Delma australis Kluge, 1974
|
publication ID |
https://doi.org/ 10.11646/zootaxa.3946.3.1 |
|
publication LSID |
lsid:zoobank.org:pub:91C0214A-7A3D-4DEC-9D24-7B10928AC127 |
|
DOI |
https://doi.org/10.5281/zenodo.6109329 |
|
persistent identifier |
https://treatment.plazi.org/id/03DA87B5-FFFC-8E6F-FF49-F9F42A7E57FF |
|
treatment provided by |
Plazi |
|
scientific name |
Delma australis Kluge, 1974 |
| status |
|
Marble-faced Delma
( Figs. 6 View FIGURE 6 , 7 View FIGURE 7 , 11)
Holotype: WAM R27359, male, Port Lincoln (34º44'S 135º52'E), South Australia, Australia, collected by G.M. Storr, 19 October 1966.
Paratype: WAM R24528, female, 37 km ENE of Wirrula (32º22'S 134º54'E), South Australia, Australia.
Other material examined: A full list of material examined is provided at the end of the paper.
Revised diagnosis. A small species of Delma ( SVL to 93 mm) with: ventral scales not markedly larger than adjacent lateral scales; one pair of supranasals; typically 18 midbody scales; 68–92 ventral scales (males average 76.3, females 83.5); six upper labials typically with fourth below eye; loreal scale row typically interrupted by a ventral extension of supraloreal scale that contacts upper labials; modally 5‒7 hindlimb scales in both sexes; strong dark variegations on upper surface of head; narrow dark bars on side of head (extending onto labial scales), nape and forebody. This revised diagnosis is essentially unchanged from those provided by previous authors ( Kluge 1974; Storr et al. 1990; Shea 1991), despite the exclusion herein of D. hebesa sp. nov.
Delma australis differs from the closely related D. torquata of southeastern Queensland in: larger adult size ( SVL to 93 mm versus to 63 mm); three precloacal scales (versus two); the fourth upper labial scale typically below the eye (versus typically the third below the eye); modally 18 midbody scale rows (versus 16); and dark variegations or narrow bars (if present) on head, neck and forebody (versus broad dark bands). It differs from D. hebesa sp. nov. in: hindlimb scale counts in both sexes modally 5‒7 (versus> 9); body colour brownish on head and tail (versus greyish on head and tail); head, nape and lateral scales of forebody with strong dark variegations or narrow barring (versus weak variegations); dark barring on head typically extends ventrally onto the chin and throat (versus indistinct dark bars or smudges present on the lower labials); and dark pigment on rostral and lower labials not aligned with sutures (versus dark smudges positioned over sutures between rostral and lower labials).
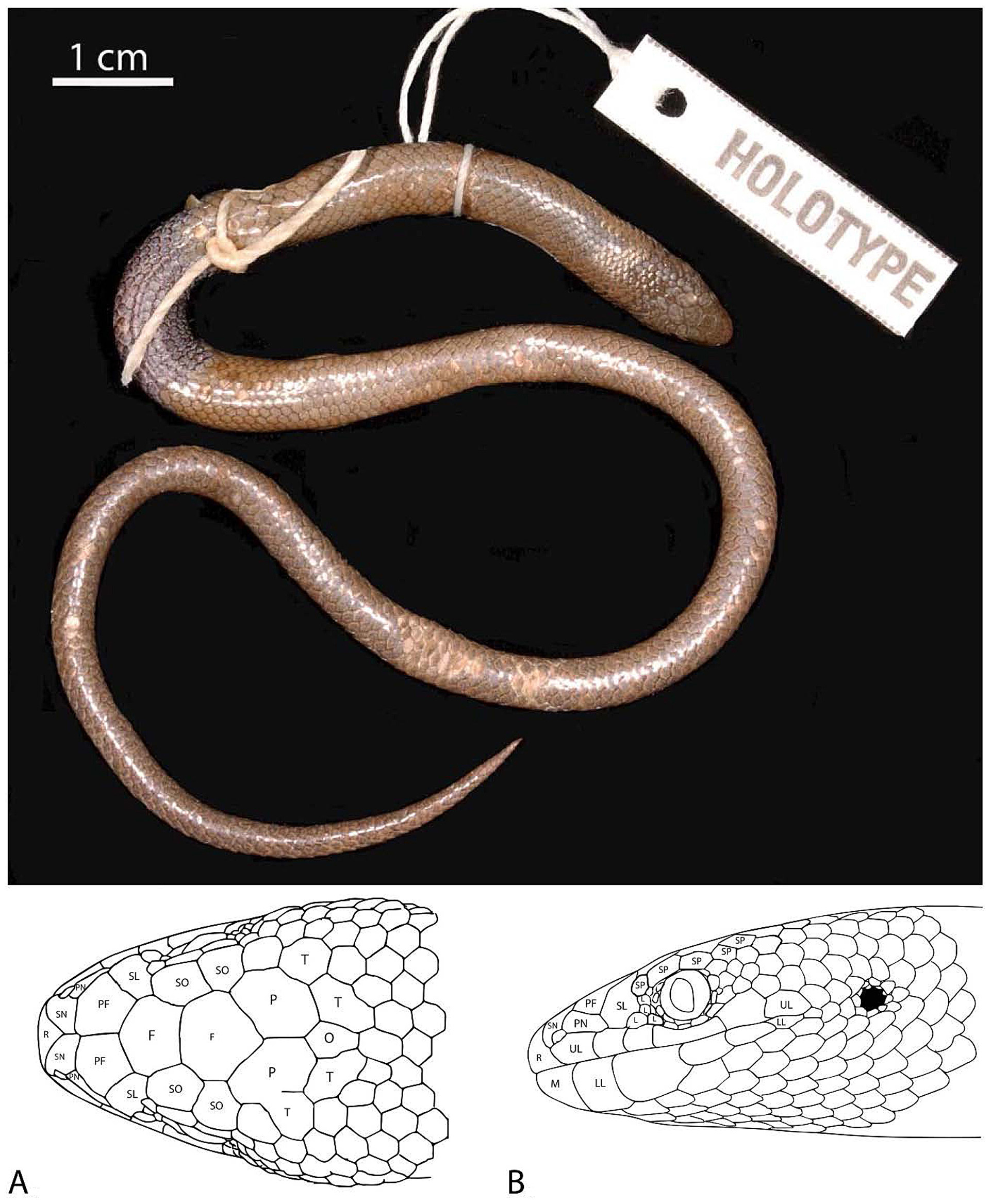
Description of holotype ( Fig. 6 View FIGURE 6 ). Measurements (in mm) and meristic values, as determined during this study: SVL 65, HD 3.5, HL 7.5, HW 5.0, HLL 3.2, ML 4.7, RL 1.0, RW 1.7, SL 2.8, EW 1.3, HLS 10, MSR 19, VE 71.
Head short and blunt, narrowing very gradually forward of eyes, of equal width to body posteriorly; obvious tympanic aperture, indicated by round opening directly posterior to corner of mouth; snout blunt and rounded in dorsal profile, rounded in lateral profile; body moderately robust of equal width and round in cross-section; hindlimbs visible as well-developed elongate, rounded flaps adpressed to body at lateral extremes of vent; tail relatively short, tapering very gradually distally to a pointed tip.
Head scales smooth, non-imbricate and heterogeneous; large rostral blunt anteriorly, wider than long, with obtuse apex projecting between supranasals; one pair of supranasals in broad contact, angled backwards posteromedially behind rostral and in short contact with first upper labial anterior to nostril; nostril positioned on posterior junction of supranasal with first upper labial and postnasal; one postnasal, much wider than high and narrower posteriorly, slightly angled posteroventrally and in broad contact with second upper labial; prefrontals symmetrical, in broad contact; one supraloreal, much higher than wide and in broad contact with second upper labial; four loreals, the anterior most much larger; five supraciliaries, first and fourth the smallest, second the largest; two supraoculars, first slightly larger and wider than second; two frontals, the posterior most slightly wider and larger; two parietals, one on left side fused (partial suture evident) with upper temporal; occipital present; two upper temporals; six upper labials, fourth the widest and positioned below eye, third the smallest and fifth the highest; five lower labials, second the largest and widest, fifth the smallest; mental wider than long with suture starting half-way along the first upper labial. General form of head and details of scalation illustrated in Fig. 6 View FIGURE 6 A, B.
Body scales smooth, non-imbricate, homogeneous, and arranged in parallel longitudinal rows; ventral scales only very marginally wider than the adjacent lateral body scales; three precloacal scales.
After more than 40 years in preservative ( Fig. 6 View FIGURE 6 ), the holotype is light brown on the dorsal surface with a slightly darker head bearing dense black variegations. Lower labials whitish with distinct black bars positioned centrally on first to third lower labial scales. Black bars continue on to corner of mouth, around ear opening and on the lateral scales of forebody. A faint dark bar is also present within mental scale. Dark bars extend ventrally onto chin and throat. Ventral surface under head and along body is whitish.
Variation in measurements and scalation. Table 5 presents the means, standard deviations and ranges of the characters counted and measured for each of the three geographic populations of D. australis , as defined in Material and Methods. Data are presented separately for each sex. Most head scales in D. australis display minimal intraspecific variation. However, the condition of the supraloreal (described as a prefrontal by Shea 1991) extending ventrally to contact the upper labials, thereby interrupting the row of small loreals, is variable in this species. The most common condition is contact with the upper labials, as noted by Storr et al. (1990: 115), Shea (1991: 115) and shown in Figure 6 View FIGURE 6 B. Our examination of 97 specimens of D. australis from Western and South Australia recorded the supraloreal (sometimes the postnasal and prefrontal) contacting the upper labials in over 70% of specimens. The opposing condition of no contact between the supraloreal and upper labial scales was mostly caused by the presence of either one large loreal (sometimes with elongate postnasal), or two smaller loreals extending continuously from the postnasal to the subocular upper labial.
We observed 0‒5 loreals in this species, with one loreal being the typical condition, as reported by Storr et al. (1990). Most specimens had six upper labials with the 4th below the eye but we saw occasional individuals with five (3rd below the eye) or seven (5th below the eye), mostly unilaterally. Over 70% of specimens examined had the typical 18 midbody scales. Most others had 20 midbody scales, with occasional counts of 17 and 19, corresponding to the range given by Storr et al. (1990). One specimen (SAMA R36487) has extensively fused head scales, with the supraloreal and prefrontal fused on the left side only and only four upper labials on the right side. The longest tail measured was 162 mm (245% of SVL) on WAM R 112667. Kluge (1974: 78) and Storr et al. (1990: 115) illustrate the general form of the head and details of scalation of D. australis .
Variation in colouration and pattern. Shea (1991: 72‒73) documented geographic variation in the intensity of the head pattern in this species in South Australia. He distinguished and mapped a ‘patterned’ form from the southern half and northwest of the state ( Fig. 7 View FIGURE 7 A; Cogger 2014: 388) and an ‘unpatterned’ form from the western Lake Eyre drainage ( Fig. 7 View FIGURE 7 B). The holotype and 15 paratypes of D. australis from the Eyre Peninsula (south of the Gawler Ranges) in South Australia ( Kluge 1974) are considered representative of the ‘patterned’ form ( Shea 1991: Fig. 1 View FIGURE 1 ) in having strong dark variegations on the head, although the pattern is reduced, particularly laterally, in a few specimens. The ‘patterned’ form is contiguous and similar to populations in northwestern Victoria and southwestern New South Wales ( Swan et al. 2004: 49; Swan & Watharow 2005: 26; Wilson & Swan 2013: 145). Kluge (1974: 78) illustrates a preserved adult male specimen (SAMA R10375) of the ‘patterned’ form from near Kokatha in South Australia. In addition, strongly patterned individuals of D. australis have been illustrated by Ehmann (1992: 87) and Henkel (2010: 141).
Western Australian populations of D. australis also display geographic patterning in colouration. Strongly patterned individuals with dark variegations and narrow bars on the lateral scales of forebody occur throughout the eastern Coolgardie Goldfields ( Fig. 7 View FIGURE 7 C) and Mallee bioregions ( Fig. 7 View FIGURE 7 D) ( Thackway & Cresswell 1995). Particularly strongly patterned individuals are found on the Houtman Abrolhos ( Fig. 7 View FIGURE 7 E), while the population found to the immediate north around Shark Bay has very reduced patterning, with some individuals almost having dark brown uniform heads ( Fig. 7 View FIGURE 7 F), similar in many respects to the unpatterned form from South Australia. By contrast, a unique representative of the group (WAM R132470) from the North West Cape has a rich brick-red body with an intensely black head flecked with small pale spots ( Fig. 7 View FIGURE 7 G).
Distribution and sympatry. Delma australis is widespread throughout the subhumid to arid areas of southern Australia, from northwestern Victoria, and southwestern New South Wales, through most of South Australia and adjacent southern Northern Territory to southern and central west Western Australia ( Wilson & Knowles 1988; Shea 1991; Swan et al. 2004; Wilson & Swan 2013; Fig. 8 View FIGURE 8 ). In Western Australia, it extends north to Shark Bay (base of Peron Peninsula), Meedo Station, Weld Range, Paynes Find, Windarling Hill and Buningonia Spring, south through the Avon Wheatbelt, Mallee and Coolgardie Goldfields bioregions, and east to Cocklebiddy. There is a disjunct population on the North West Cape, represented by a single specimen from Shothole Canyon in the Cape Range ( Fig. 8 View FIGURE 8 ). Other possible outlier populations in the mid-west of Western Australia are Walyering Hill, Oakajee and near Kalbarri. Insular populations occur on Rat and Middle Islands in the Houtman Abrolhos.
Recorded instances of sympatry involving D. australis include D. butleri , D. fraseri , D. grayii Smith , D. nasuta Kluge and D. petersoni Shea ( Dell & Chapman 1981; Dell & How 1984; Chapman & Dell 1985; Shea 1991; Mckenzie et al. 1993; B. Maryan & G. Shea, per. obs.). For instance, in the vicinity of Poochera in South Australia, D. australis , D. butleri and D. petersoni have all been recorded ( Shea 1991: 82). Four other species of Delma are recorded on the North West Cape. Delma nasuta and D. tincta are known from several localities (Maryan et al. 2007), while D. tealei Maryan, Aplin & Adams has been collected at Shothole Canyon and other localities on the Cape Range. Delma butleri is known from a single specimen collected at the Learmonth Air Weapons Range, immediately south of the Cape Range National Park.
Habitat. In northwestern Victoria and southwestern New South Wales D. australis mainly occupies mallee habitats with a spinifex ( Triodia ) understorey ( Swan et al. 2004; Swan & Watharow 2005). This habitat association is repeated in South Australia where the majority of the southern and western populations are from Triodia or mallee habitats, or combination of both ( Shea 1991). The western Lake Eyre Basin population lives on gibber plain with Atriplex , on watercourses lined with Eucalyptus , and on low, stony hills with drainage channels and Acacia ( Shea 1991; B. Maryan, pers. obs.).
In Western Australia, D. australis occupies a variety of habitats growing on different soils, including mallee and/or other Eucalyptus woodlands and Acacia with a spinifex (Triodia and Plectrachne) or shrubland understorey ( Storr et al. 1990; Smith et al. 1997; Bush et al. 2007). The habitat at Cape Range on the North West Cape consists of a heavily dissected limestone plateau sparsely vegetated with Triodia, shrubs and low eucalypts; gorges within the range are more heavily vegetated ( Storr & Hanlon 1980).
These diverse vegetation communities provide ample cover for D. australis , where most specimens have been pit-trapped, found in and under spinifex and sedge tussocks, raked from leaf litter, spoil-heaps, mats of dead vegetation and found under logs, mallee roots, rocks (including coral slabs on the Houtman Abrolhos) and rubbish, especially pieces of corrugated iron in disturbed areas adjacent to uncleared vegetation. When found in sympatry with other Delma species, D. australis tends to occur in moister microhabitats ( Shea 1991; Wilson & Swan 2013). Interestingly, nocturnal observations of D. australis on sealed roads or tracks are rare, unlike other, larger-bodied species of Delma .
Remarks. Using morphology alone, Kluge (1974) recognized and assessed differences between three geographic samples of male D. australis : a southwestern Western Australia sample (south of 32°30’S, west of 120°E), an Eyre Peninsula of South Australia sample (south of 32°S), and a Victorian sample. He found significant mean differences in number of preorbital, preanal, hindlimb and caudal scales; as well as throat pattern and visceral pigmentation. A multivariate analysis of these six characters also revealed significant differentiation among the three regions. However, Kluge’s character definitions were different to those used in this study, and were based on a very small sample size (n=3 per locality = 7, 8 and 12 respectively, with the Western Australian sample potentially including both D. australis and D. hebesa sp. nov.). Further studies in the laboratory and field are needed to determine whether or not the geographic sub-populations identified in each of South Australia and Western Australia correspond with the ‘northern’ and ‘southern’ groups distinguished by allozyme differences in the present study. Large gaps remain in the geographic sampling of all regional populations and the collection of additional specimens at or near potential contact zones would be particularly valuable to establish the status of the various forms.
| WAM |
Western Australian Museum |
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.