Erythrolamprus reginae ( Linnaeus 1758 )
|
publication ID |
https://doi.org/10.11646/zootaxa.4586.1.3 |
|
publication LSID |
lsid:zoobank.org:pub:7BBCFF79-DE38-4A79-8905-7840A1C1955F |
|
persistent identifier |
https://treatment.plazi.org/id/03DC1517-FF80-FF83-C7FD-2B08FAF1F9B2 |
|
treatment provided by |
Plazi (2019-04-16 20:05:44, last updated 2023-10-31 03:12:17) |
|
scientific name |
Erythrolamprus reginae ( Linnaeus 1758 ) |
| status |
|
Erythrolamprus reginae ( Linnaeus 1758)
Figures 1 View FIGURE 1 A–B, 2A, 3A, 6A.
Coluber reginae Linnaeus 1758 ; Systema Naturae 10:219. Terra typica: “Indis”. Holotype: NRM 44 View Materials .
Coluber graphicus Shaw 1802 ; General zoology or systematic natural history. 3:474. Terra typica: “America”. Holotype: BMNH 1946.1 .5.73.
Natrix reginae — Merrem 1820; Versuch eines Systems der Amphibia:115.
Natrix semilineata Wagler 1824 ; Serpentum Braziliensium species novae.: 33. Terra typica: Rio Solimoes. Lectotype: ZSM 1832 View Materials –0–1.
Liophis reginae — Wagler 1830; Natürliches System der Amphibien:188.
Coronella reginae — Schlegel 1837; Essai sur la Physignomie des Serpens:134.
Liophis reginae — Duméril, Bibron & Duméril 1854; Erpétologie Générale:704.
Liophis wagleri Jan, 1863 ; Iconografia generale degi ofidi:297 (part.).
Leimadophis reginae reginae — Peters & Orejas-Miranda 1970; Un. St. Nat. Mus View in CoL . Bull. 297:148.
Liophis reginae reginae — Dixon 1980; Milwaukee Publ. Mus .31:24.
Liophis reginae semilineatus — Dixon 1983a; Ann. Carnegie. Mus . 52:3 (part.).
Liophis miliaris intermedius Henle & Ehrl 1991 ; Bonner Zoog. Beit. 42:191. Terra typica: Madre de Dios, Peru. Holotype: ZFMK 34343 About ZFMK p.
Erythrolamprus reginae reginae — Grazziotin et al. 2012; Cladistics. 1:21.
Erythrolamprus reginae semilineatus — Grazziotin et al. 2012; Cladistics. 1:21.
Liophis reginae — Wallach et al. 2014; Snakes of the World:393 (part.).
Lectotype. Juvenile specimen, NRM 44, from “Indiis” (in error). Andersson (1899) designated the lectotype and Dixon (1983a) restricted the type locality to Surinam (photographs of the specimen examined; Fig. 1A View FIGURE 1 ).
Paralectotype. Juvenile specimen, NRM 45 View Materials , with same data as the lectotype ( Fig. 1B View FIGURE 1 ) .
Diagnosis. Erythrolamprus reginae can be distinguished from all congeners by unique combination of the following characters: (1) dorsal scales rows 17, reducing to 15 after midbody; (2) apical pit single; (3) ventrals 125–164 in females, 131–162 in males; (4) subcaudals 54–96 in females, 56–84 in males; (5) dorsum of head olive green, in preservative, extending from anterior third of body, changing gradually to grayish-brown at midbody; (6) upper edges of supralabials with distinctive dark postorbital stripe; (7) belly creamish-white with black spots with squared or rhomboid shape arranged in a checkered pattern; (8) black lateral spots extending from anterior third of the body, between 2–3 th dorsal scale rows, to form a lateral stripe extending until the end of the tail; (9) ventral surface of tail creamish-white without black spots; (10) intrasulcal region of hemipenial body with spines slightly elongated, arranged in a row extending from distal region of lobes to the level of bifurcation of sulcus spermaticus; (11) medial region of hemipenial body, on the asulcate side, ornamented by spinules homogeneously distributed, or elongated spines arranged in one or three longitudinal rows; (12) sulcus spermaticus bifurcates at half length of the hemipenial body; and (13) moderate body size (SVL 87–709 mm).
Comparisons. Erythrolamprus reginae shares a lateral stripe along the posterior region of body and tail and usually a cream belly scattered of squared or rhomboid black spots with E. macrosomus , E. dorsocorallinus , and E. zweifeli . Erythrolamprus reginae differs from E. oligolepis by having 17 dorsal scale rows at midbody and ventral surface with squared black spots (vs. 15 dorsal scales rows at midbody and belly usually without spots); from E. macrosomus by having ventral region of tail without black spots (vs. ventral surface of the tail with black spots); from E. zweifeli and E. dorsocorallinus by having dorsal ground color regularly pigmented of olive green with a dorsal-lateral black stripe (vs. dorsal scales with apical half black, with the basal portion yellowish, reddish or
bluish-cream) ( Figs 4 View FIGURE 4 and 5 View FIGURE 5 ). Additionally, E. reginae differs from E. epinephelus epinephelus and E. e. albiventris ( Jan 1863) by having ventral surface with squared black spots (vs. belly usually without spots), and from E. e. juvenalis ( Dunn 1937), E. e. pseudocobella ( Peracca 1914), E. e. opisthotaenius ( Boulenger 1908), E. e. bimaculatus ( Cope 1899), E. e. lamonae ( Dunn 1944), and E. e. fraseri ( Boulenger 1894) by having dorsal ground color of the head regularly pigmented of olive green, extending from anterior third of the body, without bands or blotches (vs. dorsum of the head cream or olive green, with bands on anterior portion of body, and a black spot and a thick postorbital stripe, on each side of head). Regarding the following sympatric taxa, E. reginae differs from E. cobella , E. taeniogaster and E. breviceps by having a cream belly scattered of black squared spots occupying an area lower than a ventral scale (vs. cream belly scattered of complete black bands, usually occupying two ventral scales); from E. miliaris by having dorsal ground color regularly pigmented of olive green with a dorsal-lateral black stripe (vs. dorsum of head, body and tail yellowish-cream with the distal half of the scales black and dorsallateral stripe absent); from E. typhlus and E. poecilogyrus by having 17 dorsal scale rows in the midbody (vs. 19 dorsal scale rows in the midbody); from E. taeniurus by having ventrals 125–164 in females, and 131–162 in males, besides of the dorsal ground color regularly pigmented of olive green with a dorsal-lateral black stripe (vs. 152–181 ventral scales and dorsum olive brown with 6–15 anterior dark body bands or blotches, posteriorly merging to form a middorsal dark stripe).
Redescription of the lectotype. Body cylindrical; SVL 185 mm, CL 65 mm (35.1% SVL); internasals two, as long as wide; prefrontals two, as long as wide, in contact with supraoculars, preocular, loreal and postnasal; frontal pentagonal; parietals two; supralabials eight, second and third contacting loreal, fourth to fifth contacting eye, and sixth and seventh taller than remaining supralabials; supraoculars longer than wide; nasal divided above and below nostril; nasal in contact with first two supralabials, internasals, prefrontals, loreal and rostral; loreal tetragonal, as long as high, contacting second and third supralabials, postnasal, prefrontals, and preocular; preocular contacting supraocular, prefrontal, nasal, third and fourth supralabials; postoculars two, upper postocular higher than lower; temporals 1+2, anterior longer than upper posterior temporal; symphysial triangular; infralabials 10, first pair in broad contact behind symphysial; first five pairs of infralabials in contact with chinshields; smooth dorsal scale rows 17/17/15; ventrals 137; subcaudals 76; cloacal plate divided;
Dorsum of head dark gray to dark brown; parietal spots on each side of head, contacting eighth supralabial and posterior region of parietal; pale nuchal collar (three scales long); edges of supralabial scales with distinctive postorbital line; lateral black spots extending from anterior third of body merging at the level of cloacal plate originating the lateral stripe, which extends to end of tail; belly with black marks of rectangular shape; ventral surface of tail without dots or spots.
Morphometric and meristic variation (n= 521). SVL 87–709 mm (mean= 348.7; SD= 98.9; n= 510), CL 19–199 mm (mean= 116.2; SD= 38; n= 485); head length 1.3–35 mm (mean= 17.1; SD= 3.52; n= 457), height 3.0– 13.1 mm (mean= 6.5; SD= 1.4; n= 447), and width 3.2–21.9 mm (mean= 9.5; SD= 2.2; n= 448); diameter of ocular orbit 1.8–5.7 mm (mean= 3.3; SD= 0.6; n= 457); distance between orbit and rostral shield 1.7–8.1 mm (mean= 4.1; SD= 0.8; n= 452); rostral triangular, 1.3–5.6 mm wide (mean= 3.2; SD= 0.7; n= 453), 1.0– 5.7 mm high (mean= 2.2; SD= 0.5; n= 452); internasals 0.6–3.0 mm length (mean= 1.4; SD= 0.3; n= 458), 0.9–4.2 mm wide (mean= 1.7; SD= 0.5; n= 456); prefrontals 1.0– 5.3 mm length (mean= 2.1; SD= 0.5; n= 458), 1.2–5.6 mm wide (mean= 2.4; SD= 0.6; n= 457); frontal pentagonal, 1.8–8.0 mm length (mean= 4.7; SD= 0.7; n= 461), 1.7–5.2 mm wide (mean= 3.0; SD= 0.6; n= 437); parietals 2.9–11.2 mm length (mean= 5.5; SD= 1; n= 439), 1.8–6.1 mm wide (mean= 3.3; SD= 0.6; n= 437); loreal 0.4–2.7 mm long (mean= 1.0; SD= 0.3; n= 436), 0.7–2.2 mm high (mean= 1.4; SD= 0.3; n= 435); infralabials 8–11 (mean= 9.9; SD= 0.5; n= 511); infralabials contacting chinshileds 4–6 (mean= 4.9; SD= 0.3; n= 512); anterior chinshields 2.0– 9.9 mm long (mean= 4.3; SD= 0.9; n= 459), posterior chinshields 1.9–7.1 mm long (mean= 4.4; SD= 1; n= 459); dorsal scale rows with reduction at the level of 55–109/61–114 ventral scales (right/left; n= 399); ventrals 125–164 in females (mean= 145.3; SD= 6; n= 317), 131–162 in males (mean= 145.2; SD= 5.2; n= 192); subcaudals 44–87 in females (mean= 68.8; SD= 0.1; n= 240), 56–84 in males (mean= 70.4; SD= 6.1; n= 144).
Color pattern in preservative. Dorsum of head dark gray to dark brown; edges of supralabials with distinctive postorbital stripe; supralabials, infralabials and gular region creamish-white; dorsal ground color of body anteriorly olive green without spots and cream on basal portion of dorsal scales with black tips; dorsal stripe dark brown, occupying vertebral row and two paravertebral rows, on each side of body, extending from posterior third of body to end of tail; lateral irregular black spots, located between 2–3 th dorsal scale rows, and merged on the posterior third of body originating the lateral-posterior stripe; lateral stripe extends to posterior portion of tail; ventral surface of body creamish-white scattered with rectangular black squared spots, spaced one to three ventral scales; subcaudals cream without dots or spots ( Fig. 2A View FIGURE 2 ).
Ontogenetic variation of color in preservative. Hatchlings and juveniles with cream white parietal elliptical spot, covereing the intersection of the temporals and parietal, besides of a pale nuchar colar spaced of two to three dorsal scales.
Color in life. Dorsum of head dark brown gradually changing to olive green for about midbody; supralabials and gular region cream white; first two dorsal scale rows creamish-grey ( Fig. 6A View FIGURE 6 ).
Hemipenial morphology (everted organ n = 26). Fully everted and maximally expanded hemipenes renders slightly bilobed, non-calyculated and non-capitate organs; hemipenial body covered with spinules more concentrated on the sulcus spermaticus edges; lobes laterally scattered of spinules on both sides; lobes ending with smooth apical disk on its apical portion; sulcus spermaticus bifurcates at half length of hemipenial body, each branch running centrifugally to apical disk; basal region of sulcus spermaticus with inflated portion covered by elongated spines; intrasulcal region with spinules distributed homogeneously or elongated spines arranged in rows, extending to distal region of lobes; proximal region of hemipenial body on the asulcate side with inflated areas on each side, ornamented with elongated spines extending to the separation of lobes by one or two rows; nude region at the lower region of the lobes, imediatelly under the elongated rows at the asulcate face; the medial region of hemipenial body, on the asulcate side, ornamented by spinules homogeneously distributed, or elongated spines arranged in one or three longitudinal rows ( Fig. 3A View FIGURE 3 ).
Geographic distribution. Erythrolamprus reginae occurs from the Guyana Shield, along the Venezuelan Llanos and Pantepui, as well as in the Amazon basin of the Brazil, Colombia, Ecuador, and Peru, on the “Brejos de altitude” rainforests in northeastern Brazil, into the coastal fragments of Atlantic forest from the Northeastern and Southeastern Brazil ( Fig. 8 View FIGURE 8 ).
Remarks. Dixon (1983a) confirmed that the holotype of Coluber violaceus Lacépède 1789 was lost, and maintained the synonimization proposed by Günther (1858). However, we propose removing C. violaceus from the synonymy of Erythrolamprus reginae , based on the incongruence between the description of E. reginae and that performed by Lacépède (1789) (25 subcaudal scales in C. violaceus vs. 62 in E. reginae ). Thus, we propose that the status nomen dubium is be applied to C. violaceus , until a further investigation clarify the identity of this taxon.
Andersson, L. G. (1899) Catalogue of the Linnaean type-specimens of snakes in the Royal Museum in Stockholm. Bihang till Konglika Svenska Vetenskaps - Akademiens Handlingar, 24, 1 - 34.
Boulenger, G. A. (1894) Catalogue of the Snakes in the British Museum (Natural History). Vol. II. Containing the Conclusion of the Colubridae Aglyphae. British Mus. (Nat. Hist.), London, xi + 382 pp. https: // doi. org / 10.5962 / bhl. title. 8316
Boulenger, G. A. (1908) Descriptions of new South-American reptiles. Annals and Magazine of Natural History, Series 8, 1 (1), 111 - 115. https: // doi. org / 10.1080 / 00222930808692365
Cope, E. D. (1899) Contributions to the herpetology of New Granada and Argentina, with descriptions of new forms. The Philadelphia Museum Bulletin, 1, 1 - 19. https: // doi. org / 10.5962 / bhl. title. 54674
Dixon, J. R. (1980) The Neotropical snake genus Liophis, the generic concept. Contributions in Biology and Geology of the Milwaukee Public Museum, 31, 1 - 40.
Dixon, J. R. (1983 a) Systematic of Liophis reginae and L. williamsi (Serpentes, Colubridae), with a description of a new species. Annals of the Carnegie Museum, 52, 113 - 138.
Dumeril, A. M. C, Bibron, G. & Dumeril, A. H. C. (1854) Erpetologie Generale ou Histoire naturelle Complete des Teptiles. Vol. 7. Librairie Encyclopedique de Roret, Paris, 1536 pp. https: // doi. org / 10.5962 / bhl. title. 112278
Dunn, E. R. (1937) New or Unnamed Snakes from Costa Rica. Copeia, 1937 (4), 213 - 215.
Dunn, E. R. (1944) A revision of the Colombian snakes of the genera Leimadophis, Liophis, Lygophis, Rhadinaea, and Pliocercus, with a note on Colombian Coniophanes. Caldasia, 11 (10), 489 - 495.
Grazziotin, F. G., Zaher, H., Murphy, R. W., Scrocchi, G., Benavides, M. A., Zhang, Ya-Ping & Bonatto, S. L. (2012) Molecular phylogeny of the New World Dipsadidae (Serpentes: Colubroidea): a reappraisal. Cladistics, 1, 1 - 23. https: // doi. org / 10.1111 / j. 1096 - 0031.2012.00393. x
Gunther, A. (1858) Catalogue of Colubrine snakes of the British Museum. Printed by order of the Trustees, London, 281 pp.
Henle, K. & Ehrl, A. (1991) Zur Reptilien fauna Perus nebst Beschreibung eines neuen Anolis (Iguanidae) und zweier neuer Schlangen (Colubridae). Bonner Zoogische Beitrage, 42, 143 - 180.
Jan, G. (1863) Enumerazione sistematica degli ofidi apparteneti al gruppo Coronellidae. Archivio per la Zoologia l'Anatomia e la Fisiologia, 2 (2), 213 - 320.
Lacepede, B. G. E. L. (1789) Historie naturelle des Quadrupedes Ovipares et des Serpens. Tome second, Imprimerie du Roi, Paris, 527 pp.
Linnaeus, C. (1758) Systema Naturae per Regna tria Naturae, Secundum Classes, Ordines, Genera, Species, cum Characteribus, Differentiis, Synonymis, Locis. Tomus I. Editio decima, reformata. 10 th Edition. Laurentii Salvii, Holmiae, 824 pp.
Merrem, B. (1820) Versuch eines Systems der Amphibien I Tentamen Systematis Amphibiorum. Johann Christian Kriegeri, Marburg, 191 pp.
Peracca, M. G. (1914) Reptiles et Batraciens de Colombie. In: Fuhrmann, O. and E. Mayor (Ed.), Voyage d'Exploration Scientifique en Colombie. Memoires de la Societe Neuchateloise des Sciences naturelles, 5, 1 - 1090. http: // doi. org / 10.5169 / seals- 100120
Peters, J. A. & Orejas-Miranda, B. (1970) Catalogue of the neotropical Squamata: part I. Snakes. United States National Museum Bulletin, 297, 1 - 347. https: // doi. org / 10.5479 / si. 03629236.297.1
Schlegel, H. (1837) Essai sur la Physionomie des serpents. Kipps & Van Stockum, La Haye, 606 pp.
Shaw, G. (1802) General Zoology or Systematic Natural History III. Thomas Davidson, London, 303 pp. [pp. 313 - 615]
Wagler, J. (1824) Serpentum Braziliensium species novae, ou histoire naturelle des especes nouvelles de serpens. In: Spix, J. (Ed.), Animalia Nova Sive Species Novae. Typis Francisci Seraph, Hubschmanni, 75 pp.
Wagler, J. (1830) Naturliches System der Amphibien, mit vorangehender Classification der Saugethiere und Vogel. J. G. Cotta, Munchen - Stuttgart - Tubingen, 354 pp.
Wallach, V., Williams, K. L. & Boundy, J. (2014) Snakes of the world: A Catalogue of Living and Extinct Species. Taylor and Francis, CRC Press, 1237 pp. https: // doi. org / 10.1201 / b 16901
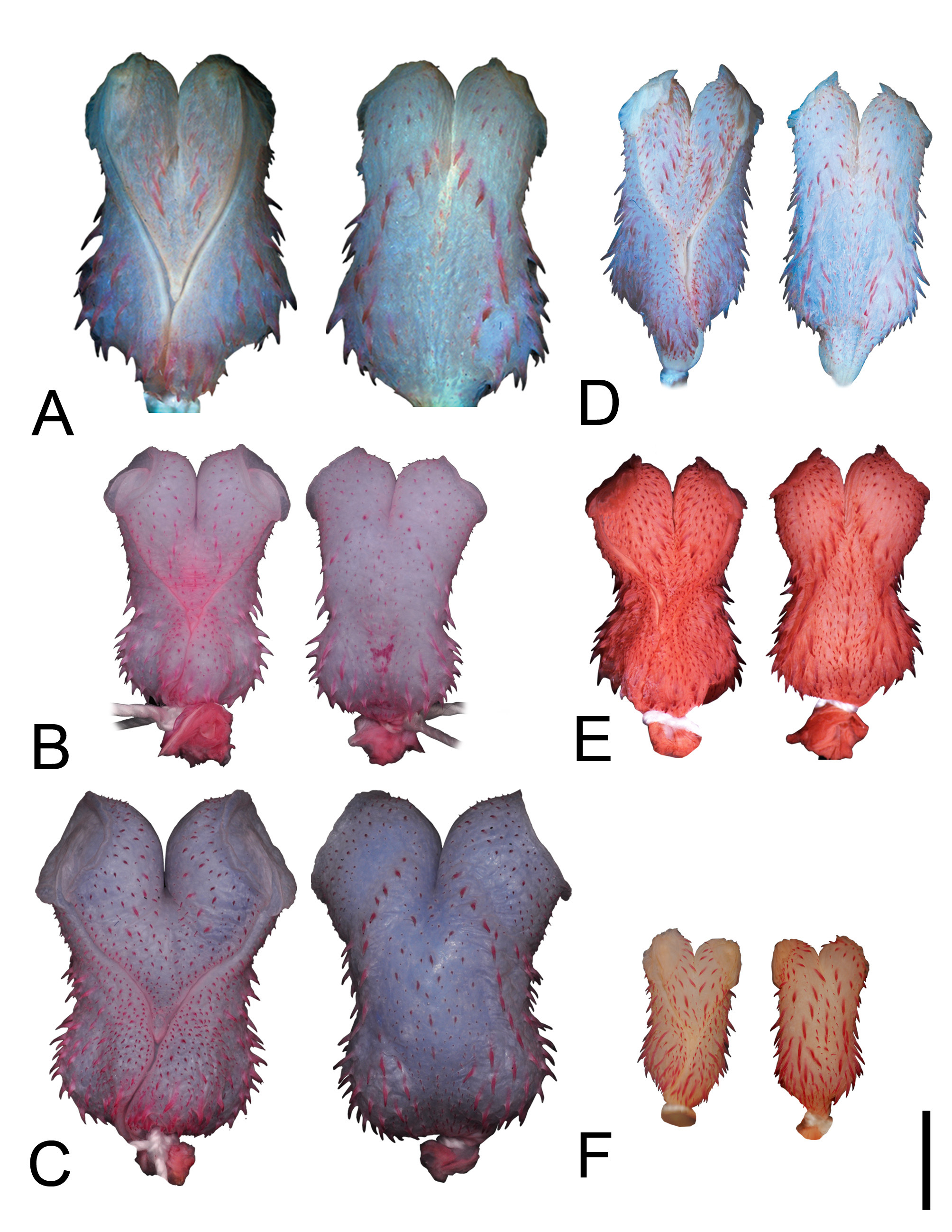
FIGURE 1. Dorsal (left) and ventral (right) views of the lectotype (NRM 44; SVL= 185 mm—A) and paralectotype of Coluber reginae (NRM 45; SVL= 165 mm—B), holotype of Coluber graphicus (BMNH 1946.1.5.73b; SVL= 340 mm—C), lectotype of Natrix semilineata (ZSM 1832-0-1; SVL= 375 mm—D); and holotype of Liophis oligolepis (BMNH 1946.1.4.66; SVL= 247 mm—E). Photos by B. Kajrup (B), P. Campbell (C, E), and M. Franzen (D).
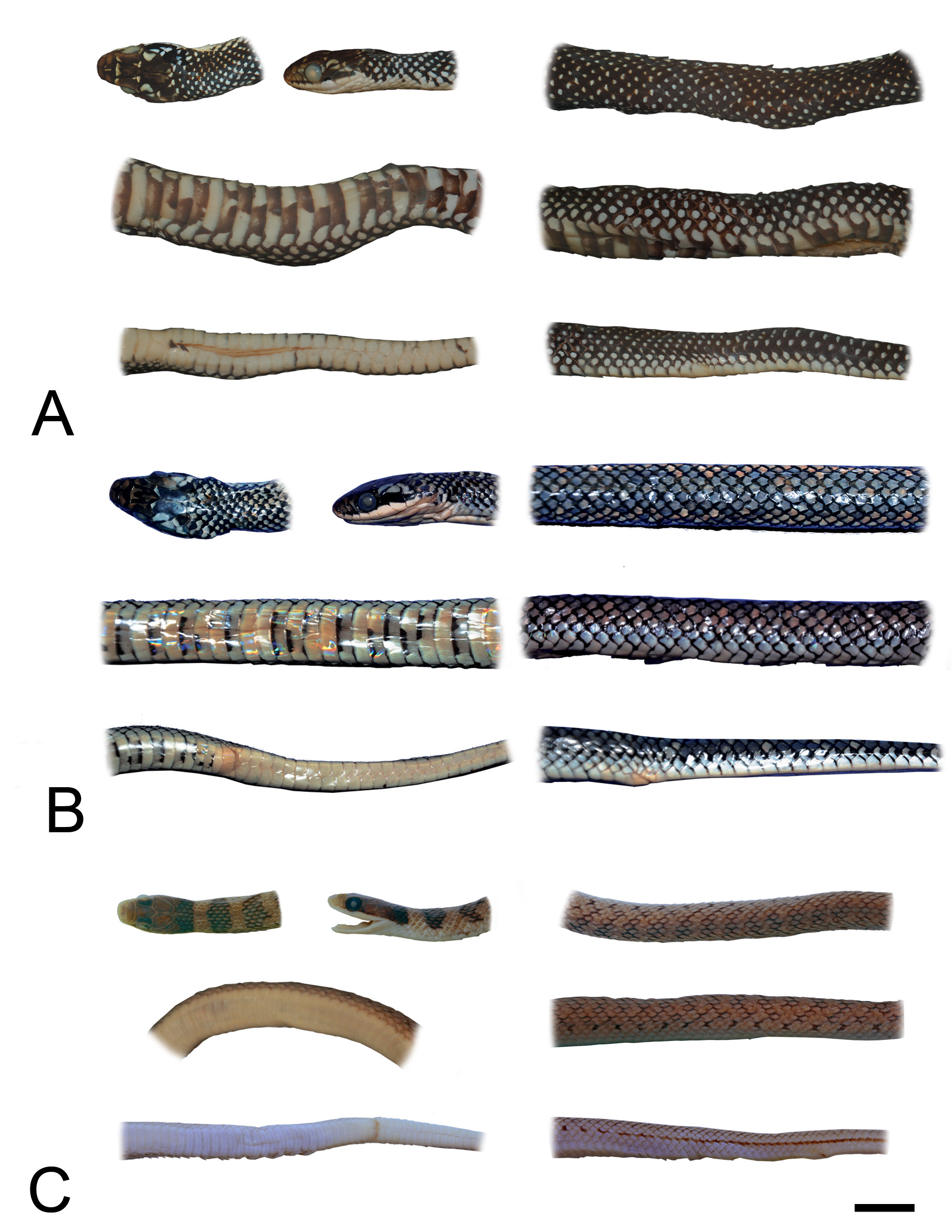
FIGURE 2. Dorsal and lateral views of head; dorsal, ventral and lateral views of midbody; and ventral and lateral views of tail of Erythrolamprus reginae (RMNH 13662—A), E. oligolepis (MPEG 25180—B), and E. macrosomus (FUNED 802—C). Scale Bar = 10 mm.
FIGURE 3. Sulcate (left) and asulcate (right) views of the hemipenis of Erythrolamprus reginae (RMNH 42042—A), E. oligolepis (MPEG 23979—B), E. macrosomus (CEPB 2125—C), E. zweifeli (AMNH 137302—D), E. dorsocorallinus (UFAC 129—E), and E. rochai (MPEG 25681—F). Scale Bar = 5 mm.
FIGURE 4. Dorsal (left) and ventral (right) views of the topotype of Erythrolamprus macrosomus (MCP 13063; SVL= 557 mm—A), holotype of Leimadophis zweifeli (MBUCV 95; SVL= 447 mm—B), holotype of Liophis dorsocorallinus (ULABG 6691; SVL= 474 mm—C), and holotype of Liophis miliaris intermedius (ZFMK 34243a; SVL= 340 mm—D). Photos by M. Rivera (A), E. La Marca (B), and M. Franzen (D).
FIGURE 5. Dorsal and lateral views of head; dorsal, ventral and lateral views of midbody; and ventral and lateral views of tail of Erythrolamprus zweifeli (AMNH 137302—A), E. dorsocorallinus (MZUSP 7362—B), and E. rochai (MPEG 25680 -C). Scale Bar = 10 mm.
FIGURE 6. General aspect of individuals in life of Erythrolamprus reginae from Trésor reserve, French Guiana (A), E. oligolepis from Maués, Amazonas, Brazil (B); E. macrosomus from San Sebastian de La Selva, Misiones, Argentina (C); E. zweifeli from Parque Nacional Henri Pittier, Rancho Grande, Aragua, Venezuela (D); E. dorsocorallinus from Boca Pariamanu, Peru (E); and E. dorsocorallinus from Barinas, Venezuela (F). Photos by Trésor Foundation (A), P. Peloso (B), J. Chiavo (C), J. Mejías (D), S. de Jong (E), and D. Sandoval (F).
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |
Erythrolamprus reginae ( Linnaeus 1758 )
| Ascenso, Alexandre C., Costa, João C. L. & Prudente, Ana L. C. 2019 |
Liophis miliaris intermedius
| Henle & Ehrl 1991 |
Liophis wagleri
| Jan 1863 |
Liophis reginae
| Dumeril, Bibron & Dumeril 1854 |
Natrix semilineata
| Wagler 1824 |
Natrix reginae
| Merrem 1820 |
Coluber graphicus
| Shaw 1802 |
Coluber reginae
| Linnaeus 1758 |
Mus
| Linnaeus 1758 |
Erythrolamprus reginae reginae
| reginae (Linnaeus 1758 |