Cerithidea Swainson, 1840
|
publication ID |
https://doi.org/ 10.11646/zootaxa.3775.1.1 |
|
publication LSID |
lsid:zoobank.org:pub:D9FF6080-0316-4433-ABB8-7D6D6F2BF24B |
|
DOI |
https://doi.org/10.5281/zenodo.5694412 |
|
persistent identifier |
https://treatment.plazi.org/id/03DA0723-6532-284A-D1A0-F983FE958F73 |
|
treatment provided by |
Plazi |
|
scientific name |
Cerithidea Swainson, 1840 |
| status |
|
Genus Cerithidea Swainson, 1840 View in CoL
Cerithidea Swainson, 1840: 198 View in CoL , 203, 342
(type by subsequent designation ( Makiyama 1936) Melania lineolata Gray in Griffith & Pidgeon, 1833 = C. obtusa View in CoL ).
Phaenommia Mörch, 1860: 80 View in CoL
(type by monotypy Cerithidea charbonieri (sic) = Cerithium charbonnieri Petit, 1851 ).
Potamides (Aphanistylus) Fischer, 1884: 682
(type by monotypy Cerithidea charbonnieri = Cerithium charbonnieri Petit, 1851 ).
Taxonomic history. When introducing the new genus Cerithidea, Swainson (1840) gave only two included species, Melania lineolata (= C. obtusa , herein) and the fossil Cerithium fragilis Brogniart. The subsequent history of the designation of the former as the type of the genus has been discussed by Bequaert (1942b), who concluded that the first valid designation (of C. obtusa ) was that of Pilsbry & Harbison (1933). This conclusion was accepted by Houbrick (1984) but, as pointed out by Petit & Coan (2008), this designation was invalid, because it did not mention either of the originally included taxa, so that the first valid designation was that of Makiyama (1936).
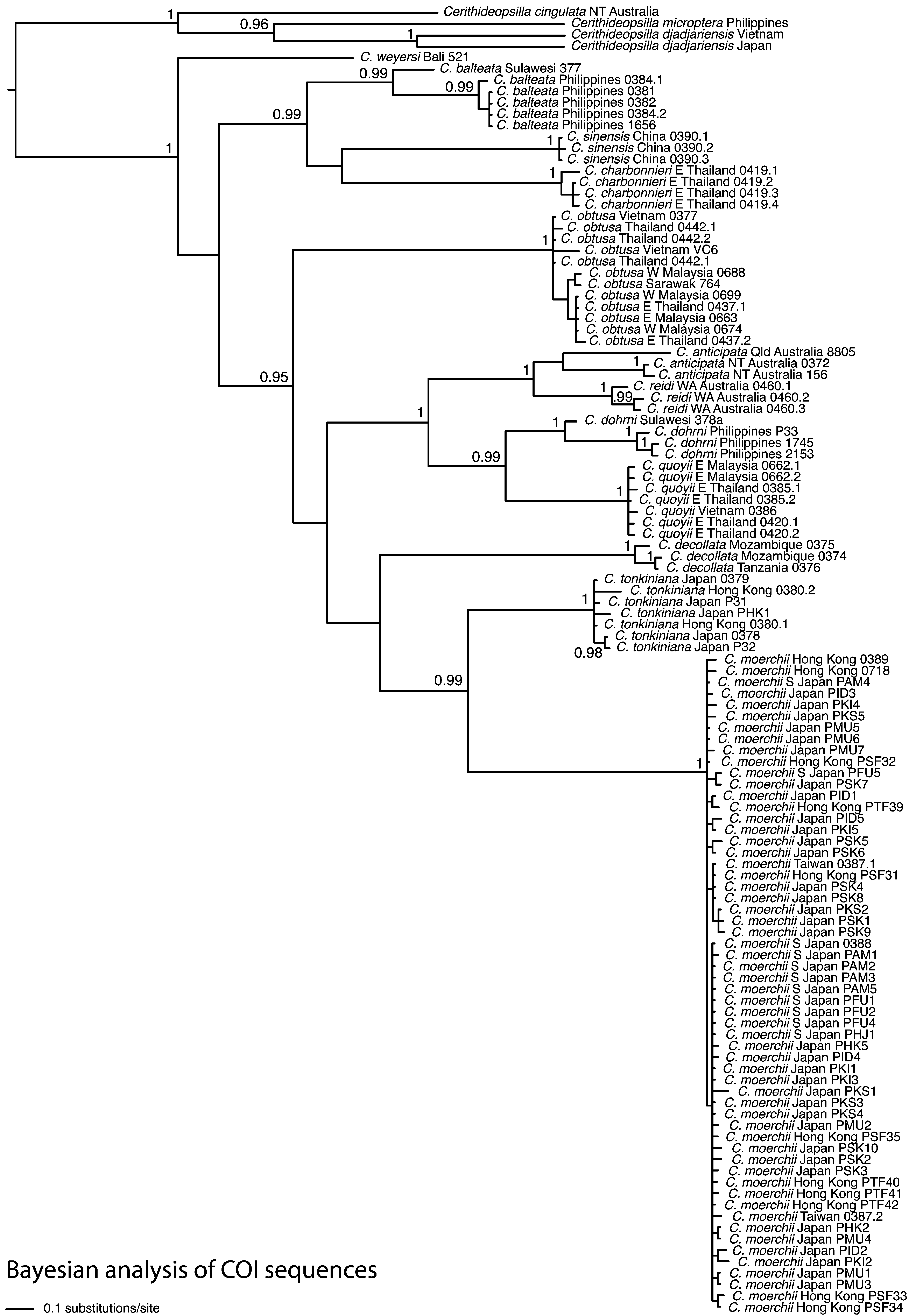
Two generic names have been based on C. charbonnieri , by Mörch (1860) and Fischer (1884). Both authors cited the long optic peduncles and short or invisible tentacles in the figure by H. Adams & A. Adams (1854: pl. 31, fig. 2). In fact the tentacles of living and preserved Cerithidea species are often folded back and not immediately visible (von Martens 1897a: pl. 10, fig. 5), and the peduncles are not unusually long in this species. The shell of C. charbonnieri is unusually smooth for the genus, with a strong peripheral carina. Aphanistylus has occasionally been used as a subgenus (Fischer 1884; Kobelt 1890a; Casto de Elera 1896; Dautzenberg 1899) and rarely as a genus ( Wattebled 1886; Morlet 1889), for one or more of C. weyersi , C. charbonnieri , C. sinensis and C. balteata . Thiele (1929) remarked that the subgenus Aphanistylus “might be hardly different” from Cerithidea s. s., but nevertheless later replaced it with the earlier name Phaenommia ( Thiele 1931) . Wenz (1939) formally listed Aphanistylus and Phaenommia in the synonymy of Cerithidea . The three species C. charbonnieri , C. sinensis and C. balteata do form a well-supported molecular clade ( Fig. 1 View FIGURE 1 ). Nevertheless, there is no known morphological synapomorphy that could justify the continued use of this subgeneric unit.
The generic name Cerithidea is here used only for those potamidids from the Indo-West Pacific that have a close, or obligate, association with mangrove trees and halophytic vegetation. This concept of Cerithidea is that of Reid et al. (2008) and before this the generic classification of its species had a complex history. Cerithidea was established by Swainson (1840) and included as a genus in the classification by Gray (1847). This early concept of the genus included American species (assigned to Cerithideopsis by Reid et al. 2008) as well as the Indo-West Pacific members (H. Adams & A. Adams 1854; Adams 1855; Sowerby 1866). The smaller, mud-dwelling Indo- West Pacific potamidids (assigned to Cerithideopsilla by Reid et al. 2008) were classified in Tympanotonos by these early authors, while Pirenella contained the diverse forms of the polymorphic P. conica from the Mediterranean and Indian Ocean. Some nineteenth-century authors maintained a much broader generic concept and included all these potamidids in Cerithium ( Kiener 1841 –1842; Sowerby 1855; Reeve 1860), sometimes with Cerithidea , Tympanotonos and Pirenella as subgenera ( Chenu 1859; Kobelt 1890 –1895). Fischer (1884) established the use of Potamides (of which the type was an Oligocene fossil) with these three potamidid groups as subgenera, and this scheme was widely followed ( Tryon 1887; von Martens 1897a; Cossmann 1906, but treating the Recent Tympanotonos s. s. as a separate genus).
The prevailing twentieth-century concept of Cerithidea dates from Thiele (1929), who introduced Cerithideopsis as a subgenus, with sections Cerithideopsis s. s. for American species and Cerithideopsilla for Indo- West Pacific ones, while continuing to recognize Pirenella as a genus. This scheme was modified only slightly by Wenz (1938), who established the tripartite division of Cerithidea in three subgenera: Cerithidea s. s., Cerithideopsis and Cerithideopsilla . This was followed by most subsequent authors including Van Regteren Altena (1940), Bequaert (1942a) and Brandt (1974), and was supported by a detailed anatomical study by Houbrick (1984). Pirenella continued to be treated as a genus, but was synonymized with Potamides by Lozouet (1986).
As a result of molecular phylogenetic analysis and a review of the shell characters of living and fossil genera, Reid et al. (2008) proposed a new classification of the Potamididae . The three former subgenera Cerithidea , Cerithideopsis and Cerithideopsilla were raised to generic rank, the enigmatic C. largillierti from the western Pacific was classified in Cerithideopsis for the first time and ‘ Potamides ’ conicus was shown to be a member of Cerithideopsilla .
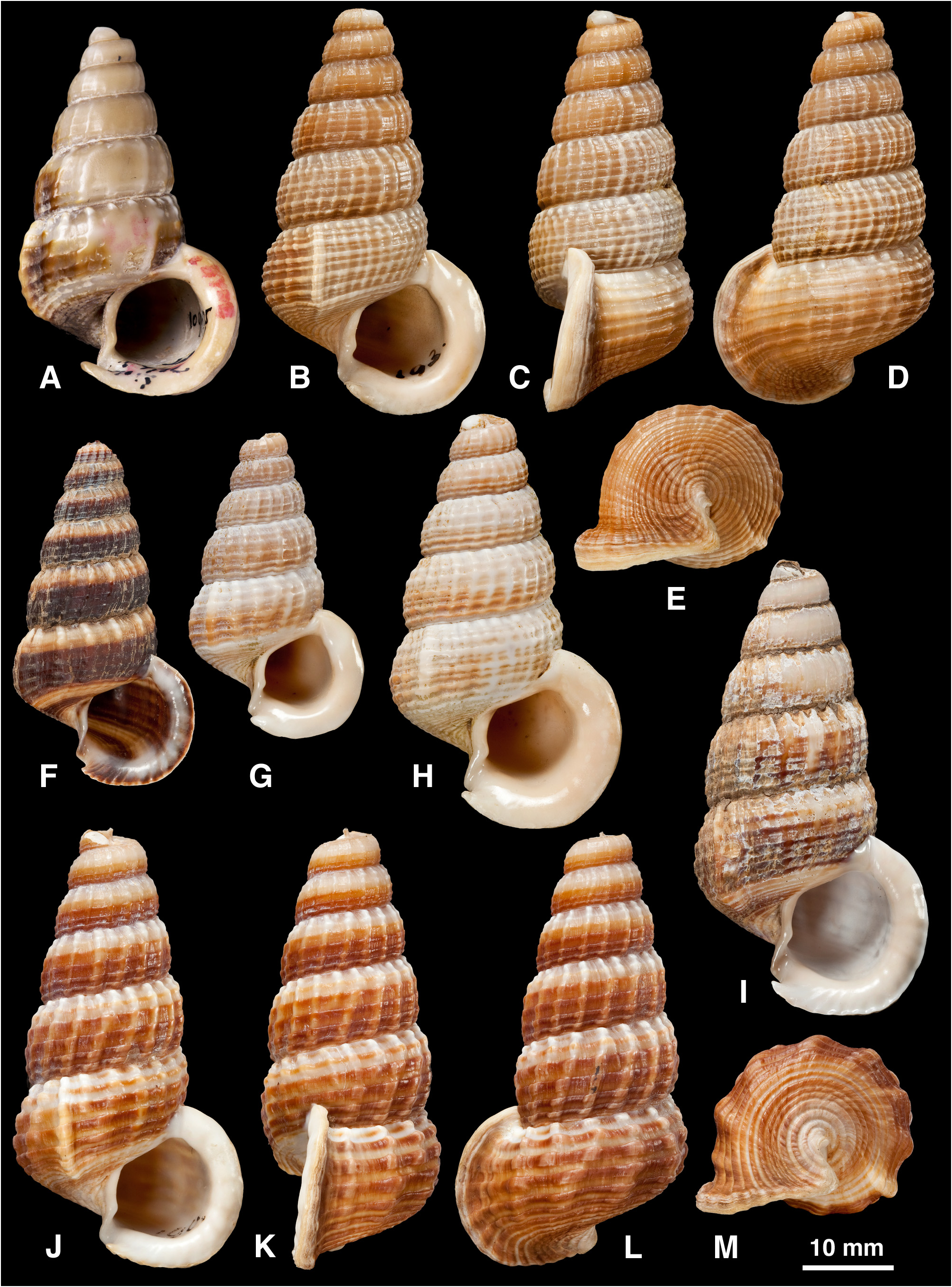
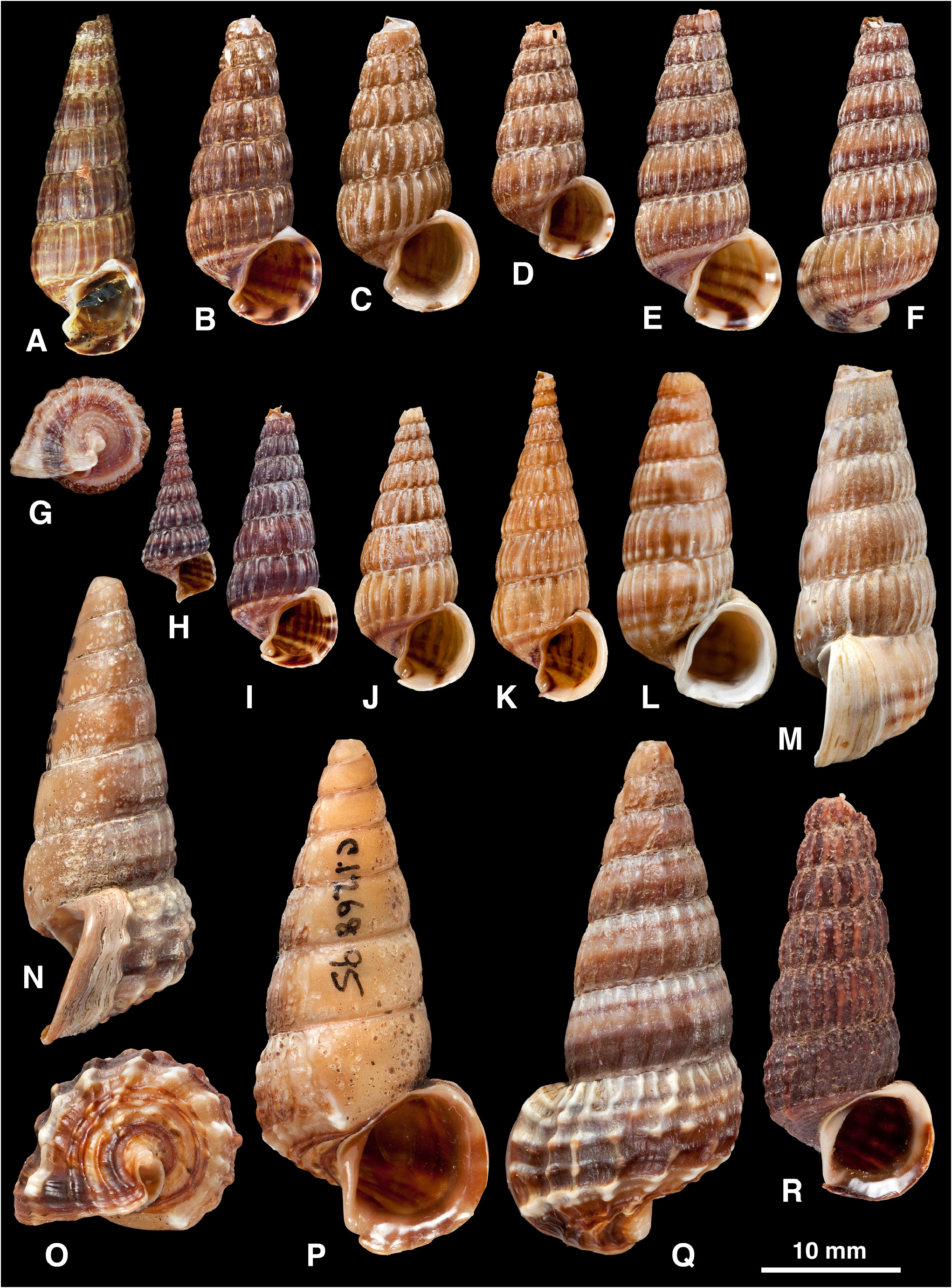
Diagnosis. Shell: large (to 61 mm), delicate to solid, elongate, spire decollate; sculpture of axial ribs, one developed as prominent ventrolateral varix on body whorl (rarely weak or absent) and weakening thereafter; varices almost always absent on spire whorls; spiral ridges present on base, present or absent above periphery; aperture circular, peristome flared and thickened in adult, planar or slightly sinuous, with short anterior canal and slight posterior groove; columella straight or slightly twisted, lacking folds; colour often with spiral bands. Radula: rachidian tooth narrow with small central cusp; outer marginal lacks cusps and has wide flange on outer side of basal shaft ( Houbrick 1984: 16).
Remarks. The diagnostic characters of the shells of the three similar potamidid genera Cerithidea , Cerithideopsis and Cerithideopsilla were summarized by Reid et al. (2008). In Cerithideopsis there are 2–3 spiral cords on the earliest spire whorls, which usually become obsolete or may increase to 5; there are numerous axial ribs that terminate at the strong spiral cord demarcating the base; varices are scattered over the spire and the ventrolateral varix is absent. In Cerithideopsilla there are 3 spiral cords, numerous axial ribs and no varices on the spire whorls; the ventrolateral varix is usually present and the anterior canal of the aperture is well defined by a basal projection of the aperture. The spire is not decollate in either of these two genera, as it is in Cerithidea .
The shell of Cerithidea is typically of light construction, as expected in relation to the habit of climbing onto trunks and vegetation, where the animals rest, attached only by mucus at the apertural lip, between periods of feeding activity on the substrate at low tide. One of the largest is C. obtusa , which is also more thick-shelled than other species; it rests on tree bases and even on the ground, where its relatively solid shell may be resistant to attack by predators such as large crabs and molluscivorous fish. Large samples of several species show dimorphism in shell size, which is possibly related to sex (e.g. Fig. 8 View FIGURE 8 G, H), although this remains to be established.
All species are decollate, the apical 5–10 mm of the spire being lost (e.g. Figs 3K, L View FIGURE 3. A – M , 15 View FIGURE 15. A – I O, P). This is a gradual process of dissolution of the delicate early whorls, which appears to continue throughout life, and the resulting opening is sealed by successive convex plugs of shell material secreted at the tip of the visceral mass. Dissolution does not appear to be an endogenous process connected with conservation of calcium carbonate or neutralization of tissue acidity during anaerobic respiration (cf. Vermeij 1973). Instead, it is likely to be the result of erosion by acidic conditions in the detritus-rich sediments on which the snails crawl to feed at low tide. The ventral surface of the adult shell, in prolonged contact with the sediment, sometimes shows signs of dissolution that may even remove the surface sculpture ( Figs 3 View FIGURE 3. A – M N–P, 12H). Similarly, dissolution pits may be seen wherever the periostracum is damaged. Decollation may, nevertheless, be adaptive. It is a derived character not seen to such an extreme in other potamidids ( Reid et al. 2008) and might reduce the weight of the shell, or reduce vulnerability to predatory attack by removing the more easily damaged apical whorls.
Growth is determinate and the final whorl is modified by reduction of axial sculpture, the formation of a single strong axial rib (the ‘ventrolateral varix’) and a final flaring and thickening of the aperture (compare juvenile and adult in Figs 12 View FIGURE 12. B – J O, K, 14I, A). This growth pattern is typical of cerithioideans and has been considered adaptive in relation to stability on soft substrates and protection against predatory crabs that attack the aperture and ‘peel’ the shell ( Sälgeback & Savazzi 2006). Vermeij (1973) pointed out that in tree-climbing potamidids the form of the shell is a compromise between achieving stability on sediment and on a hard substrate. The flared aperture generally has a planar margin and is inclined at an angle to the axis of coiling (i.e. opisthocline) so that the shell opening can be held closely against a flat surface such as the trunk of a tree ( Fig. 12 View FIGURE 12. B – J T, V); in the potamidid Terebralia sulcata this has been considered as an adaptation against desiccation ( Sälgeback & Savazzi 2006), but in Cerithidea it is likely that this shape also functions to improve attachment by dried mucus. In five species ( C. sinensis , C. charbonnieri , C. tonkiniana , C. rhizophorarum , C. moerchii ) the apertural margin is slightly sinuous in side view so that the aperture cannot be applied tightly to a flat surface ( Fig. 7G View FIGURE 7. A – L , Q). Three of these five species ( C. sinensis , C. tonkiniana , C. moerchii ) extend to the highest latitudes in the entire genus, beyond the mainly tropical range of mangrove trees, and can be found attached to salt marsh plants and reeds, so this form of the aperture could be functional in relation to attachment to stems of narrow diameter. Whether the ventrolateral varix improves stability by preventing rolling is doubtful, because its position is rather variable (e.g. Fig. 12 View FIGURE 12. B – J Q–S, W–Y, AA–CC); stability would only be enhanced if this varix were always close to 270° behind the apertural edge. Instead, it is likely that the varix serves mainly as a second line of defence against predators that break the thickened aperture and peel away the thin body whorl. Evidence of such damage that stops at the ventrolateral varix is frequent in Cerithidea but, as observed in Terebralia ( Sälgeback & Savazzi 2006) , sublethal breakages do not extend past this barrier, presumably because they are always lethal. In Cerithidea the ventrolateral varix is not only thickened, but also bears two internal teeth (pers. obs. in C. moerchii ). In some specimens this varix appears to be formed by the fusion of two axial ribs.
The anatomy and radula of Cerithidea have been described by Houbrick (1984, 1986). A notable peculiarity is the presence of a pallial eye on the mantle margin, which protrudes into the anterior canal of the shell when the animal is at rest. In C. obtusa the eye has an epithelial lens and cup-shaped cornea ( Pflugfelder 1930) and pallial eyes of varying complexity are found in Cerithideopsis , Cerithideopsilla and other cerithioideans ( Houbrick 1984). As expected in snails that live mainly out of water, the gill and osphradium are reduced and the mantle cavity is vascularized ( Houlihan 1979; Houbrick 1984). The living animal is sometimes brightly coloured, as first recorded by Adams (in Adams & Reeve 1848; see also Fukuda 2000; Fig. 2 View FIGURE 2 ).
In Southeast Asia several common species (especially C. obtusa and C. quoyii ) are of local economic importance and sold in food markets ( Brandt 1974; Poutiers 1998; Hamli et al. 2013).
In the following accounts of the species, the arrangement follows an approximate phylogenetic sequence. The COI phylogeny ( Reid et al. 2013; Fig. 1 View FIGURE 1 ) identifies the following monophyletic groups: (1) C. weyersi ; (2) C. balteata , C. sinensis , C. charbonnieri ; (3) C. obtusa ; (4) C. anticipata , C. reidi , C. quoyii , C. dohrni ; (5) C. decollata ; (6) C. tonkiniana , C. moerchii . The species C. houbricki , C. andamanensis and C. rhizophorarum are known only from shell material and have been added to groups 1, 4 and 6 respectively, on the basis of shell resemblance.
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
Cerithidea Swainson, 1840
| Reid, David G. 2014 |
Phaenommia Mörch, 1860 : 80
| Morch 1860: 80 |
Cerithidea
| Swainson 1840: 198 |