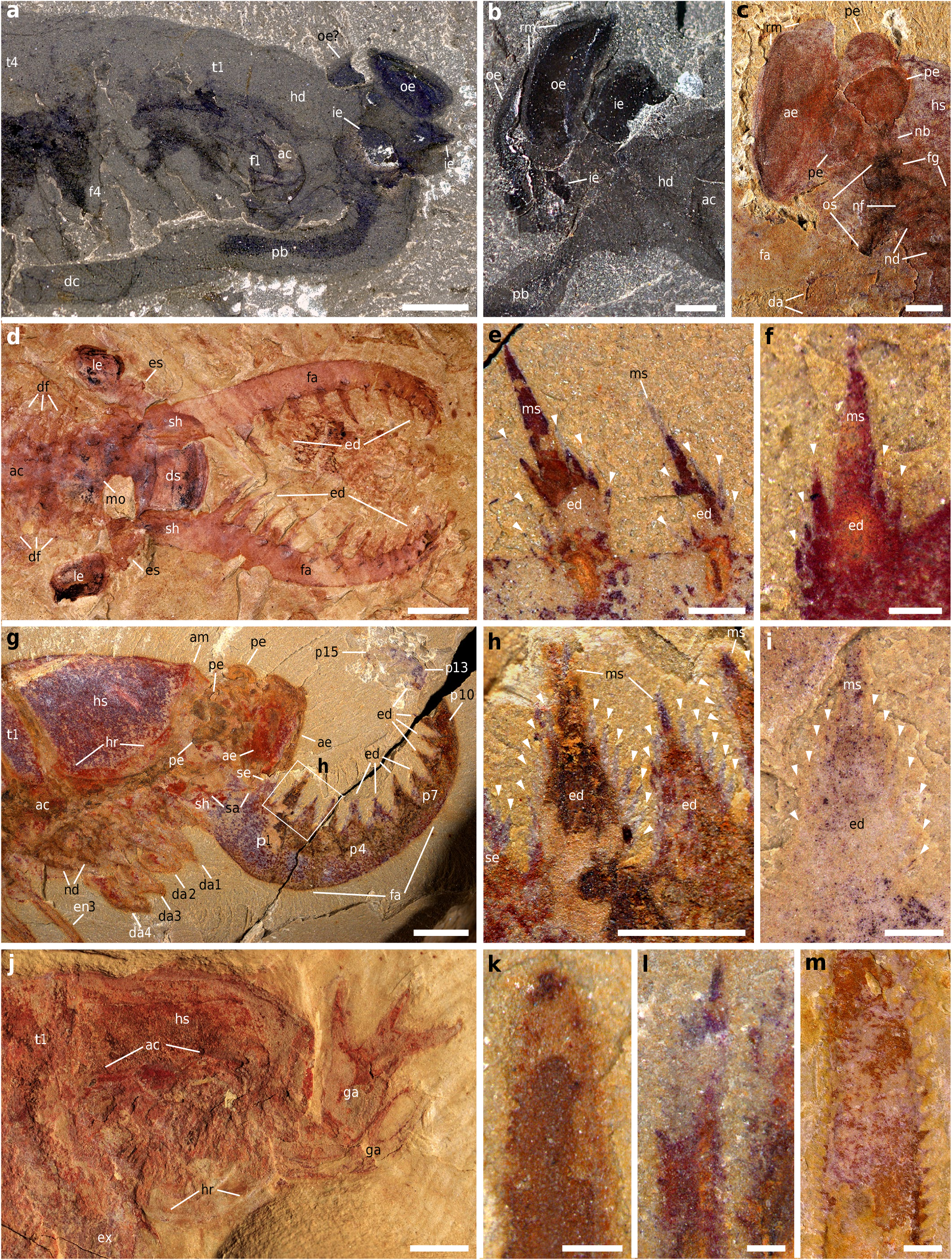
Etymology. Kylin:CHIMERICCREATUREINCHINESEMYTHOLOGY; xia:CHINESE WORDFORSHRIMP-LIKEARTHROPOD; AND zhang: AFTERYEHUIZHANG, WHO CONTRIBUTED THE PARATYPE. Holotype. NANJINGINSTITUTEOFGEOLOGYANDPALAEONTOLOGY (NIGP) 171304, PARTANDCOUNTERPART (FIGS. 1A, C, D, G, 2C, IANDEXTENDEDDATA View Fig FIGS.1A, B, D, 3N, O View Fig , 4G View Extended , 5 View Extended , 6B, G, I View Extended ). Referredmaterial. PARATYPE:YINGLIANGSTONENATURALHISTORYMUSEUM (YLSNHM) 01124 (FIGS. 1B, E, F, H, 2G, HANDEXTENDEDDATAFIGS View Fig . 1C, E, 4A, F View Extended , 6A, H View Extended ). OTHERSPECIMENS:NIGP 171305 ( EXTENDEDDATAFIGS.2A, B View Fig , 4D View Extended , 6C View Extended ), NIGP 171306 ( EXTENDEDDATAFIGS.2C, D View Fig , 4B View Extended , 6D View Extended ), NIGP 171307 ( EXTENDEDDATAFIGS. 2E–G View Fig , 4C View Extended , 6E View Extended ) ANDNIGP 171308 (EXTENDEDDATA FIGS.2H, I View Fig , 4E View Extended , 6F View Extended ). Localityandhorizon. JIANSHAN,HAIKOU,KUNMING,YUNNAN,CHINA;MAO- TIANSHANSHALEMEMBER,YU’ANSHANFORMATION, WutingaSpiS – EoredliChia BIOZONE (CAMBRIANSTAGE 3). Diagnosis. AN EUARTHROPOD POSSESSINGA PAIR OF UPWARD-ORIENTATED FRONTALMOST APPENDAGES,EACHCONSISTING OF A STOUTSHAFT AND A DISTAL ARTICULATED REGION COMPOSED OF 15 PODOMERES BEARING ELONGATE TRI- ANGULARENDITESWITHTWOROWSOFUPTOSEVENSHARPAUXILIARYSPINES. FIVESTALKEDCOMPOUNDEYES. HEADSHIELDWITHROUNDEDGENALANGLES. ANTERIORMOSTFOURPOST-ORALAPPENDAGESDIFFERENTIATEDFROMPOSTERIOR ONES.TRUNKCONSISTINGOFUPTO 25 TERGITES.PYGIDIUMMERGEDFROMAT LEASTFIVESOMITES.TAILFANCOMPRISINGTHREELOBES. Descriptionandcomparisons Kylinxia ISKNOWNFROMSIXSPECIMENSWITHWELL-PRESERVEDSOFTPARTS (ADETAILEDDESCRIPTIONISPROVIDEDINTHESUPPLEMENTARYDISCUSSION). ITHASFIVESTALKEDCOMPOUNDEYESONTHEHEAD, OFWHICHTHEANTERIOR TWO ARE AT LEAST TWICE AS LARGE AS THE POSTERIOR THREE (‘AE’ AND ‘PE’ IN FIGS.1C, 2C, GANDEXTENDEDDATAFIGS View Fig . 1D, E, 2B, D, F, I View Fig , 3N, O View Fig , 4A–G View Extended , 5B View Extended ). THISCONFIGURATIONOFEYESISREMINISCENTOFTHEPECULIARFIVEEYESIN OpabiniaregaliS 10. THEEYEARRANGEMENTBETWEENTHETWOTAXAIS COMPARABLE,WITH THE ANTERIORAND POSTERIOR EYES IN Kylinxia CORRE- SPONDINGTOTHEOUTERANDINNEREYESIN Opabinia , RESPECTIVELY 10 (‘AE’, ‘PE’, ‘OE’ AND ‘IE’, IN FIG. 2A–C View Fig , EXTENDEDDATA FIG. 3G, H, N, O View Fig ). IN BOTH Kylinxia AND Opabinia , THEEYES AREBORDEREDWITHMARGINALRIMS (‘RM’ IN FIGS. 1C View Fig , 2B, C View Fig ).THETWOORTHREEMEDIANEYESINEUARTHROPODSINCLUDING CAMBRIANHELMETIIDS ANDHYMENOCARINES 11 (‘LE’ AND ‘ME’ INEXTENDED DATAFIG.3J–M View Fig ) MAYTHUSBEHOMOLOGOUSTOTHEPOSTERIORORINNERCOM- POUNDEYES IN Kylinxia OR Opabinia , RESPECTIVELY,BYREFERRING TOTHEIR COMPARABLEANATOMICAL POSITIONS AND SMALLERSIZES.
THE FRONTALMOST APPENDAGESOF Kylinxia ARERADIODONT-LIKE (‘FA’ IN FIGS.1C View Fig , 2GANDEXTENDEDDATAFIGS View Fig .2B, D, F,I, 4A–G View Extended ).THESEAPPENDAGES SHARE KEY MORPHOLOGICALFEATURESOF THE RADIODONTFAMILIES ANOMALO- CARIDIDAEAND AMPLECTOBELUIDAEREPRESENTEDBY AnomaloCariS 12 AND RamSkoeldia 13, RESPECTIVELY(‘FA’ INFIG.2D, GANDEXTENDEDDATAFIG View Extended View Fig .4F–I; DETAILEDCOMPARISONS ARE PROVIDEDIN THESUPPLEMENTARYDISCUSSION). THEIRSHAREDSIMILARITIESINCLUDE12–15DISTALARTICULATEDPODOMERES(‘P1’– ‘P15’ INFIGS.1C View Fig , 2GANDEXTENDEDDATAFIGS View Fig .2D,F, 4A–C,F–I View Extended ),ASHAFTREGION WITHAN OBLIQUEARTHRODIAL MEMBRANEAND A SINGLEPAIROF ENDITES (‘SH’, ‘SA’ AND ‘SE’ INFIGS.1C View Fig , 2 GANDEXTENDEDDATAFIGS View Fig View Fig .1D, E, 2B,F,I View Fig , 4A–K View Extended , 5B View Extended ), ENDITESOFALTERNATINGLENGTHS (‘SE’AND‘ED’INFIGS.1C,2D–HANDEXTENDED DATAFIGS.1D, E View Fig , 2B, I View Fig , 4A, D–I View Extended ) ANDROUGHLY SYMMETRICALLY ARRANGED AUX- ILIARYSPINESONEACHENDITE ( ARROWHEADSINFIG.2E,F,H,I View Fig );WEALSOINFERA
SIMILARFUNCTIONALMORPHOLOGY 12, 13 ( FIG.2D View Fig ,GANDEXTENDEDDATAFIG.4F–I). HOWEVER,IN CONTRAST TO RADIODONTS,THE UPWARD ORIENTATION ANDTHE ABSENCEOF OUTERSPINESIN THEFRONTALMOST APPENDAGES OF Kylinxia (‘FA’ INFIGS.1C View Fig , 2 GANDEXTENDEDDATAFIGS View Fig View Fig .1D, E, 2B,D,F,I View Fig , 4A–G View Extended )AREFEATURESOF MEGACHEIRANS 14 ( FIG.2 JANDEXTENDEDDATAFIG View Extended View Fig .7A–F,H),GREAT-APPENDAGE BIVALVEDEUARTHROPODS 15 ANDISOXYIDS 16, 17 ( EXTENDEDDATAFIG.7J–O View Extended ).THE PRESENCE OF AUXILIARY ENDITIC SPINESONTHE FRONTALMOST APPENDAGES OF RADIODONTS ( ARROWHEADSINFIG.2E,F View Fig ), Kylinxia ( ARROWHEADSINFIG.2H,I View Fig ) ANDMEGACHEIRANS( FIG.2K– MANDEXTENDEDDATAFIG View Extended View Fig .7D–I)STRENGTHENS THE MORPHOLOGICAL SIMILARITIESOF THEFRONTALMOST APPENDAGES AMONG THESETAXA,BECAUSESUCHAUXILIARYENDITICSPINESAREABSENTINISOXYIDS 16, 17 ANDOTHERCAMBRIANEUARTHROPODS 18 ( EXTENDEDDATAFIG.7J–O View Extended ).
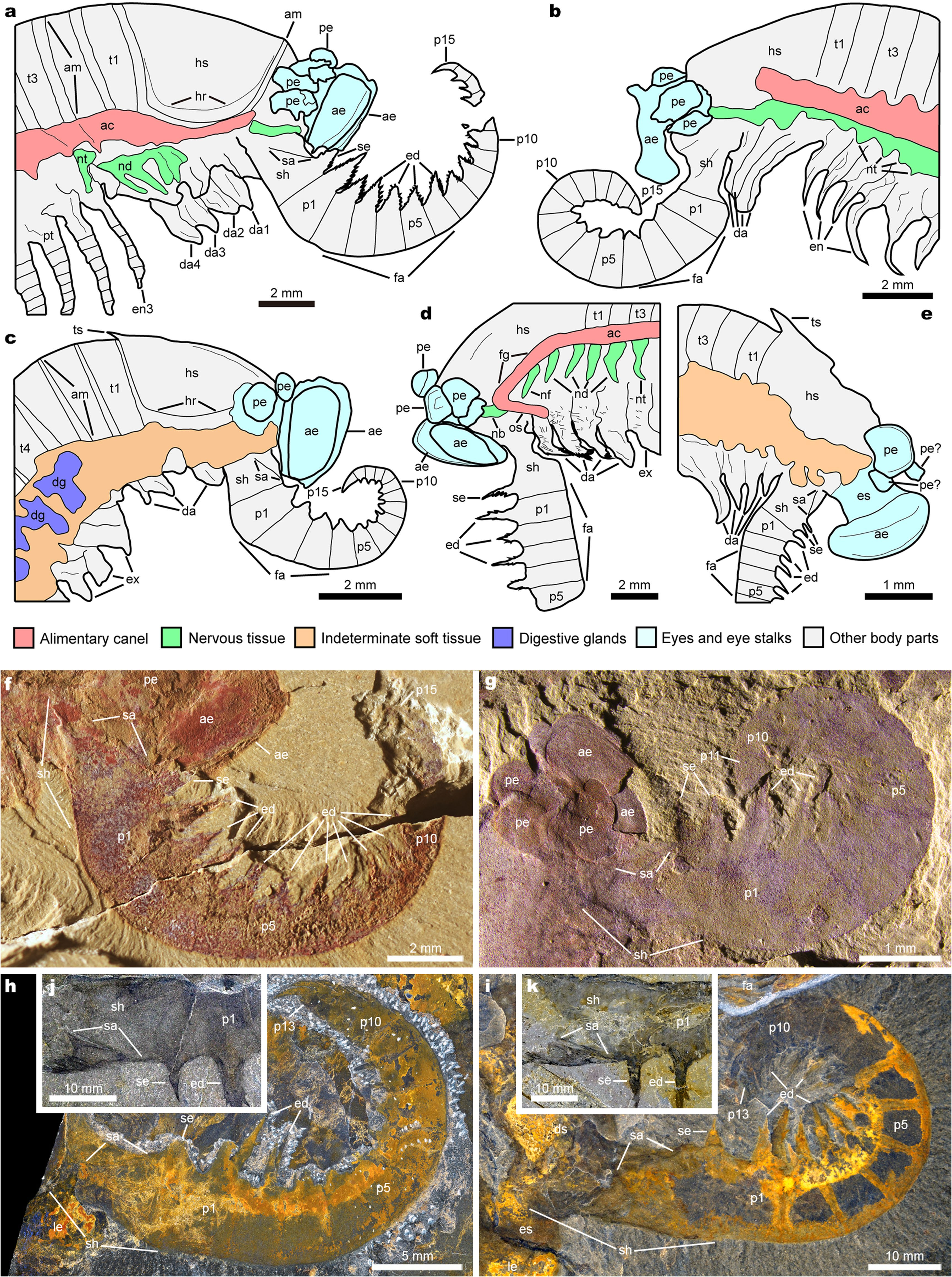
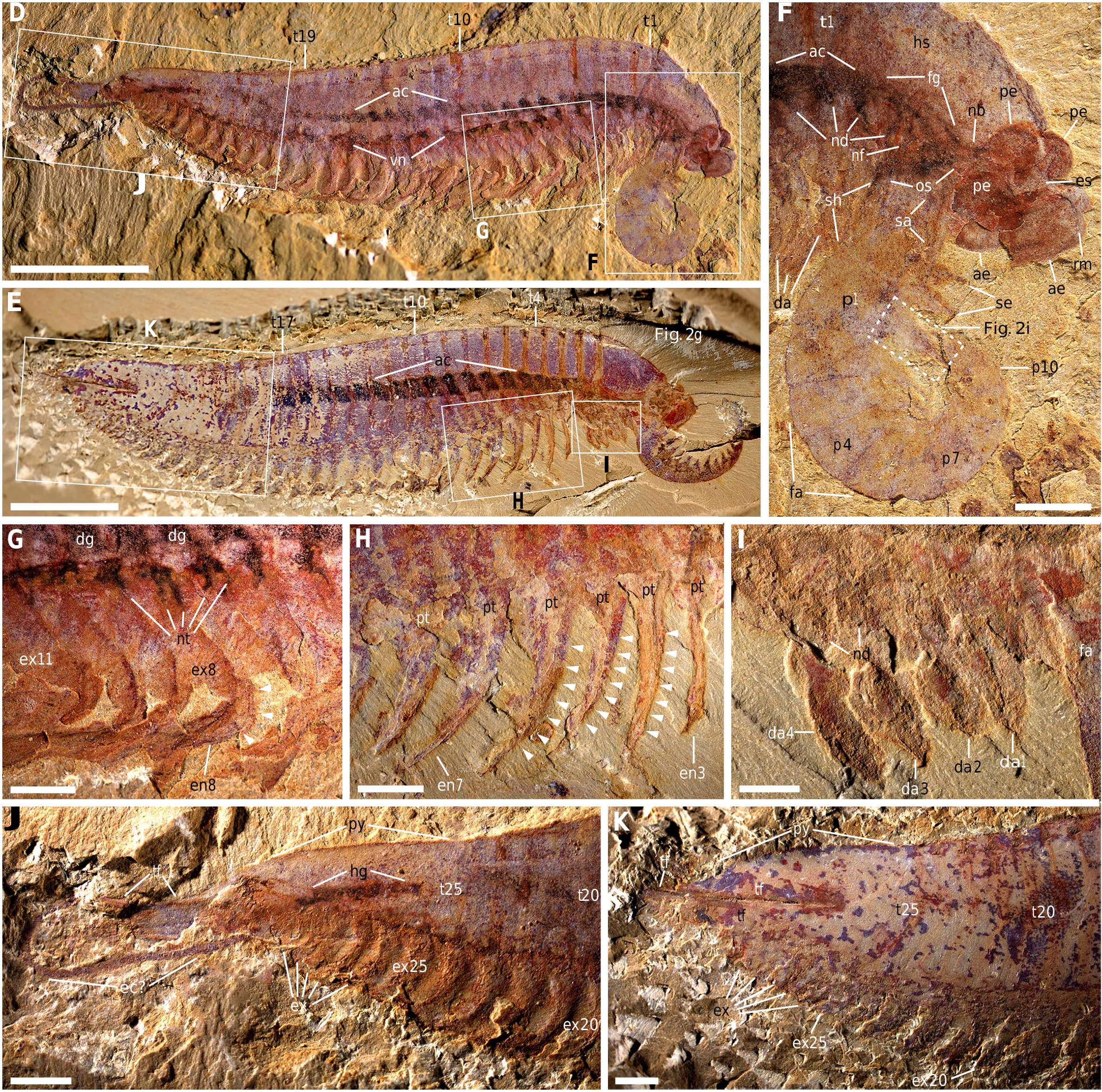
THE BODY PLANOF Kylinxia —WHICH CONSISTS OF A FUSED HEAD SHIELD, A MULTI-SEGMENTED TRUNK, A PYGIDIUM AND ARTHRODIZED POST-ORAL BIRAMOUS APPENDAGES—IS TYPICAL OF DEUTEROPODS ANDIS PARTICULARLY MEGACHEIRAN-LIKE ( FIG. 1A, B View Fig AND EXTENDED DATAFIGS. 1 View Fig , 2A, C, E, H View Fig ). THE SEMI-CIRCULARHEADSHIELDOF Kylinxia WITHROUNDEDGENALANGLESISVERY SIMILARTOTHEHEADOFTHEMEGACHEIRAN HaikouCariS 14 (‘HS’ INFIG.1C View Fig , 2G, J View Fig AND EXTENDEDDATA FIGS. 1D, E View Fig , 2D, F, I View Fig , 4A–E View Extended , 7A View Extended ). Kylinxia HASUP TO 25 TRUNK TERGITES (‘T1’–‘T25’IN FIG. 1A, B View Fig AND EXTENDEDDATA FIGS. 1 View Fig , 2A, C, E, H View Fig ), WHICHFALLSWITHINTHE RANGEOF 20–33 TERGITESIN ‘MULTI-SEGMENTED’ MEGACHEIRANSINCLUDING Sklerolibyon, Jianfengia AND FortiforCepS 19
( EXTENDEDDATAFIG. 7B View Extended ). ALTHOUGHAPYGIDIUMTHATCOVERSMULTIPLE APPENDAGES IN Kylinxia IS CHARACTERISTIC OF ARTIOPODANS 18 AND THE THREE-LOBED TAIL FANCOMPRISING A MIDDLE AND A PAIR OF LATERAL FLAPS OF Kylinxia HASFOUND COUNTERPARTSIN MEGACHEIRANS 19, FUXIANHUIIDS 18 ANDHYMENOCARINES 17, THEFUSEDPYGIDIUMARTICULATEDWITHATAILFANOF Kylinxia ISUNIQUEAMONGCAMBRIAN EUARTHROPODS (‘PY’ AND ‘TF’ INFIG.1G, HANDEXTENDEDDATAFIGS View Fig .1D,E, 6E–H View Extended ).THETRUNKAPPENDAGESOF Kylinxia AREBIRAMOUS,COMPRISINGANENDOPODITEOF ATLEASTSEVENPODOMERES (‘EN’ AND ARROWHEADSIN FIG. 1D, EANDEXTENDEDDATA FIGS.1D View Fig , 2C, D View Fig , 4A View Extended ) AND ANOVALEXOPODITEFLAPFRINGEDWITHLONGLAMELLAE (‘EX’ INFIG.1D, G, H View Fig AND EXTENDED DATA FIGS. 1D, E View Fig , 2F View Fig , 4C View Extended ). THE POST-ORAL APPENDAGES IN
Kylinxia AREHOMONOMOUS,EXCEPTTHATTHEANTERIORMOSTFOURAPPEND- AGE PAIRS (TWO IN THEHEAD ANDTWOIN THE TRUNK) AREDIFFERENTIATED AND SMALLER (‘DA’ INFIGS.1C,F View Fig , 2GANDEXTENDEDDATAFIGS View Fig .2B,D, F,I, 3N View Fig , 4A–E View Extended , 5B View Extended ). THEARRANGEMENT ANDMORPHOLOGYOFTHE APPENDAGESOF Kylinxia AREMOSTSIMILARTO THOSEOFMEGACHEIRANS AMONGCAMBRIANEUARTHRO- PODS 19 (‘DA’, ‘EN’ AND ‘EX’ INEXTENDEDDATA FIG. 7B View Extended ).
THE SPECIMENS OF Kylinxia EXHIBIT TWO TOPOLOGICALLY CONSISTENT STRANDS OF DARK MATTERTHROUGH THE BODY, ONE CENTRAL (‘AC’ IN FIG.1A–C View Fig AND EXTENDEDDATA FIGS. 1 View Fig , 2A, C View Fig , 6A–D View Extended ) AND THE OTHER VENTRAL (‘VN’ IN FIG. 1A ANDEXTENDEDDATAFIGS. 1A, B, D View Fig , 2A, C View Fig , 6B–D View Extended ). THE TWOSTRANDS CORRESPOND WELL IN POSITION AND MORPHOLOGY TO THE EUARTHROPOD
ALIMENTARYCANALANDVENTRALNERVECORD,RESPECTIVELY 20, 21. THEALIMEN- TARYCANALISASSOCIATEDWITHPAIREDDIGESTIVEGLANDS (‘DG’ INFIG.1D View Fig AND EXTENDEDDATAFIGS.1D, E View Fig , 2D, G View Fig , 4C View Extended ).INEACHTRUNKSEGMENTOF Kylinxia , BIFURCATING STRUCTURESINNERVATE APPENDAGES FROM THE VENTRAL NERVE CORD (‘NT’ INFIG. 1D View Fig ANDEXTENDEDDATAFIG. 6B–D View Extended ) AND ARECOMPARABLE TOTHEPAIREDLEGNERVESINOTHERCAMBRIAN EUARTHROPODS 20, 22.
INALATERAL VIEW,THEALIMENTARYCANALANDTHE VENTRALNERVECORDMEET ATTHE HEAD REGION (‘AC’,‘FG’,‘ND’ AND ‘NT’ IN FIGS. 1A–C View Fig , 2CAND View Fig EXTENDED DATAFIGS.1A,B,D View Fig , 2B, D View Fig , 3N, O View Fig , 4A,B,D View Extended , 5B View Extended ).MORPHOLOGICALINTERPRETATION AIDEDBYELEMENTALANALYSIS ( EXTENDEDDATAFIG.5 View Extended ANDSUPPLEMENTARY DISCUSSION) OF THIS REGION SHOWSOESOPHAGUS,FOREGUT (‘OS’ AND ‘FG’, RESPECTIVELY, INFIGS.1C View Fig , 2 CANDEXTENDEDDATAFIGS View Fig View Fig .1D, 2B View Fig , 3N, O View Fig , 4D View Extended , 5B, D View Extended )ANDPOSSIBLEASSOCIATEDNERVOUSTISSUES (‘NB’,‘ND’ AND ‘NF’ INFIGS. 1C View Fig , 2C, GANDEXTENDEDDATAFIGS View Fig View Fig .1A, 2B View Fig , 3N,O View Fig , 4A,D View Extended , 5B,D View Extended ). THEANTERIORMOST OFTHESEISPUTATIVENERVOUSTISSUEBETWEENTHEOESOPHAGEALANDOCULAR REGIONS (‘NB’ INFIGS. 1C View Fig , 2 CANDEXTENDEDDATAFIGS View Fig View Fig .1D, 2B View Fig , 3N, O View Fig , 4D View Extended , 5B, D View Extended ),ANDSITUATEDPOSTERIORLY ARENERVESINTOTHEFRONTALMOST APPENDAGES ANDDIFFERENTIATED POST-ORALAPPENDAGES (‘NF’ AND ‘ND’ INFIGS. 1C View Fig , 2C,G View Fig ANDEXTENDEDDATAFIGS. 1D View Fig , 2B View Fig , 3N, O View Fig , 4D View Extended , 5B, D View Extended ). TAKEN TOGETHER,THE POST-OCULARFRONTALMOST APPENDAGESOF Kylinxia ANDTHE OESOPHAGEAL POSITION OF THEIR NERVES ARE MOST CONSISTENTWITH ADEUTEROCEREBRAL IDENTITY,THEDEFININGFEATUREOF DEUTEROPODA 7, 22.
Phylogeneticimplicationsof Kylinxia
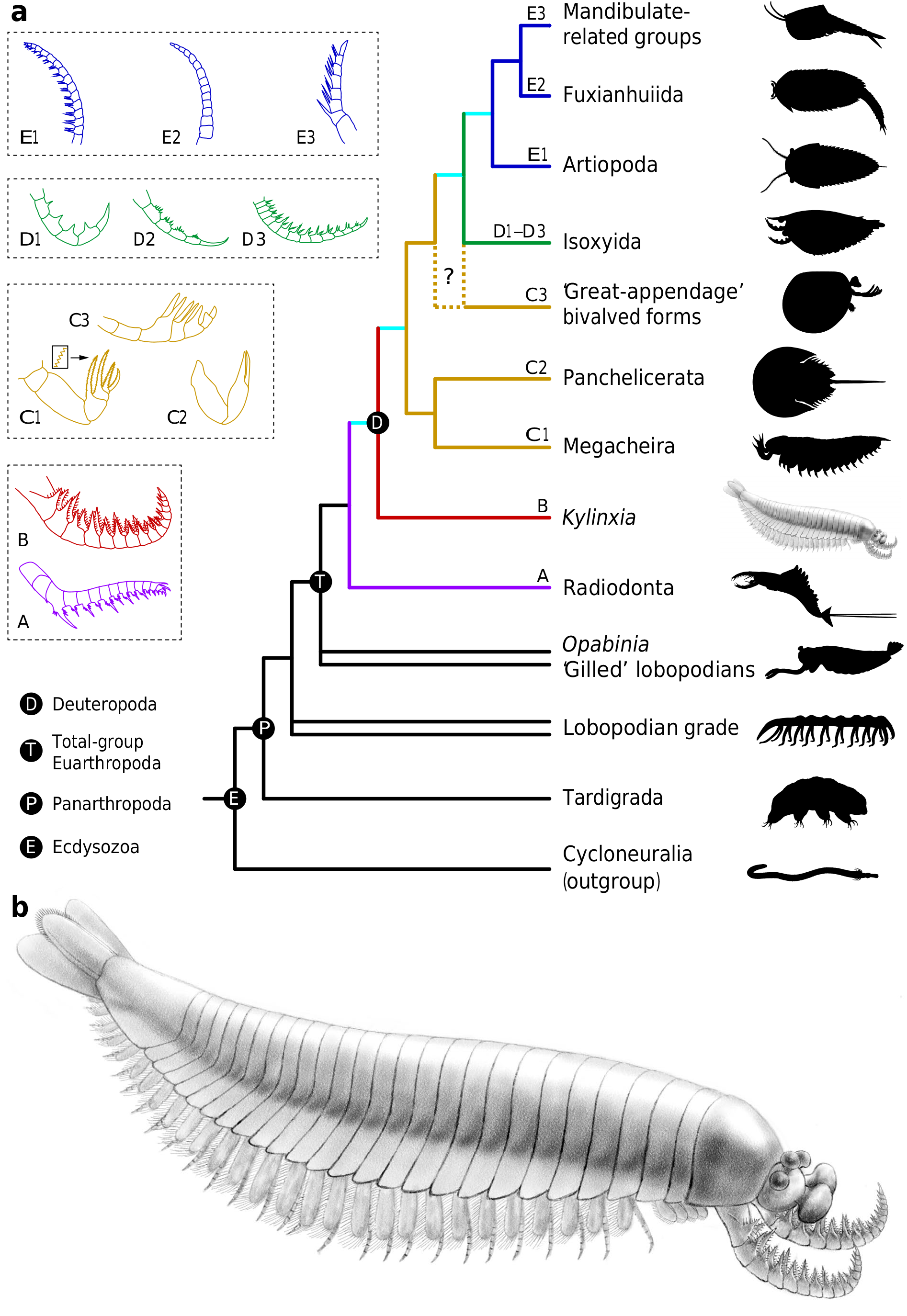
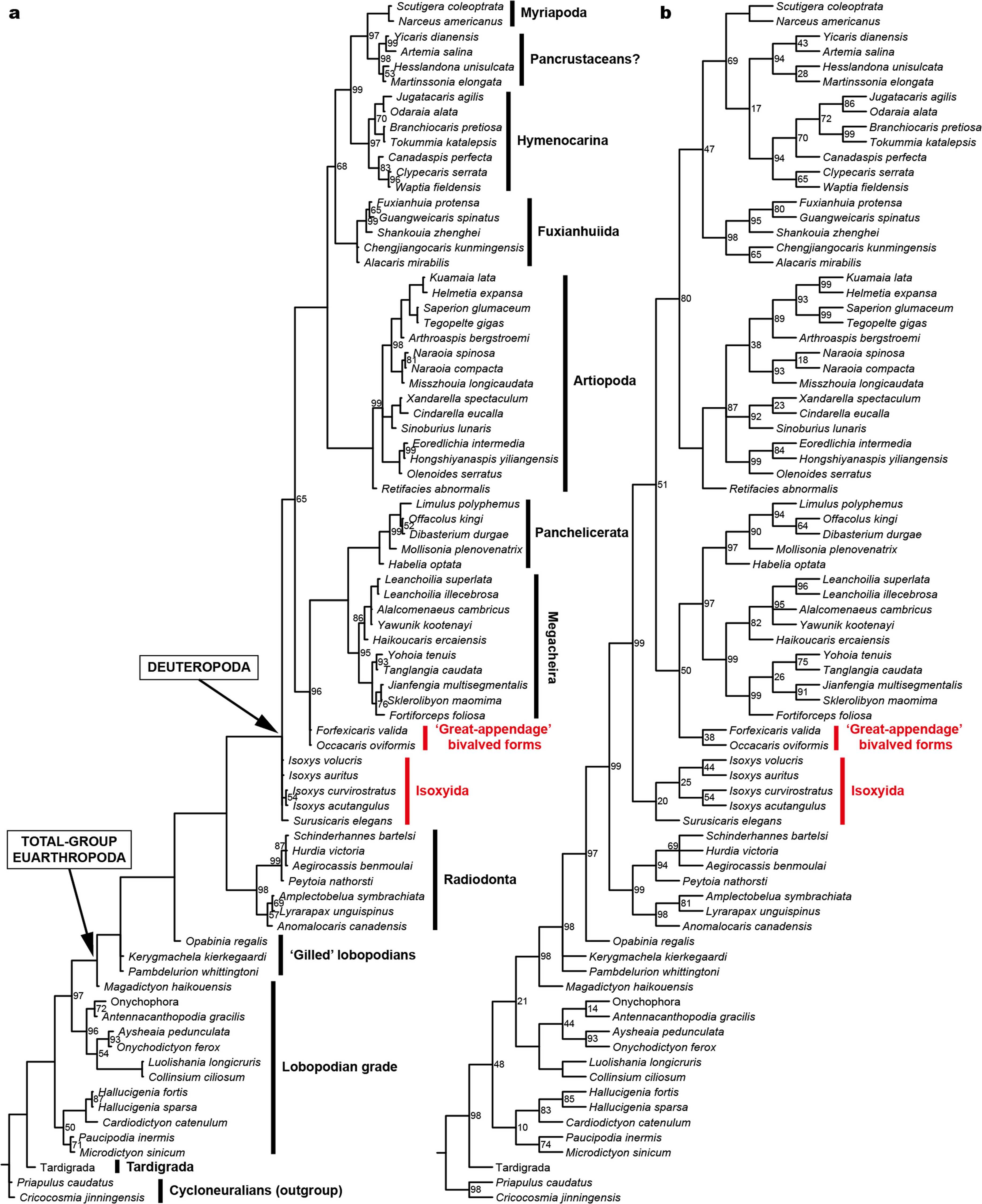
IN SUMMARY, Kylinxia HAS A CHIMERIC BODY PLAN THAT COMBINES KEY MORPHOLOGICALFEATURES OF Opabinia , RADIODONTA ANDDEUTEROPODA ( ESPECIALLYMEGACHEIRA) ( FIG. 3B View Fig ANDEXTENDEDDATAFIG. 8 View Extended ). TORESOLVE ITS PHYLOGENETIC POSITION AMONG EUARTHROPODS, WEBUILT A MORPHO- LOGICAL MATRIXBY ASSEMBLING CHARACTERSFROM PUBLISHED DATASETS OF PANARTHROPODPHYLOGENY 6, 8 (THECHARACTERLISTANDCOMPLETEREFERENCE LIST AREPROVIDEDINTHESUPPLEMENTARYDISCUSSION).OURPHYLOGENETIC RECONSTRUCTIONRESOLVES Kylinxia ASTHEMOSTBASALDEUTEROPOD AND AS A TRANSITIONALTAXONTHATBRIDGESBETWEENRADIODONTAANDDEUTEROPODA ( FIG.3A View Fig , EXTENDEDDATAFIG.9 View Fig ).THISPHYLOGENETICPLACEMENTOF Kylinxia IS STRONGLYSUPPORTEDBY ACONFIGURATIONOFEYESSIMILARTOTHATOF Opabinia, RADIODONT-LIKEFRONTALMOST APPENDAGES AND ADEUTEROPOD BODY THAT FEATURES AFUSEDHEAD SHIELD,AN ARTHRODIZED TRUNK,AFUSED PYGIDIUM ANDJOINTEDENDOPODITES.PLACEDNEARTHEROOTOFDEUTEROPODA, Kylinxia OFFERS A KEY REFERENCENODE FOR EXPLORINGTHE ORIGINS OFSEVERAL CRITICAL EVOLUTIONARYNOVELTIES DURING THE EARLY EVOLUTION OF EUARTHROPODS, INCLUDING THECOMPLETE ARTHRODIZATION OFTHE BODY,ARTHROPODIZATION OFTHETRUNKAPPENDAGES ANDCEPHALIZATIONOF AMULTI-SEGMENTEDHEAD, WHICHAREABSENTIN RADIODONTA 7 BUTPRESENTIN Kylinxia .
Kylinxia ISHELPFULINRESOLVINGTHE INTERRELATIONSHIPS AMONGMAJOR DEUTEROPODASTEM GROUPS.OUR PHYLOGENY RECOVERS A VERY BASALPAR- APHYLETIC LINEAGE OF DEUTEROPODA FEATURING TYPICAL RAPTORIAL FRON- TALMOST APPENDAGES AND CONSISTINGOF MEGACHEIRA, PANCHELICERATA, GREAT-APPENDAGEBIVALVEDFORMS ANDISOXYIDA ( FIG. 3AANDEXTENDED View Fig DATA FIG. 9 View Fig ). SUCH ABASAL POSITION OF MEGACHEIRA IS LARGELY A CONSE- QUENCE OF INCLUDING Kylinxia , WHICH COMBINES Opabinia , RADIODONT ANDMEGACHEIRANCHARACTERISTICS,INOUR ANALYSIS.A CLOSEPHYLOGENETIC LINKBETWEEN RADIODONTAAND MEGACHEIRAHASBEEN SUGGESTEDONTHE BASISOFTHEIRMORPHOLOGICALSIMILARITIESINFRONTALMOST APPENDAGES 14, 23, BUT IN THAT CONTEXT, RADIODONTA ISCONSIDERED A GROUP THATLEADS TO THE ORIGINOFONLYTHE CHELICERATA 14, 23 RATHERTHANTOTHE ORIGINOF ALL DEUTEROPODS 6, 8, 9. ACOMPARABLEBASALPLACEMENTOFPARAPHYLETICMEG- ACHEIRANS AND A TIGHT PARAPHYLETIC LINK BETWEEN MEGACHEIRANS AND PANCHELICERATESHAVEALSOBEENREPORTEDINARECENT STUDY 9. HOWEVER,IN THAT ANDOTHERCONTRIBUTIONS,ISOXYIDS 8, 9 ANDEVEN HYMENOCARINES 6 ARE RECONSTRUCTED ASMOREBASALTHANMEGACHEIRANS,ALTHOUGHTHISPLACE- MENTREMAINSUNDER DEBATE 4, 24. OURPHYLOGENETICINFERENCEINSTEAD FAVOURS A POSITIONOFISOXYIDA ABOVEMEGACHEIRA ANDPANCHELICERATA, WITHISOXYIDAANDGREAT-APPENDAGE BIVALVEDFORMSEITHERPARAPHYLETIC OR MONOPHYLETIC ( FIG. 3A View Fig , EXTENDED DATA FIG. 9 View Fig AND SUPPLEMENTARY
DISCUSSION).OUR EXPERIMENTALANALYSISWITH Kylinxia OMITTEDRECOVERS THEBASALPLACEMENTOFISOXYIDSFOUNDINPREVIOUSSTUDIES 8, 9 (EXTENDED DATA FIG. 10 View Extended , SUPPLEMENTARYDISCUSSIONAND SUPPLEMENTARY DATA 2), EMPHASIZINGTHEINFLUENCEOF Kylinxia ONTHEEUARTHROPODPHYLOGENY.
Evolutionof arthropodfirstappendages
THE EVOLUTION OF THE FRONTALMOST APPENDAGES HAS BEEN A KEY ISSUE IN RESOLVING THE ORIGIN AND EARLY EVOLUTION OF EUARTHROPODS 3, 24, 25.
THE RADIODONT-LIKEFRONTALMOST APPENDAGES ON AMEGACHEIRAN-LIKE BODYIN Kylinxia PROVIDES STRONGEVIDENCEFORTHE HOMOLOGYBETWEEN RADIODONT ANDMEGACHEIRAN FRONTALMOST APPENDAGES.UNDER THEPAR- SIMONYCRITERION,THESHAREDCHARACTERSINFRONTALMOST APPENDAGESOF RADIODONTAAND Kylinxia ARE VERY UNLIKELYTO BE CONVERGENT.DESPITE THEMISMATCHEDSEGMENTALAFFINITIESOFTHEFRONTALMOST APPENDAGESIN RADIODONTA 26, AND Kylinxia ANDTHEMEGACHEIRANS 22,THEIRHOMOLOGYIS HERECONSIDEREDMOSTLIKELY(SEECONTROVERSIES AND ALTERNATIVEHYPOTH- ESES 14, 19, 23 INTHESUPPLEMENTARYDISCUSSION).NOTABLY,OURPHYLOGENETIC TESTS SHOW THAT THEUNCERTAINTIES IN CODING THE SEGMENTAL AFFINITY OF RADIODONTFRONTALMOST APPENDAGESDONOTINFLUENCETHEPHYLOGENETIC POSITION OF Kylinxia NORTHE OVERALLTREETOPOLOGY.
THEVERY BASALPHYLOGENETIC PLACEMENT OF Kylinxia AND OTHER DEU- TEROPODS THATHAVE TYPICALRAPTORIALFRONTALMOST APPENDAGESFAVOURS THEIDEATHATRAPTORIALFRONTALMOST APPENDAGES WEREAPLESIOMORPHIC FEATUREOF ANCESTRALDEUTEROPODS 8, 9, 14, 23 (‘A’–‘D’ INFIG. 3A View Fig ), INCONTRAST TO THE ALTERNATIVE ANTENNIFORM HYPOTHESIS 6, 21. SUCH ABASAL GROUP- ING ALSOCASTS NEW LIGHTON THE ORIGINS OF FRONTALMOST APPENDAGES IN MAJOR EUARTHROPOD CROWN GROUPS:THE CHELICERAE INCHELICERATA AND THE(FIRST) ANTENNAEINMANDIBULATA.THESISTERGROUPINGOFMEGACHEIRA AND PANCHELICERATA IN OUR PHYLOGENY HAS GAINED PALAEONEUROLOGICAL EVIDENCEFROMACHELICERATEGROUNDPATTERNINTHEMEGACHEIRANBRAIN 22, ALTHOUGHALTERNATIVE SCENARIOS SUCH AS A CLOSE PARAPHYLETIC GROUPING OFMEGACHEIRANS ANDPANCHELICERATES 9 AND ATRADITIONALARTIOPODAN AFFINITYOFCHELICERATA EXIST 6. THUS, OURPHYLOGENETICTOPOLOGYSUP- PORTSTHEORIGINOF CHELICERATAFROM BASALDEUTEROPODSWITH RAPTORIAL FRONTALMOST APPENDAGES AND, IN THISSENSE,CHELICERAEWEREMODIFIED FROMMEGACHEIRANGREAT APPENDAGES 14, 22, 23 (‘C1’ AND ‘C2’ INFIG. 3A View Fig ), IN CONTRAST TO THE MODEL INWHICH CHELICERAE EVOLVED FROM SMALL SENSO- RIALANTENNAE 9.
OURPHYLOGENETICRECONSTRUCTIONRETRIEVES ANUPTREECLADEOFDEUTER- OPODSWITHTYPICALANTENNAEASTHEIRFIRST APPENDAGES,WHICHCOMPRISES ARTIOPODA,FUXIANHUIIDA ANDMOREMANDIBULATE-RELATEDGROUPS(‘E1’– ‘E3’ IN FIG. 3AANDEXTENDEDDATAFIG View Fig .9; SUPPLEMENTARY DISCUSSION).A SIMILARMONOPHYLYOFTHESEGROUPSWASREVEALEDRECENTLY 9, DESPITETHE FACTTHATOTHERIDEASHAVEBEENPROPOSED 6.ITISNOTABLETHATTHEANTENNAE INSOMEARTIOPODANS 18, 27 (‘E1’ INFIG.3 AANDEXTENDEDDATAFIG View Extended View Fig .7P, Q) AND HYMENOCARINES 28 (‘E3’ INFIG.3A View Fig ) POSSESSPAIREDNEEDLE-SHAPEDENDITIC SPINES AND THESEHAVE BEENINTERPRETEDTOHAVE A PREDATORYFUNCTION. HOWEVER,ALMOSTIDENTICALANTENNALFORMS AREPRESENTIN ISOXYIDSSUCH AS ISoxySVoluCriS AND ISoxySaurituS 17, 29 (‘D3’ INFIG. 3AANDEXTENDED View Fig DATA FIG. 7L–O View Extended ), WHEREAS THE FRONTALMOST APPENDAGES OF OTHER ISOXY- IDSEXHIBITLESS ANTENNIFORM FEATURES 16, 17, 30 (‘D1’ AND ‘D2’ INFIG. 3A View Fig AND EXTENDED DATAFIG. 7J, K View Extended ). GIVEN THE INTERMEDIATE POSITION OF ISOXYIDA BETWEENTHEDEUTEROPODSWITHRAPTORIALFRONTALMOST APPENDAGES AND THOSE WITH TYPICAL ANTENNAE ON OUR TREES ( FIG. 3A View Fig AND EXTENDEDDATA FIG. 9 View Fig ), THE REMARKABLE MORPHOLOGICAL VARIATIONS SHOWN BY ISOXYID FRONTALMOST APPENDAGESINDICATETHATTHEFIRST ANTENNAEOFMANDIBULATA MIGHTHAVE BEENDERIVEDFROMRAPTORIALPROTOTYPESRESEMBLINGTHOSE IN ISOXYIDA (‘D1’–‘D3’ IN FIG. 3A View Fig ).
THE Kylinxia DEMONSTRATES THE IMPORTANT ROLE OFTRANSITIONAL FOS- SILS IN RESOLVING THE EARLY HISTORY OF EUARTHROPODS. THE EMERGENCE OF STEM-GROUP EUARTHROPODS WITH MORPHOLOGICALLY AND FUNCTIONALLY DIVERSEFRONTALMOST APPENDAGES ILLUSTRATESTHE BROADEXPLORATION OF MORPHOSPACEAND ECOSPACE BY EARLYEUARTHROPODS DURING THECAM- BRIAN EXPLOSION 30, WHICHPROBABLYLAIDTHEFOUNDATIONFORTHEIR LATER EVOLUTIONARY SUCCESSES.
30. Aria, C. & Caron, J. - B. Cephalic and limb anatomy of a new isoxyid from the Burgess Shale and the role of stem bivalved arthropods in the disparity of the frontalmost appendage. PLoS ONE 10, e 0124979 (2015).
8. Aria, C. & Caron, J. - B. Burgess Shale fossils illustrate the origin of the mandibulate body plan. Nature 545, 89 - 92 (2017).
9. Aria, C. & Caron, J. - B. A middle Cambrian arthropod with chelicerae and proto-book gills. Nature 573, 586 - 589 (2019).
19. Aria, C., Zhao, F., Zeng, H., Guo, J. & Zhu, M. Fossils from South China redefine the ancestral euarthropod body plan. BMC Evol. Biol. 20, 4 (2020).
14. Chen, J., Waloszek, D. & Maas, A. A new ' great-appendage' arthropod from the Lower Cambrian of China and homology of chelicerate chelicerae and raptorial antero-ventral appendages. Lethaia 37, 3 - 20 (2004).
26. Cong, P., Ma, X., Hou, X., Edgecombe, G. D. & Strausfeld, N. J. Brain structure resolves the segmental affinity of anomalocaridid appendages. Nature 513, 538 - 542 (2014).
13. Cong, P. et al. New radiodonts with gnathobase-like structures from the Cambrian Chengjiang biota and implications for the systematics of Radiodonta. Pap. Palaeontol. 4, 605 - 621 (2018).
12. Daley, A. C. & Edgecombe, G. D. Morphology of Anomalocaris canadensis from the Burgess Shale. J. Paleontol. 88, 68 - 91 (2014).
4. Daley, A. C., Antcliffe, J. B., Drage, H. B. & Pates, S. Early fossil record of Euarthropoda and the Cambrian Explosion. Proc. Natl Acad. Sci. USA 115, 5323 - 5331 (2018).
3. Edgecombe, G. D. & Legg, D. A. Origins and early evolution of arthropods. Palaeontology 57, 457 - 468 (2014).
16. Fu, D., Zhang, X. & Shu, D. Soft anatomy of the early Cambrian arthropod Isoxys curvirostratus from the Chengjiang biota of south China with a discussion on the origination of great appendages. Acta Palaeontol. Pol. 56, 843 - 852 (2011).
29. Fu, D., Zhang, X., Budd, G. E., Liu, W. & Pan, X. Ontogeny and dimorphism of Isoxys auritus (Arthropoda) from the Early Cambrian Chengjiang biota, South China. Gondwana Res. 25, 975 - 982 (2014).
23. Haug, J. T., Waloszek, D., Maas, A., Liu, Y. & Haug, C. Functional morphology, ontogeny and evolution of mantis shrimp-like predators in the Cambrian. Palaeontology 55, 369 - 399 (2012).
15. Hou, X. New rare bivalved arthropods from the Lower Cambrian Chengjiang Fauna, Yunnan, China. J. Paleontol. 73, 102 - 116 (1999).
18. Hou, X. et al. The Cambrian Fossils of Chengjiang, China: The Flowering of Early Animal Life 2 nd edn (John Wiley & Sons, 2017).
17. Legg, D. A. & Vannier, J. The affinities of the cosmopolitan arthropod Isoxys and its implications for the origin of arthropods. Lethaia 46, 540 - 550 (2013).
6. Legg, D. A., Sutton, M. D. & Edgecombe, G. D. Arthropod fossil data increase congruence of morphological and molecular phylogenies. Nat. Commun. 4, 2485 (2013).
11. Ortega-Hernandez, J. Homology of head sclerites in Burgess Shale euarthropods. Curr. Biol. 25, 1625 - 1631 (2015).
7. Ortega-Hernandez, J. Making sense of ' lower' and ' upper' stem-group Euarthropoda, with comments on the strict use of the name Arthropoda von Siebold, 1848. Biol. Rev. 91, 255 - 273 (2016).
24. Ortega-Hernandez, J., Janssen, R. & Budd, G. E. Origin and evolution of the panarthropod head - a palaeobiological and developmental perspective. Arthropod Struct. Dev. 46, 354 - 379 (2017).
20. Ortega-Hernandez, J., Lerosey-Aubril, R. & Pates, S. Proclivity of nervous system preservation in Cambrian Burgess Shale-type deposits. Proc. R. Soc. Lond. B 286, 20192370 (2019).
25. Scholtz, G. & Edgecombe, G. D. The evolution of arthropod heads: reconciling morphological, developmental and palaeontological evidence. Dev. Genes Evol. 216, 395 - 415 (2006).
27. Stein, M. A new arthropod from the Early Cambrian of North Greenland, with a ' great appendage' - like antennula. Zool. J. Linn. Soc. 158, 477 - 500 (2010).
22. Tanaka, G., Hou, X., Ma, X., Edgecombe, G. D. & Strausfeld, N. J. Chelicerate neural ground pattern in a Cambrian great appendage arthropod. Nature 502, 364 - 367 (2013).
10. Whittington, H. B. The enigmatic animal Opabinia regalis, Middle Cambrian, Burgess Shale, British Columbia. Phil. Trans. R. Soc. Lond. B 271, 1 - 43 (1975).
21. Yang, J., Ortega-Hernandez, J., Butterfield, N. J. & Zhang, X. Specialized appendages in fuxianhuiids and the head organization of early euarthropods. Nature 494, 468 - 471 (2013).
28. Yang, J., Ortega-Hernandez, J., Lan, T., Hou, J. & Zhang, X. A predatory bivalved euarthropod from the Cambrian (Stage 3) Xiaoshiba Lagerstatte, South China. Sci. Rep. 6, 27709 (2016).
Fig.2 |Comparative anatomy ofhead structures inKylinxia zhangi, Opabiniaregalis, radiodonts andmegacheirans. a, b, O. regaliS. a, USNM (NATIONAL MUSEUMOF NATURAL HISTORY)155600B,HEAD REGION.b, USNM205258, EYES.c, K.Zhangi, NIGP 171305B,HEAD REGION ANDEYES.d–f, RADIODONTS. d, AnOmalOCariS SP.,ELRC (EARLYLIFE RESEARCH CENTRE) 20001A,HEAD REGION. e, f, ENDITES OF RADIODONTFRONTAL APPENDAGES.e, RamSkOeldia COnSimiliS, NIGP 162527A.f, ParanOmalOCariS multiSegmentaliS, NIGP 154564B.g–i, K.Zhangi. g, YLSNHM 01124,HEAD REGION INFIG.1B.THE BOXEDREGION IS SHOWN INh. h, i, ENDITES OF THE FRONTALMOSTAPPENDAGES.h, YLSNHM 01124,ENDITES FROM g. i, NIGP 171305A,ENDITE FROM FIG.1C. j–m, MEGACHEIRANS.j, HaikOuCariS erCaienSiS, NIGP EC44154A,HEAD REGION.k–m,ENDITES OF MEGACHEIRANGREAT APPENDAGES.NOTE THENUMEROUS AUXILIARY SPINES ON THEENDITES.k, H. erCaienSiS, NIGP 171309.l, FOrtifOrCepSfOliOSa, NIGP 169954.m, ParapeytOia yunnanenSiS, NIGP 171310.ARROWHEADS INDICATE AUXILIARY SPINES.SCALE BARS, 5 MM (d), 2 MM(a, g, j), 1MM (b, c, e, h, m), 200 ΜM(f, i, k, l). AM,ARTHRODIAL MEMBRANE;DC,DISTAL CLAW;DF,DIFFERENTIATEDFLAPS;DS,DORSAL SCLERITE;ED,ENDITE ONDISTAL ARTICULATEDPODOMERE;F,BODYFLAP;GA,GREATAPPENDAGE;HD,HEAD; HR,MARGINAL RIMOF HEAD SHIELD;IE,INNER EYE;LE,LATERAL EYE;MO,MOUTHOPENING; MS,MAIN SPINE;OE,OUTER EYE;PB,PROBOSCIS.OTHER ABBREVIATIONS AS INFIG.1. NUMBERSREFER TOTHE TOPOLOGICAL POSITION OF THE CORRESPONDINGSTRUCTURE.
Fig. 3|Reconstruction ofKylinxia and evolutionof thefrontalmost appendages inearly euarthropods. a, SIMPLIFIED CLADOGRAMOF PANARTHROPODS BASED ON AMATRIX OF 81 TAXAAND 283 CHARACTERS (EXTENDED DATAFIG.9 AND SUPPLEMENTARY DATA 1)SHOWING KylinxiaIN RED.DOUBLE LINES INDICATE PARAPHYLETIC GROUPINGS.THEGROUPING OF GREAT-APPENDAGEBIVALVED FORMS AND ISOXYIDA IS RECONSTRUCTED AS PARAPHYLETIC AND MONOPHYLETICIN BAYESIAN AND PARSIMONY ANALYSES,RESPECTIVELY (INDICATEDBY THE DASHEDLINES AND QUESTION MARK).DIAGRAMSONTHE LEFTDEPICTTHE FRONTALMOSTAPPENDAGESIN REPRESENTATIVE TAXA.THE BOXES,COLOURS AND LETTERSA–E INDICATE DIFFERENT APPENDAGEFORMS. A,B,RADIODONT-LIKE FORMS.A,RADIODONT (PURPLE),RamSkOeldiaCOnSimiliS; B,Kylinxia Zhangi(RED).C,GREAT-APPENDAGE FORMS (YELLOW).C1,MEGACHEIRAN, FOrtifOrCepS fOliOSa; C2, PANCHELICERATE,LimuluS pOlyphemuS; C3, GREAT- APPENDAGE BIVALVED EUARTHROPOD,OCCaCariSOVifOrmiS. D,ANTENNA-LIKE RAPTORIAL APPENDAGES INISOXYIDA,SHOWINGINTERSPECIFIC VARIATIONS(GREEN). D1, ISOxySaCutanguluS; D2, ISOxySCurVirOStratuS; D3, ISOxyS aurituS. E,TYPICAL ANTENNAE INCAMBRIAN EUARTHROPODS,INCLUDING TYPICALSENSORIAL FORMS AND SOME PUTATIVERAPTORIAL FORMS.E1,ARTIOPODAN,Kuamaia lataAND KiiSOrtOQia SOperi; E2,FUXIANHUIID,ShankOuia ZhengheiAND OTHERS;E3,HYMENOCARINE, ClyperCariS Serrata. b, ARTISTIC RECONSTRUCTIONBY J.SUN.MORPHOLOGICAL DETAILS OF THE LATERAL TAIL FLAPSWERE INFERRED FROM MEGACHEIRANS SUCH AS FOrtifOrCepS.
ExtendedData Fig.4 |Drawingsof thehead regionin Kylinxia and comparison offrontalmost appendages in Kylinxia andAnomalocaris. a–g, K.Zhangi. a–e, CAMERALUCIDA DRAWINGSOF HEAD REGIONS,SEE COLOURS OF DIFFERENT ANATOMICALSTRUCTURES AT THE BOTTOM.a, YLSNHM 01124,AS IN FIG.2G. b, NIGP 171304A,AS IN EXTENDED DATAFIG.2D. c, NIGP 171307A,AS IN EXTENDED DATA FIG.2F.d, NIGP 171305A,AS IN EXTENDEDDATA FIG.2B.e, NIGP 171308,AS IN EXTENDED DATA FIG.2I. f, g, FRONTALMOST APPENDAGES OF Kylinxia. f, YLSNHM 01124.g, NIGP 171304A.h–k, FRONTAL APPENDAGESOF AnOmalOCariS CanadenSiS. h,i, FULLAPPENDAGES.h, GSC 75535.i, ROMIP 51212.j, k, SHAFT REGION.j, ROMIP 51215.k,ROMIP 59947.ABBREVIATIONS AS IN FIGS.1, 2 ANDEXTENDED DATA FIG.2.
ExtendedData Fig.5 | Anatomyand geochemical analysisof cephalic soft tissuesin Kylinxia zhangi,holotypeNIGP 171304a. a, MAGNIFICATION OF THE HEAD ANDANTERIORMOST TRUNK UNDER VISIBLE LIGHT.b, INTERPRETIVE DRAWINGOF a BY INTEGRATING OBSERVATIONS UNDER VISIBLE LIGHT ANDFROM GEOCHEMICAL ANALYSIS, SEE EXTENDED DATAFIG.4 FOR ACOLOUR KEY.c, BACKSCATTER SCANNING ELECTRON IMAGE OF a UNDER ASCANNING ELECTRONIC MICROSCOPE.d–h, ELEMENTAL MAPS OF a FROM ENERGY-DISPERSIVE X-RAY SPECTROSCOPY.d, CMAP,SHOWING ALIMENTARY CANAL WITHPOSITIVESIGNAL.e, FE MAP,SHOWINGNERVOUS TISSUEWITH POSITIVE SIGNAL.f, OVERLAY IMAGE OF CAND FE MAPS WITHTHE BLENDING MODE OF FILTERING COLOUR IN ADOBE PHOTOSHOP CS6,SHOWING THECOMPOSITIONAL DIFFERENCES BETWEEN ALIMENTARY CANAL AND NERVOUS TISSUES.g, PMAP,SHOWINGPUTATIVE DIGESTIVE GLANDS WITH POSITIVESIGNAL.h, SI MAP,SHOWING SOFTTISSUES INNEGATIVE RELIEF.PR,PROTOCEREBRAL TISSUE.OTHER ABBREVIATIONS AS INFIGS.1, 2.
ExtendedData Fig.6 |Morphological details oftrunk in Kylinxiazhangi. d, NIGP 171306.e–h, TAIL REGION.e, NIGP 171307A.f, NIGP 171308.g, NIGP a–d, SOFT TISSUEIN TRUNK.ARROWHEADS INDICATE PUTATIVEBIFURCATING NERVESINTO 171304A.h, YLSNHM 01124.i, MAGNIFICATIONOF THE TAIL FLAPS INg, SHOWINGSETAE. PAIREDTRUNK APPENDAGES.a, YLSNHM 01124.b, NIGP 171304A.c, NIGP 171305A. A,TRUNK APPENDAGE;ST,SETAE.OTHERABBREVIATIONS AS INFIGS.1, 2.
Fig.1 |Anatomy ofKylinxiazhangi from the earlyCambrian Chengjiang biota. a, NIGP 171304A.BOXED REGIONSARESHOWN INc, d, g. b, YLSNHM 01124. BOXED REGIONS ARESHOWN IN e, f, h. THE REGIONWITH ADASHED OUTLINEIS SHOWN IN FIG.2G.c,HEAD REGION INa. THEREGION WITHA DASHEDOUTLINE IS SHOWN INFIG.2I. d, e,TRUNK APPENDAGESFROM a, b, RESPECTIVELY.f, DIFFERENTIATED APPENDAGES FROM b. g, h, POSTERIORBODY REGION FROMa, b, RESPECTIVELY.ARROWHEADS INDICATE ARTHRODIAL MEMBRANES BETWEEN ENDOPODITEPODOMERES.SCALEBARS,10 MM
Extended Data Fig.7 | Frontalmost appendages of Cambrian megacheirans, isoxyids and artiopodans. a–i, GREAT APPENDAGESOF MEGACHEIRANS.a, d, HaikOuCariS erCaienSiS, NIGP 171309.a, WHOLE SPECIMEN. d, GREAT APPENDAGEIN a.b, e,FOrtifOrCepS fOliOSa,NIGP 169954.b, WHOLE SPECIMEN.e, GREAT APPENDAGEIN b. c, f, g, YOhOia tenuiS, USNM179053.c, WHOLE SPECIMEN.f, GREATAPPENDAGE INc. g, ENDITES IN f, ARROWHEADS INDICATE AUXILIARY SPINES.h, i,ParapeytOia yunnanenSiS, NIGP 171310.h, GREATAPPENDAGE. i, ENDITE SHOWING AUXILIARY SPINES INh. j–q, FRONTALMOST APPENDAGESSHOWING
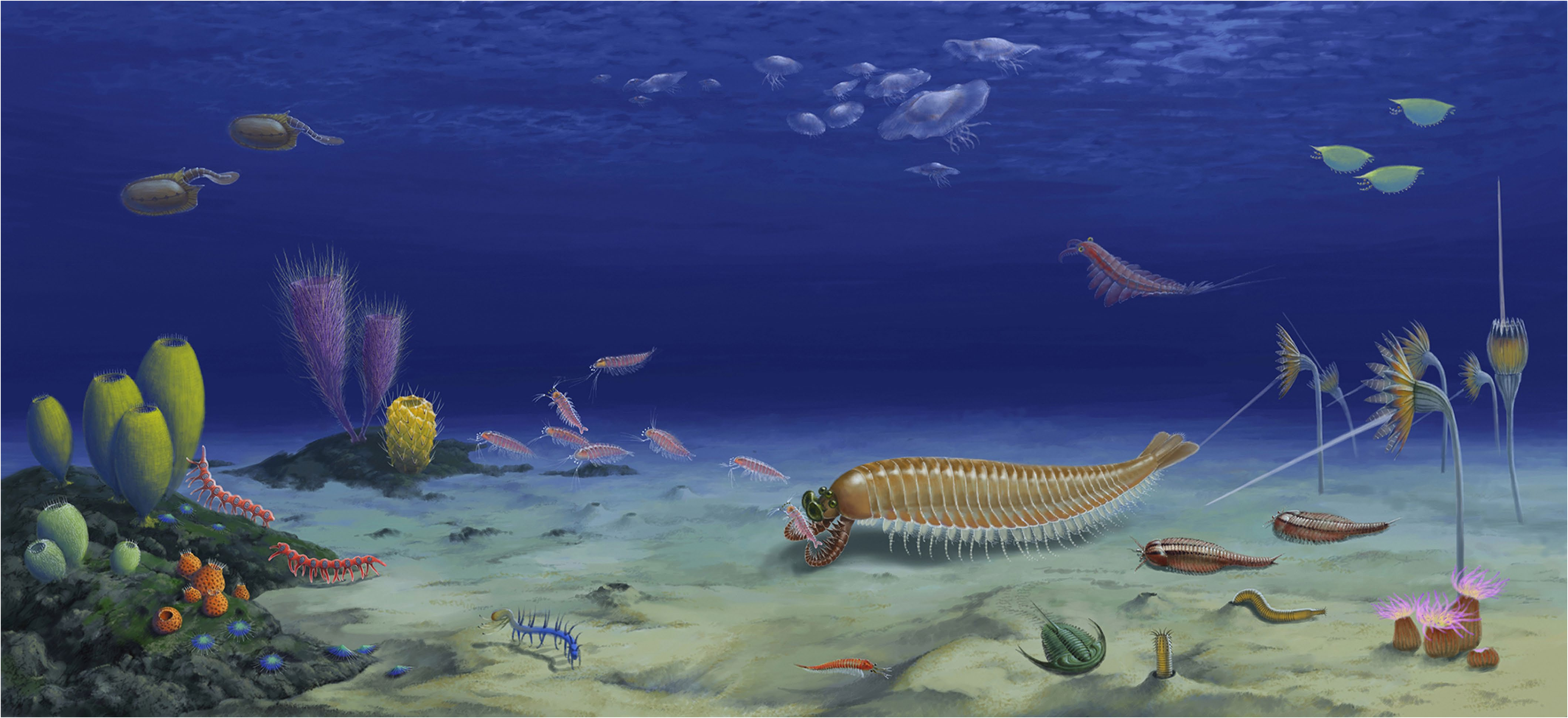
ExtendedData Fig.8 |Ecological reconstructionof Kylinxiazhangi in the earlyCambrian Chengjiang biota. ARTISTIC RECONSTRUCTIONBY J.SUN.
ExtendedData Fig.10 | Consensus trees fromBayesianand parsimony analyses ofpanarthropod relationshipswith Kylinxia zhangiomitted. a,THE 50%MAJORITY-RULE CONSENSUS TREEFROM A BAYESIAN ANALYSIS.NODAL SUPPORTSARE POSTERIORPROBABILITIES.MAJOR TAXONOMICGROUPS ARE INDICATED BY BARS ONTHE RIGHTOF TIPS.b, STRICT CONSENSUS OF ASINGLE MOST PARSIMONIOUSTREE OF 49.60546 STEPS(CONSISTENCY INDEX =0.518,RETENTION INDEX= 0.874) FROM A PARSIMONY ANALYSIS USING IMPLIED CHARACTER WEIGHTING (CONCAVITY CONSTANT k = 3). NODAL SUPPORTS AREGROUPPRESENT ANDCONTRADICTED FREQUENCY DIFFERENCES.IN a, b, NODALSUPPORTS OF 100% ARENOT SHOWN.NOTE THATISOXYIDA AND GREAT-APPENDAGE BIVALVED FORMS(BOTHIN RED) ARE PLACEDAT A MORE BASAL POSITION THANMEGACHEIRA AND PANCHELICERATA WHEN K.Zhangi IS OMITTED,WHICH IS A MAJORDIFFERENCE FROM THE RESULTSWITH K.Zhangi INCLUDED (EXTENDED DATA FIG.9).
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Genus |