Amphibola crenata ( Martyn, 1786 )
|
publication ID |
https://doi.org/ 10.5281/zenodo.176773 |
|
DOI |
https://doi.org/10.5281/zenodo.6240258 |
|
persistent identifier |
https://treatment.plazi.org/id/03F887BA-9906-543D-118F-C241FDEAFB60 |
|
treatment provided by |
Plazi |
|
scientific name |
Amphibola crenata ( Martyn, 1786 ) |
| status |
|
Amphibola crenata ( Martyn, 1786) View in CoL
Nerita nux avellana Chemnitz, 1781: 272 , figs. 1919, 1920 (not binominal).
Limax crenata Martyn 1786 , pl. LXIX (validated by ICZN Opinion 479, 1959).
Bulimus avellana Bruguière, 1789: 297 View in CoL . Refers to Chemnitz (1781) figs. 1919, 1920 (subjective synonym).
Helix crenata ; Gmelin 1791: 3623.
Helix avellana ; Gmelin 1791: 3640.
Amphibola australis Schumacher 1817: 190 View in CoL . Refers to Chemnitz (1781) figs. 1919, 1920 and Spengler (1775) pl. 9, fig. 4 (subjective synonym).
Ampullaria avellana ; Lamarck 1822: 179.
Ampullacera avellana ; Quoy & Gaimard 1832: 196, pl. XV, figs. 1–9.
Thallicera avellana ; Swainson 1840: 339. Refers to Chemnitz (1781) figs. 1919, 1920.
Ampullarina avellana ; Sowerby 1842: 64, fig. 638.
Amphibola obvoluta Jonas 1846: 35 View in CoL (subjective synonym).
Amphibola crenata View in CoL ; Suter 1913: 596, pl. LXIX, fig. 9; Farnie 1919, fig. 1; Thiele 1931: 470, fig. 661; Powell 1979: 294, pl. 54.
Amphibola avellana View in CoL ; Hubendick 1945: 106, fig. 6.
Type material: Location not known, material not examined (see Remarks).
Other material examined: New Zealand: Aotea, mudflats, 31 Dec 2003, W.F. Ponder (AMS C.446521); Otago Peninsula, Hoopers Inlet, 5 Apr 2005, J. Waters (AMS C.446522); Waimakariri, river estuary, 20 Dec 1955, W.C. Clark (AMS C.446523 ex MNZ M. 100845).
Redescription: Shell ( Fig. 2 View FIGURE 2 A): Diameter to 35 mm, globose, thick, spire short, exterior surface roughened with coarse axial sculpture. Last whorl of shell with angulated square shoulder and flat upper surface. Sinus notch corresponding with position of pneumostome and anal lobe on animal, raised and indented. Aperture circular to elongate, outer lip of aperture thickened at base opposite columella; umbilicus sometimes with vertical fold in shell surface adjacent to umbilicus, opposite aperture. Exterior grey to light brown.
Operculum ( Fig. 3 View FIGURE 3 A): Corneous, elongate, thick, brown. Exterior surface with raised striae; paucispiral nucleus marginal, basal near columellar edge, with incomplete spiral rib encircling nucleus on interior surface.
External morphology: Anterior foot dark grey to black in formalin-preserved specimens, protruding only slightly beyond shell when animal crawling. Head grey in formalin-preserved specimens with black pigmented longitudinal stripe on snout; dorsal surface of mantle black. Columellar muscle visible externally as large, white structure, occupying ventral surface between foot and digestive gland. Ovotestis visible externally as bright yellow to orange multi-lobed structure in formalin-preserved specimens, embedded in digestive gland.
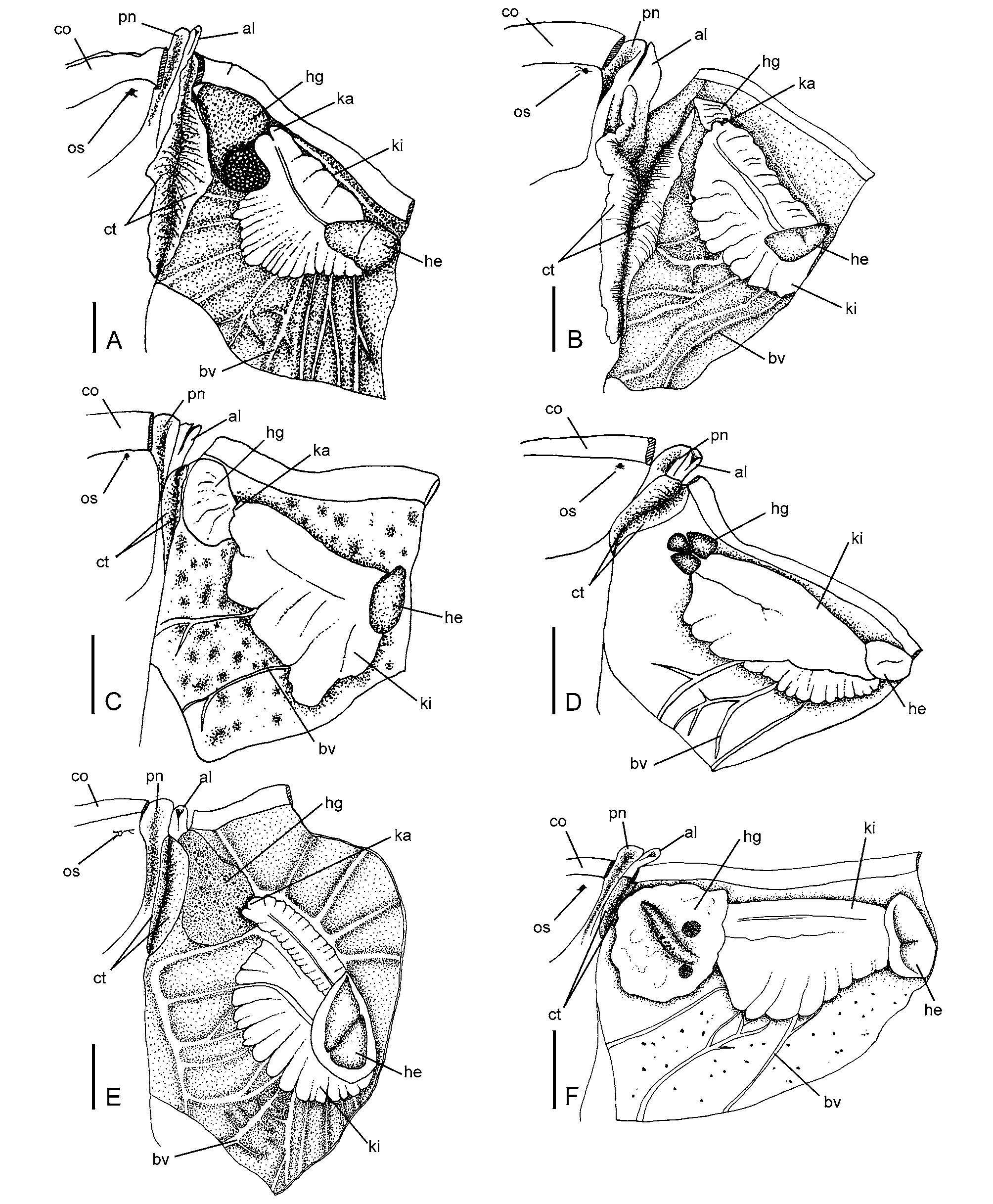
Mantle organs ( Fig. 7 View FIGURE 7 A): Opposed ciliary tracts raised, orange in formalin-preserved specimens, with ciliated epithelium, occupying full length of dorsal and ventral surfaces of right mantle cavity. Hypobranchial gland at anterior right of mantle cavity, approximately 1/4 length of mantle cavity, dome-shaped, dark red in formalin-preserved specimens with black pigmented ducts leading to embedded glandular cells. Kidney occupying roof of mantle cavity from posterior edge of hypobranchial gland to left posterior of mantle cavity; interior of kidney folded, lined with numerous spherical cells; aperture simple, with two lips. Numerous blood vessels (unpigmented) radiate from kidney towards border of mantle and through kidney towards heart.
Digestive system: Oesophagus running posteriorly from mouth to stomach at posterior left of visceral mass; intestine coiled clockwise and anticlockwise around albumen and oviductal glands of reproductive system; rectum lying along right side of body leading to anal lobe at right anterior edge of mantle. Buccal mass small, cream in formalin-preserved specimens. Salivary glands tubular, pale yellow in formalin-preserved specimens, opening to posterior ventral surface of buccal mass. Oesophagus long, thin-walled, ventral to anterior reproductive structures. Stomach thin-walled, highly folded, with two broad, longitudinal strips of cilia running along inner edge; ciliary action in live specimens moving particles towards typhlosole at posterior end of stomach. Muscular bilobed gizzard separated from stomach by short duct, with two hemispheres of muscle surrounding small, sand-filled compartment; typhlosole ventral to opening of gizzard on posterior left of stomach. Stomach narrows anteriorly to form intestine. Digestive gland dark brown to olive in formalinpreserved specimens, with numerous small lobes, occupying upper whorls of visceral mass, opening to posterior right of ventral cavity adjacent and posterior to junction of oesophagus with stomach.
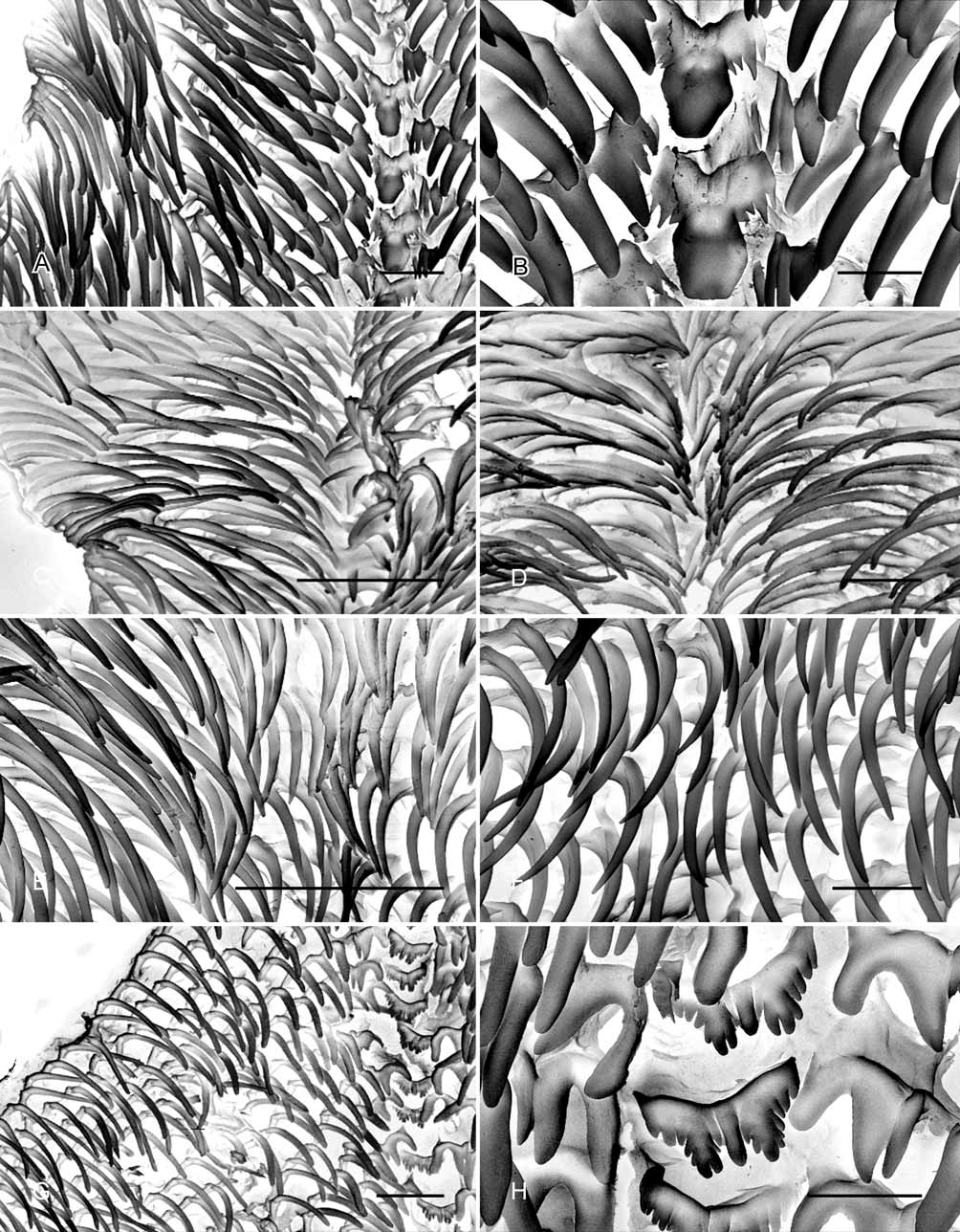
Radula ( Figs. 10 View FIGURE 10 A, B): Each row with central tooth, two pairs of lateral teeth and, on each side, approximately 30 marginal teeth. Central tooth with nine cusps; mesocone large, flattened, hexagonal in outline, flanking cusps flattened, short, triangular. Inner lateral teeth short, narrow, bicuspid. Outer lateral teeth larger than inner lateral teeth, with three cusps; outer cusp large, triangular, inner cusp narrow. Marginal teeth long, narrow, curved towards posterior end of radula; increasing in length and narrower towards outer edge of radula.
Central nervous system ( Fig. 12 View FIGURE 12 A): Cerebral ganglia equal in size to pedal ganglia; cerebral commissure long, moderately thick. Subcerebral commissure very thin, ventral to oesophagus. Parapedal commissure thin, long, anterior to thicker pedal commissure. Pleural ganglia small, with short connectives to pedal, parietal and cerebral ganglia. Visceral ganglionic chain moderately long. Parietal ganglia asymmetrical, right larger than left. Right parietal-visceral connective shorter than left.
Reproductive system ( Figs. 13 View FIGURE 13 A, 14H, 17F): Running from posterior visceral mass along right side of body to genital aperture on right side of head. Ovotestis composed of numerous small lobes leading to converging ducts which join hermaphrodite duct at a single point. Upper hermaphrodite duct highly convoluted, cream with russet pigmentation in formalin-preserved specimens, containing unorientated sperm. Albumen and oviductal glands large with central duct and peripheral lobes, both opening to carrefour at a single point. Seminal receptacle small, elliptical, unpigmented; containing sperm orientated towards walls of structure; opening via a short duct to carrefour. Spermoviduct following zigzag course through right body wall distally, leading to hairpin fold in cephalic region before junction with proximal end of copulatory organ. Elongate, tubular prostatic gland joining spermoviduct at junction with copulatory organ. Distal prostatic gland wrapped around retractor muscle of copulatory organ creating false appearance of two separate structures. Spermovipositor long, cylindrical, with hood-shaped aperture of spermoviduct sub-terminal. Additional narrow, blindending duct opens adjacent to spermoviduct aperture at apex of spermovipositor; runs back through spermovipositor to just below proximal base but does not connect to spermoviduct or prostatic gland. Spermovipositor covered by thin-walled spermovipositor sheath which lacks folds or pockets. Egg masses deposited on substrate as a collar, elliptical in cross-section, sometimes with substratum like sand or mud embedded in the matrix.
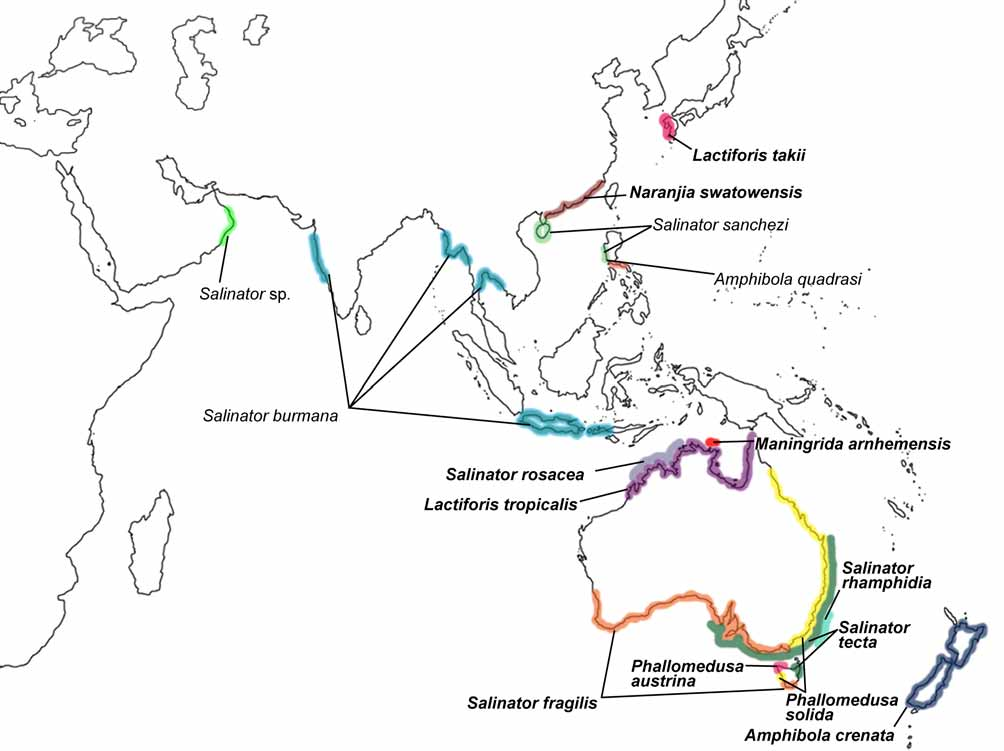
Distribution and habitat ( Fig. 1 View FIGURE 1 ): Widely distributed in coastal mainland New Zealand, in tidal estuaries, mud flats and amongst saltmarsh vegetation. Often occurring in very high densities ( Watters 1964; Powell 1979).
Remarks: The type material for A. crenata could not be located and may be lost. Some of Chemnitz’s specimens are believed to be lodged in the Zoological Museum, Copenhagen, but Cernohorsky’s (1974) catalogue of the Chemnitz specimens held in that collection does not include this species. The type location of this species is simply ‘New Zealand’.
Like Farnie (1919), we have referred all New Zealand material to Amphibola crenata ( Martyn, 1786) . Furthermore, we have found Farnie’s description of the species to be largely accurate. Amphibola obvoluta ( Jonas, 1846) was synonymised with A. crenata by Hubendick (1945) and this interpretation is followed here. Amphibola obvoluta was originally distinguished as possessing a shell with a shorter (more depressed) spire, lacking an umbilicus and with columellar callus or fold ( Jonas 1846). The specimens examined in this study and attributed to A. crenata varied in the height of the spire, presence of a callus on the columella and presence of a prominent fold adjacent to the umbilicus. We also observed that in the radula, the mesocone on the central tooth varied in relative size and outline. For the present, all the New Zealand material is assumed to be A. crenata , but further studies of the taxon are required to determine if these differences are simply variation or are indicative of cryptic taxa.
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
SubClass |
Heterobranchia |
|
Order |
|
|
Family |
|
|
Genus |
Amphibola crenata ( Martyn, 1786 )
| Golding, Rosemary E., Ponder, Winston F. & Byrne, Maria 2007 |
Amphibola avellana
| Hubendick 1945: 106 |
Amphibola crenata
| Powell 1979: 294 |
| Thiele 1931: 470 |
| Suter 1913: 596 |
Amphibola obvoluta
| Jonas 1846: 35 |
Ampullarina avellana
| Sowerby 1842: 64 |
Thallicera avellana
| Swainson 1840: 339 |
Ampullacera avellana
| Quoy 1832: 196 |
Ampullaria avellana
| Lamarck 1822: 179 |
Amphibola australis
| Schumacher 1817: 190 |
Helix crenata
| Gmelin 1791: 3623 |
Helix avellana
| Gmelin 1791: 3640 |
Bulimus avellana Bruguière, 1789 : 297
| Bruguiere 1789: 297 |
Nerita nux avellana
| Chemnitz 1781: 272 |