Labops, BURMEISTER, 1835
|
publication ID |
https://doi.org/10.1111/j.1096-3642.2011.00770.x |
|
persistent identifier |
https://treatment.plazi.org/id/03E8878D-FFDC-FFD4-5D8F-FC7AB734FB10 |
|
treatment provided by |
Marcus (2021-08-30 17:59:36, last updated by Plazi 2023-11-06 02:33:14) |
|
scientific name |
Labops |
| status |
|
LABOPS BURMEISTER View in CoL View at ENA ( FIGS 4 View Figure 4 , 33–36 View Figure 33 View Figure 34 View Figure 35 View Figure 36 )
Labops Burmeister, 1835: 279 View in CoL [gen. nov.; type species: Labops diopsis Burmeister, 1835 View in CoL by monotypy (junior synonym of Capsus sahlbergii Fallén, 1829 View in CoL )]; Fieber, 1858: 316 (key); Fieber, 1860a: 294; 1861 (key, descr.); Flor, 1860: 448 (key); Thompson, 1871: 432 (key); Walker, 1873: 44 (cat.); Reuter, 1875a: 24 (key); Reuter, 1875b (1): 86 (2):97 (key, descr.); Provancher, 1887: 135 (descr.); Atkinson, 1890: 120 (cat.); Reuter, 1891: 80, 160 (descr., key); Hueber, 1906: 5 (key); Kirkaldy, 1906: 131 (cat.); Oshanin, 1910: 786 (cat.); Reuter, 1910: 148 (cat.); Van Duzee, 1916: 211 (key); Van Duzee, 1917: 373 (cat.); Knight, 1922: 258 (key, n. spp.); Blatchley, 1926: 797 (key, spp. nov.); Stichel, 1933: 235 (key); Knight, 1941: 81; Slater, 1950: 51 (genitalia); Kiritshenko, 1951: 126 (key); Carvalho, 1952: 74 (cat.); Slater, 1954: 57 (note); Carvalho, 1955: 66 (key); Carvalho, 1958: 18 (cat.); Kerzhner, 1964a: 964 (diag., key); Kelton, 1980: 189 (diag., key); Kerzhner, 1988: 43 (illustrated key to east Asian spp.); Schuh, 1995: 58 (world cat.).
Ophthalmocoris Zetterstedt, 1838: 280 gen. nov. (syn. by Herrich-Schäeffer, 1850:166).
Diagnosis: Readily distinguished from other Halticini by the following combination of characters: tall head and stylate eyes; hemelytra of brachypterous individuals nearly covering abdomen; aedeagus with mass of thin, needle-like spicules suspended in the membrane anterior to secondary gonopore; posterior wall of bursa copulatrix with paired inter-ramal sclerites similar to those found in other Orthotylinae ; DLP often with heavily sclerotized inter-ramal bridge.
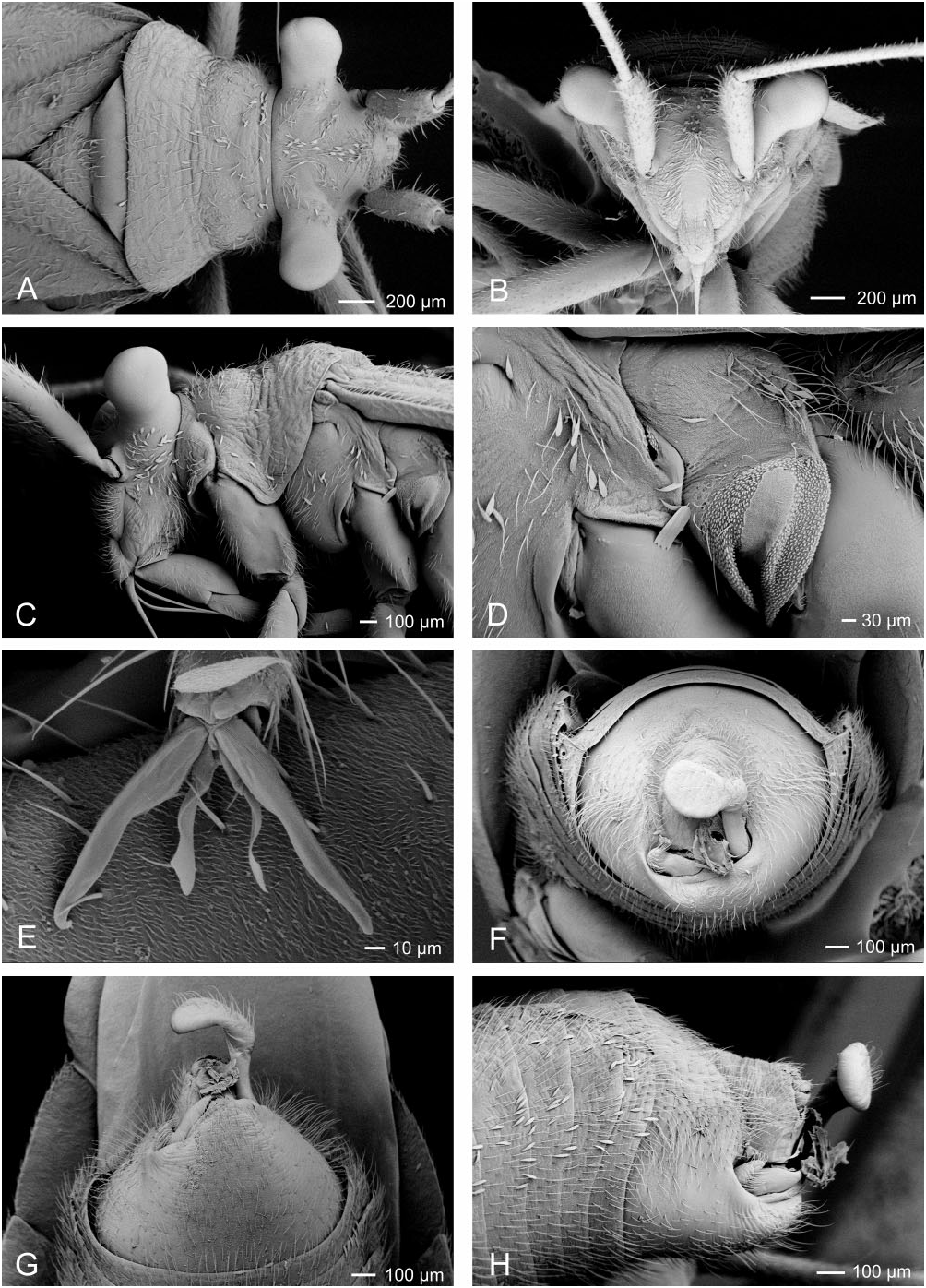
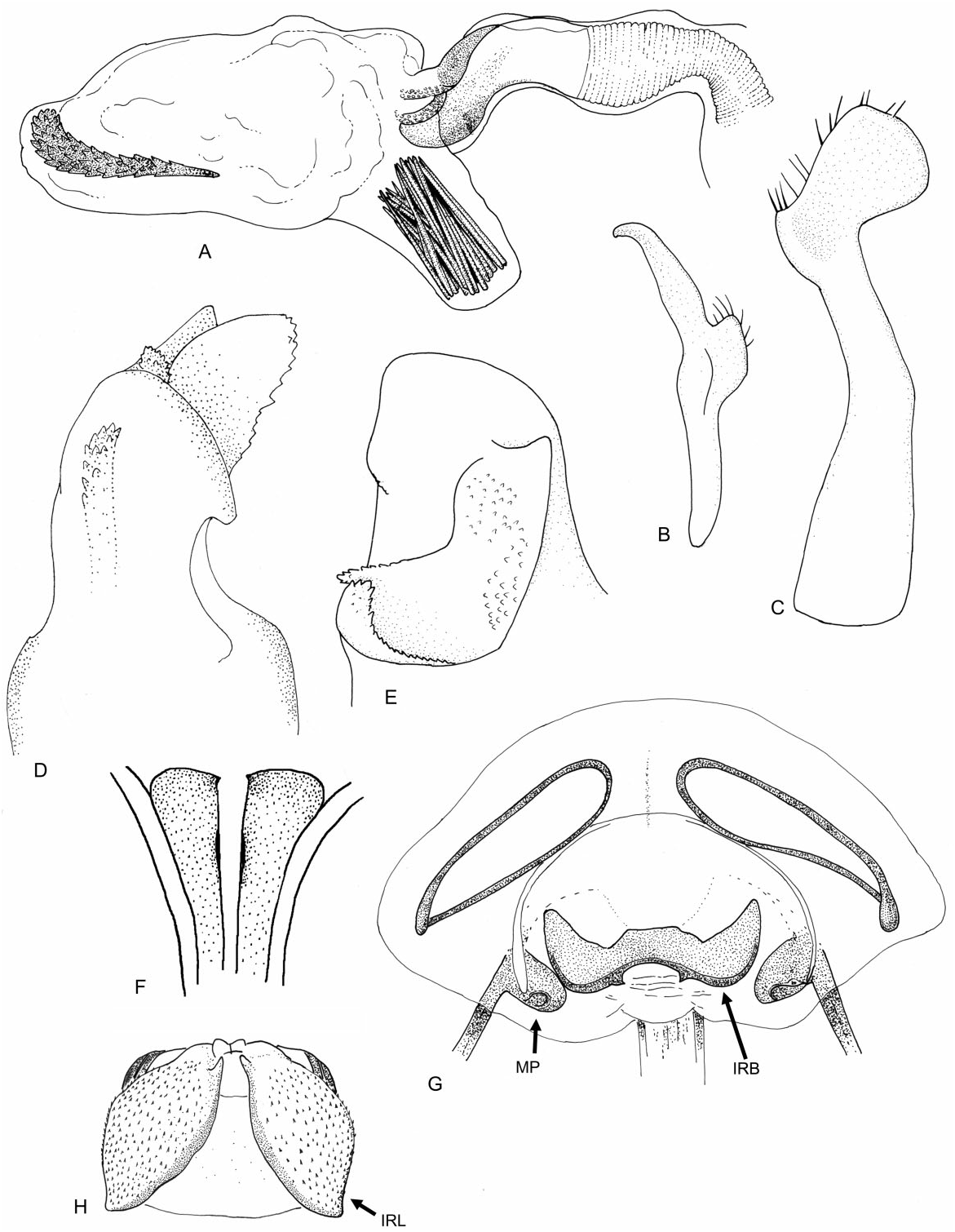
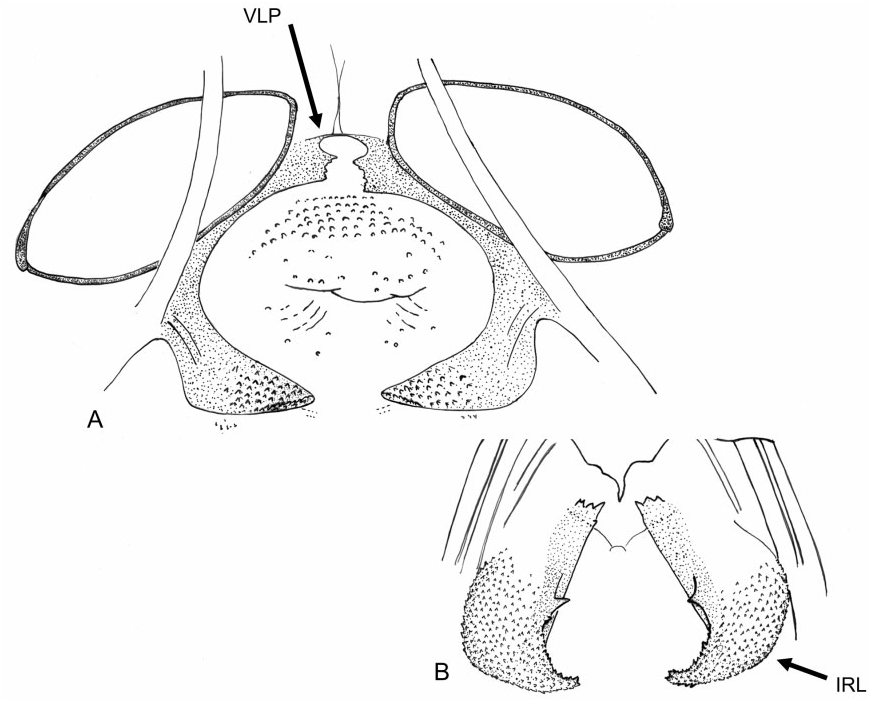
Redescription: Coloration ( Fig. 4 View Figure 4 ): mostly dark, dull brown to black, usually with yellow, orange, or reddish markings. Head: black with yellow markings, particularly on vertex, along midline, and on genae, mandibular and maxillary plates. Hemelytra black, sometimes with white or yellow along costal margins. Surface and vestiture ( Figs 4 View Figure 4 , 33A–H View Figure 33 ): clothed in simple setae and pale, scale-like setae. Head smooth and setaceous. Antennae and legs with erect and semi-erect spines, particularly on AI and tibiae. Posterior of pronotum and scutellum rugulose. Hemelytra clothed in simple setae or both simple and scale-like setae. Structure: body elongate-oval. Head ( Figs 4 View Figure 4 , 33A–C View Figure 33 ): transverse and tall; genae height> two times eye height; eyes stylate, projecting laterally and slightly upward, extending well beyond the anterolateral margins of the pronotum; frons steep, with faint folds radiating from midline; mandibular plate sometimes tumescent, especially males. Labium ( Fig. 33C View Figure 33 ): variable, sometimes reaching metacoxae; LI swollen. Antennae ( Figs 4 View Figure 4 , 33A–C View Figure 33 ): insertion below eye; shorter than insect; AI swollen, shorter to longer than pronotum, approximately twice as long as eye height. Thorax ( Figs 4 View Figure 4 , 33A, C, D View Figure 33 ): pronotum campanulate, collar thin and flat, callosite region well defined, posterior margin weakly concave; mesoscutum broad; scutellum small, triangular; metathoracic spiracle small and exposed, thinly surrounded with evaporative bodies; MTG external efferent system broad, swollen and triangular, encompassing lower half of tergite, ostiole prominent and elongate, peritreme tongue-shaped, situated above ostiole in middle of evaporative area, angled rearward parallel to posterior margin of tergite. Hemelytra ( Fig. 4 View Figure 4 ): both sexes with brachypterous and macropterous morphs, degree of brachyptery varying across species ( Slater, 1954); length variable in brachypterous morphs but generally covering much if not all of the abdomen, cuneus sometimes differentiated, membrane either absent or vestigial; in macropterous morphs hemelytra extend beyond abdomen, margins gently rounded; membrane with two cells. Legs: long, metafemur not greatly swollen; pretarsi without fleshy pulvilli. Abdomen: elongate-oval. Male genitalia ( Figs 33F–H View Figure 33 , 34A–E View Figure 34 , 36A–D View Figure 36 ): pygophore simple and conical, ventral margin extending slightly caudad, left ventrolateral margin sulcate below left paramere; left paramere either straight or with obtusely angled apophysis, apophysis weakly curved to strongly angled apically, sensory lobe often prominent; right paramere extends caudally out of pygophore, considerably larger than left paramere, with long base, apex with laterally deflected concave club; apex of phallotheca with serrate, plate-like process of variable size projecting from the left of phallothecal opening; ductus seminis elongate, secondary gonopore sclerotized, vaguely bowl-shaped with a prised operculum, with scale-like texturing along opening margin; endosoma with tight bundle of sclerotized needles, sometimes also with other sclerotized processes, including thin strips of sclerotized teeth. Female genitalia ( Figs 34D–G View Figure 34 , 35A–B View Figure 35 , 36E–G View Figure 36 ): sclerotized rings large, elongateelliptical, diagonal, medially subcontiguous, lateral margins somewhat upturned; margin of VLP adjoining rami sclerotized, sometimes medially with paired teeth, lateral-most region of VLP joined with rami to form paired, medially projecting, sclerotized processes, sometimes covered with fields of spines; DLP sometimes with heavily sclerotized, bilaterally ventrally pointed inter-ramal bridge, sometimes reduced to paired pincer-like lateral sclerites, a simple band of
THE HALTICINI OF THE WORLD 611
tissue or completely membranous; posterior wall of bursa copulatrix with bilaterally symmetrical interramal lobes of varying complexity projecting from posterior wall; margins of first gonapophyses symmetrical, swollen, and sclerotized.
Diversity and distribution: Labops is comprised of 12 species and exhibits a Holarctic distribution.
Included species: Labops bami Kulik, 1979 Russia Labops brooksi Slater, 1954 Canada
Labops burmeisteri Stål, 1858 Holarctic View in CoL
Labops chelifer Slater, 1954 View in CoL Canada
Labops hesperius Uhler, 1872 View in CoL * Canada; USA Labops hirtus Knight, 1922 View in CoL Canada; USA Labops nivchorum Kerzhner, 1988 View in CoL Russia
Labops sahlbergii ( Fallén, 1829) View in CoL * Scandinavia; Siberia (Ural)
Labops setosus Reuter, 1891 View in CoL Russia
Labops tumidifrons Knight, 1922 View in CoL * Canada
Labops utahensis Slater, 1954 View in CoL * USA
Labops verae Knight, 1929 View in CoL USA
Biology and host plant associations: Species of Labops are associated with grasses in arid conditions ( Slater, 1954). In North America, L. hesperius has been recorded on Agropyron cristatum , various range grasses, Rosa arkansana ( Kelton, 1980) , Koeleria cristata , Poa secunda , Stipa comata , Stipa williamsi , Hordeum sp. , Triticum sativum ( Mills, 1939: in Slater, 1954), and wheat ( Mills, 1941), whereas L. hirtus has been recorded on wheat in Montana ( Mills, 1939) . In Yakutia, Vinokurov (1979) recorded the genus on cereals and sedges ( Carex stenophylla , Critesion jubatum , and Leymus sp. ), and suggested that it may decrease productivity of pastures and hay fields ( Table 1).
Remarks: Labops has previously been treated as its own tribe, Labopini, owing to various characteristics unique to the genus ( Knight, 1923). Slater was the first to note the unusual female genitalia of Labops – both the posterior wall (1950, 1954) and the interramal bridge (1954). Slater (1950) questioned whether the sclerotized inter-ramal tumescences on the posterior wall might be homologous to the interramal lobes (i.e. K-structures) found in other Orthotylinae – the absence of which has been considered a synapomorphy for the Halticini ( Schuh, 1974) . However, with the discovery of inter-ramal tumescences in both Anapus and Scirtetellus , we consider these to be homologous in origin. Despite the presence of inter-ramal lobes in Labops , Scirtetellus , and Anapus , our phylogeny instead places Labops as sister to Myrmecophyes based on four unambiguous characters, including one synapomorphy (56–1: apex of phallotheca with a prominent flange).
The inter-ramal bridge ( Kullenberg, 1947; Slater, 1950, 1954) is another interesting structure otherwise absent in the Halticini (although Anapus , Barbarosia , and Euryopicoris possess a sclerotized plate on the DLP, which may be homologous).
Atkinson ET. 1890. Catalogue of the Insecta. Order Rhynchota. Suborder Hemiptera-Heteroptera. Family Capsidae. Journal of the Asiatic Society of Bengal (Natural Science Supplement) 58: 25 - 200.
Blatchley WS. 1926. Heteroptera or True Bugs of Eastern North America, with especial reference to the faunas of Indiana and Florida. Indianapolis: Nature Publishing Company.
Burmeister HCC. 1835. Handbuch der Entomologie. Zweiter Band. Erste Abtheilung-Rhynchota. Berlin: Theodore Christian Friedrich Enslin.
Carvalho JCM. 1955. Keys to the genera of Miridae of the world (Hemiptera). Bolletim do Museu Paraense Emilio Goeldi 11: 5 - 151.
Carvalho JCM. 1958. Catalogo dos Mirideos do Mundo. Parte III. Subfamilia Orthotylinae. Arquivos do Museo Nacional, Rio de Janeiro 47: 161.
Fallen CF. 1829. Hemiptera Sueciae. Cimicides eorumque familae affines. London.
Fieber FX. 1858. Criterien zur generischen Theilung der Phytocoriden (Capsini auct.). Wiener Entomologische Monatschrift 2: 289 - 327, 329 - 347, 388.
Fieber FX. 1860 a. Die europaischen Hemipteren Halbflugler (Rhynchota Heteroptera). Nach der analytischen Methode bearbeitet. Part I. Wien: C. Gerold.
Fieber FX. 1861. Die europaischen Hemiptera Halbflugler (Rhynchota Heteroptera). Nach der analytischen Methode bearbeitet. Part II. Wien: C. Gerold.
Flor G. 1860. Die Rhynchoten Livlands in Systematischer Folge bescreiben. Erster Theil: Rhynchota frontirostria Zett. (Hemiptera Heteroptera Ast.). Archiv fur die Naturkunde Liv-, Ehst-, und Kurlands. II Serie, Biologische Naturkunde (1860 - 1861). Dorpat: Carl Schulz.
Herrich-Schaeffer GAW. 1850. Die wanzenartigen insekten, vol. XIV. Nurnberg: C. H. Zeh'schen Buchhandlung.
Hueber T. 1906. Synopsis der deutschen Blindwanzen (Hemiptera Heteroptera Fam. Capsidae). Jahreshefte des Vereins fur Vaterlandische Naturkunde in Wurttemberg 2: 201 - 262.
Kelton LA. 1980. The plant bugs of the Prairie Provinces of Canada. Heteroptera: Miridae. The insects and arachnids of Canada. Part 8. Hull, Quebec: Canadian Government Publishing Centre.
Kerzhner IM. 1964 a. New and little known Heteroptera from Kazakhstan and some other regions of USSR. Trudy Zoologicheskogo Instituta Akademii Nauk SSSR 34: 113 - 130.
Kerzhner IM. 1988. Novye i maloizvestnye poluzhestkiokrylye nasekomye (Heteroptera) Dal'nego Vostoka SSSR (1987). Akademiya Nauk USSR, Vladivostok 1987: 1 - 84.
Kiritshenko AN. 1951. [True bugs of the European parts of the USSR (Hemiptera): Key and bibliography]. Opredeliteli po Faune SSSR 42: 1 - 423.
Kirkaldy GW. 1906. List of the genera of the pagiopodous Hemiptera-Heteroptera, with their type species from 1758 to 1904 and also of the aquatic and semi-aquatic Trochalopoda. Transactions of the American Entomological Society 32: 117 - 156, 156 a - 156 b.
Knight HH. 1922. The North American species of Labops (Heteroptera-Miridae). Canadian Entomologist 54: 258 - 261.
Knight HH. 1923. Guide to the insects of Connecticut. Part
Knight HH. 1929. Labops verae, new species, with Labopella, Nicholia, and Pronotocrepis, new genera of North American Miridae (Hemiptera). Canadian Entomologist 61: 214 - 218.
Knight HH. 1941. The plant bugs, or Miridae of Illinois. Bulletin of the Illinois Natural History Survey 22: 1 - 234.
Kullenberg B. 1947. Der Kopulationsapparat der Insekten aus Phylogenetischen Gesicktspunkt. Zoologische Bijdragen 25: 79 - 90.
Mills HB. 1939. Montana insect pests for 1937 and 1938. Bulletin of the Montana Agricultural Experiment Station 366: 1 - 32.
Mills HB. 1941. Montana insect pests for 1939 and 1940. Bulletin of the Montana Agricultural Experiment Station 384: 1 - 27.
Oshanin B. 1910. Verzeichnis der Palaearktischen Hemipteren. Kaiserlichen Akademie der Wissenschaften, St. Petersburg 3: 1 - 218.
Provancher L. 1887. Petite Faune Entomologique du Canada et Particulierement de la Province de Quebec. Vol. 3, Cinquieme ordre. Les Hemipteres. Quebec: C. Darveau.
Reuter OM. 1875 a. Revisio critica Capsinarum, praecipue Scandinaviae et Fenniae. Forsok till de Europaiska Capsinernas naturelinga uppstallning jamte kritisk ofversigt af de Skandinavisk-finska arterna. Akademisk Afhandling Helsingfors 1: 1 - 101.
Reuter OM. 1875 b. Genera Cimicidarum Europae. Bihang till Kongliga Svenska Vetenskapsakademiens Forhandlingar 3: 1 - 66.
Reuter OM. 1891. Hemiptera Gymnocerata Europae. Hemipteres Gymnocerates d'Europe du bassin du Mediterranee et de l'Asie Russe. Tome IV. Acta Societatis Scientiarum Fennicae 23: 1 - 179.
Reuter OM. 1910. Neue Beitrage zur Phylogenie und Systematik der Miriden nebst einleitenden Bemerkungen uber die Phylogenie der Heteropteren-Familien. Acta Societatis Scientiarum Fennicae 37: 1 - 167.
Schuh RT. 1974. The Orthotylinae and Phylinae (Hemiptera: Miridae) of South Africa with a phylogenetic analysis of the ant-mimetic tribes of the two subfamilies for the world. Entomologica Americana 47: 1 - 332.
Slater JAS. 1950. An Investigation of the female genitalia as taxonomic characters in the Miridae (Heteroptera). Iowa State College Journal of Science 25: 1 - 81.
Slater JAS. 1954. Notes on the genus Labops, Burnmeister in North America, with the descriptions of three new species (Hemiptera: Miridae). Bulletin of the Brooklyn Entomological Society 49: 1 - 15.
Stal C. 1858. Beitrag zur Hemipteren-Fauna Sibiriens und des Russischen Nord-Amerika. Stettiner Entomologische Zeitung 19: 175 - 198.
Stichel W. 1933. Illustrierte Bestimmungstabellen der Deutschen Wanzen (Hemiptera-Heteroptera). Fasc. 8, 9. Berlin- Hermsdorf: W. Stichel.
Thompson CG. 1871. Opuscula entomologica. Hemiptera, Fasc IV. Lund.
Uhler PR. 1872. Notices on the Hemiptera of the western territories of the United States, chiefly from the surveys of Dr. F. V. Hayden. In: Heydon FV, ed. Preliminary report of the United States Geological survey of Montana and portions of adjacent territories, being a fifth annual report of progress. Washington, DC: Government Printing Office, 392 - 423.
Van Duzee EP. 1916. Check list of the Hemiptera (excepting the Aphididae, Aleurodidae and Coccidae) of America, North of Mexico. New York: New York Entomological Society.
Van Duzee EP. 1917. Catalogue of the Hemiptera of America north of Mexico, excepting the Aphididae, Coccidae and Aleurodidae. University of California College of Agriculture, Agricultural Experiment Station, Technical Bulletin, Entomology 2: 1 - 902.
Vinokurov NN. 1979. Identification manual of the fauna of the USSR. No. 123. Heteroptera of the Yakutia. Leningrad: Zoological Institute of the USSR.
Walker F. 1873. Catalogue of specimens of Hemiptera Heteroptera in the collection of the British Museum. Part VI. London: The British Museum.
Zetterstedt JW. 1838. Insecta Lapponica descripta. Leipzig: L. Voss.
Figure 4. Photographs of Halticini genera: Labops–Strongylocoris. Abbreviations: F, female; M, male.
Figure 33. Scanning electron micrograph images of Labops sahlbergii (male). A, head and pronotum, dorsal view; B, head, anterior view; C, head and thorax, lateral view; D, meso- and metathorax, lateral view; E, tarsus; F, pygophore, posterior view; G, pygophore, ventral view; H, pygophore, lateral view.
Figure 34. Male and female genitalia of Labops sahlbergii. A, aedeagus, phallotheca removed; B, left paramere; C, right paramere; D, apex of phallotheca, ventral view; E, apex of phallotheca, left side; F, first gonapophyses, ventral view; G, bursa copulatrix; H, posterior wall. Abbreviations: IRB, inter-ramal bridge; IRL, inter-ramal lobe; MP, medial process of ventral labiate plate and ramus.
Figure 35. Female genitalia of Labops hesperius. A, bursa copulatrix; B, posterior wall. Abbreviations: IRL, inter-ramal lobe; VLP, ventral labiate plate.
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
Labops
| Tatarnic, Nikolai J. & Cassis, Gerasimos 2012 |
Ophthalmocoris
| Herrich-Schaeffer GAW 1850: 166 |
| Zetterstedt JW 1838: 280 |
Labops
| Kerzhner IM 1988: 43 |
| Kelton LA 1980: 189 |
| Kerzhner IM 1964: 964 |
| Carvalho JCM 1958: 18 |
| Carvalho JCM 1955: 66 |
| Slater JAS 1954: 57 |
| Kiritshenko AN 1951: 126 |
| Slater JAS 1950: 51 |
| Knight HH 1941: 81 |
| Stichel W 1933: 235 |
| Blatchley WS 1926: 797 |
| Knight HH 1922: 258 |
| Van Duzee EP 1917: 373 |
| Van Duzee EP 1916: 211 |
| Oshanin B 1910: 786 |
| Reuter OM 1910: 148 |
| Hueber T 1906: 5 |
| Kirkaldy GW 1906: 131 |
| Reuter OM 1891: 80 |
| Atkinson ET 1890: 120 |
| Provancher L 1887: 135 |
| Reuter OM 1875: 24 |
| Walker F 1873: 44 |
| Thompson CG 1871: 432 |
| Fieber FX 1860: 294 |
| Flor G 1860: 448 |
| Fieber FX 1858: 316 |
| Burmeister HCC 1835: 279 |