Echinolittorina angustior ( Mörch, 1876 )
|
publication ID |
https://doi.org/ 10.11646/zootaxa.2184.1.1 |
|
persistent identifier |
https://treatment.plazi.org/id/03D3606F-A536-FFD6-FF26-FC2DFBEEFD81 |
|
treatment provided by |
Felipe |
|
scientific name |
Echinolittorina angustior ( Mörch, 1876 ) |
| status |
|
Echinolittorina angustior ( Mörch, 1876) View in CoL
( Figures 2A–C View FIGURE 2 , 27–30 View FIGURE 27 View FIGURE 28 View FIGURE 29 View FIGURE 30 )
Littorina ziczac View in CoL — Potiez & Michaud, 1838: 280, pl. 28, figs 11, 12 (not Gmelin, 1791). Bequaert, 1943: 14–18, pl. 5, fig. 5 (in part, includes E. lineolata View in CoL , E. ziczac View in CoL , E. jamaicensis View in CoL , E. interrupta View in CoL , E. placida View in CoL ; not Gmelin, 1791). Lewis, 1960: 415–416, fig. 11G, H (egg capsule) (in part, includes E. jamaicensis View in CoL ; not Gmelin, 1791).
Litorina ziczac View in CoL — Weinkauff, 1883: 220 (in part, includes E. interrupta View in CoL , E. jamaicensis View in CoL , E. ziczac View in CoL ; not Gmelin, 1791).
Littorina (Melarhaphe) ziczac View in CoL — Tryon, 1887: 251, pl. 45, fig. 6 (in part, includes E. interrupta View in CoL , E. jamaicensis View in CoL , E. lineolata View in CoL , E. ziczac View in CoL , Littoraria glabrata View in CoL ; not Gmelin, 1791; as Melaraphe View in CoL ). Warmke & Abbott, 1961: 52–53, pl. 9l (in part, includes E. interrupta View in CoL ; not Gmelin, 1791).
Litorina (Melarhaphe) ziczac View in CoL — Dall & Simpson, 1901: 429 (in part, includes E. ziczac View in CoL ; not Gmelin, 1791; as Melaraphe View in CoL ).
Littorina lineata View in CoL —d’Orbigny, 1842: 208–209, pl. 14, figs 24, 26, 27 (in part, includes E. jamaicensis View in CoL , E. lineolata View in CoL ; not Lamarck, 1822 = Littoraria tessellata View in CoL ). T.V. Borkowski & M.R. Borkowski, 1969: 409, fig. 1 (map), fig. 4B (egg capsule), pl. 66, figs 1, 2 (not Lamarck, 1822). Borkowski, 1971: fig. 2 (egg capsule) (not Lamarck, 1822). Flores, 1973a: 14–15, pl. 2, figs 6–10 (not Lamarck, 1822). Borkowski, 1975: 369–377, fig. 1A (radula) (in part, includes E. interrupta View in CoL , E. lineolata View in CoL ; not Lamarck, 1822).
Littorina (Melarhaphe) lineata View in CoL —von Martens, 1900: 583 (in part, includes E. interrupta View in CoL , E. jamaicensis View in CoL ; not Lamarck, 1822).
? Littorina (Melarhaphe) ziczac lineata View in CoL — Johnson, 1934: 102 (as Melaraphe View in CoL ; not Lamarck, 1822).
Littorina (Austrolittorina) lineata View in CoL — Rosewater, 1970: 423 (not Lamarck, 1822).
Littorina carinata d’Orbigny, 1841: 209–210 View in CoL , pl. 15, figs 1–4, 4’ (operculum) ( Cuba; lectotype ( Bandel & Kadolsky 1982) + 12 paralectotypes BMNH 1854.10 .4.128, seen).
Litorina carinata View in CoL —Philippi, 1847: 164, Litorina View in CoL pl. 3, fig. 19. Küster, 1856: 19, pl. 2, figs 28, 29 (pl. 2 1853). Weinkauff, 1878, 1882: 31, 107 (in part, includes E. jamaicensis View in CoL ). Weinkauff, 1883: 220 (in part, includes E. jamaicensis View in CoL ).
Littorina (Melarhaphe) carinata View in CoL — Mörch, 1876: 139 (as Melaraphe View in CoL ).
Littorina (Melaraphis) carinata View in CoL —Arango, 1880: 158.
Littorina (Melarhaphe) angustior Mörch, 1876: 139 View in CoL (Havanna [La Habana, Cuba] (restricted by Bandel & Kadolsky 1982); lectotype ( Bandel & Kadolsky 1982) ZMK, Fig. 27A View FIGURE 27 herein + paralectotype ZMK, specimens both lost, not seen; as Melaraphe View in CoL ). Nevill, 1885: 140 (as Melaraphe View in CoL ).
Litorina angustior —Weinkauff, 1882: 67, pl. 8, fig. 16. Weinkauff, 1883: 220 (in part, includes E. jamaicensis View in CoL ).
Littorina (Austrolittorina) angustior View in CoL — Abbott, 1974: 68–69, fig. 560. H.E. Vokes & E.H. Vokes, 1983: 14, pl. 4, fig. 3.
Nodilittorina (Nodilittorina) angustior View in CoL — Bandel & Kadolsky, 1982: 25–26, figs 5 (shell, egg capsule, operculum, radula), 8 (map), 27–29 (shells and radulae).
Nodilittorina angustior View in CoL — Bandel, 1984: fig. 13 (radula). Britton & Morton, 1989: 86, fig. 4-5J. Espinosa & Ortea, 2001: 15, fig. 80. Redfern, 2001: 28, pl. 14, fig. 113. Reid, 2002a: 259–281, fig. 1E.
Littorina angustior View in CoL — Janson, 1985: 871–879. Sterrer, 1986: 408, pl. 135. De Jong & Coomans, 1988: 19. Díaz & Puyana, 1994: 124 (in part, includes E. interrupta View in CoL ).
Nodilittorina (Echinolittorina) angustior View in CoL — Reid, 1989: 99, fig. 11o (egg capsule).
Echinolittorina angustior View in CoL — Williams, Reid & Littlewood, 2003: 60–86. Williams & Reid, 2004: 2227–2251, fig. 6D (map).
Littorina lineolata View in CoL — Abbott, 1964: 65–66 (in part, includes E. placida View in CoL , E. jamaicensis View in CoL ; not d’Orbigny, 1840). Abbott, 1968: 82–83, fig. (not d’Orbigny, 1840).
Littorina jamaicensis View in CoL — Bandel, 1974a: 99, 103, figs 10 (shell), 17 (egg capsule) (in part, includes E. jamaicensis View in CoL ; not C.B. Adams in Philippi, 1847).
Taxonomic history: Bequaert (1943) noted that the name L. carinata d’Orbigny, 1841 View in CoL was a junior secondary homonym of Turbo carinatus J. Sowerby, 1819 , because he considered the latter to be a Littorina View in CoL . He did not replace the name, considering it to be a synonym of L. ziczac View in CoL (so the name is not permanently invalid; ICZN 1999: Art. 59.3). In fact, as pointed out by Bandel & Kadolsky (1982), Sowerby’s species is not a member of the Littorinidae View in CoL , removing the homonymy. However, they went on to propose a new secondary homonymy with Delphinula carinata Woodward, 1833 and Turbo carinatus Woodward, 1833 , which are both forms of L. littorea View in CoL ; this is incorrect, because they placed L. carinata in Nodilittorina View in CoL , thereby removing the homonymy (ICZN 1999: Art. 59.2). Junior secondary homonyms rejected after 1960 are to be reinstated if the homonymy is removed (ICZN 1999: Art. 59.4), so the valid name of the present species should be E. carinata . However, for stability it is desirable to maintain existing usage of the more familiar junior synonym L. angustior View in CoL . Mandatory reversal of precedence requires not only that the senior synonym has not been used since 1899 (as is the case with L. carinata ) but also that the junior synonym has been used as the valid name in 25 works by at least ten authors published in the preceding 50 years (ICZN 1999: Art. 23.9). This criterion is only partially met; the synonymy above lists 15 works by 12 authors since 1974. Nevertheless, current usage will be maintained here.
As with all black-and-white-striped western Atlantic Echinolittorina species , the name L. ziczac was employed when a broad concept of that species was current ( Tryon 1887; Dall & Simpson 1901; Lewis 1960; Warmke & Abbott 1961). Following the recognition of three species within the ‘ ziczac group’ in Florida, the name ‘ L. lineata d’Orbigny, 1841 ’ was adopted (T.V. Borkowski & M.R. Borkowski 1969; Rosewater 1970; Flores 1973a). However, the attribution to d’Orbigny is incorrect. Although d’Orbigny (1841) himself gave the name as ‘ Littorina lineata, d’Orb. ’, in the same work he cited in the same way no less than six littorinid names that had been described by earlier authors, so he was probably not intending to introduce a new name. The name was first introduced as Phasianella lineata by Lamarck (1822) and is not correctly applied to the present species (see Taxonomic History of E. interrupta ). As discussed above, the name angustior has been widely used following the monograph by Bandel & Kadolsky (1982).
Diagnosis: Shell of medium size; 6–7 primary spiral grooves; secondary grooves usually absent; dark oblique axial lines or zigzags, usually no spiral band above suture on spire. Penis with long tapering filament, not glandular at base. Caribbean Sea, Bahamas, E Florida, Bermuda. COI: GenBank AJ622975 View Materials , AJ622976 View Materials .
Material examined: 173 lots (including 33 penes, 14 sperm samples, 5 pallial oviducts, 2 spawn samples, 5 radulae).
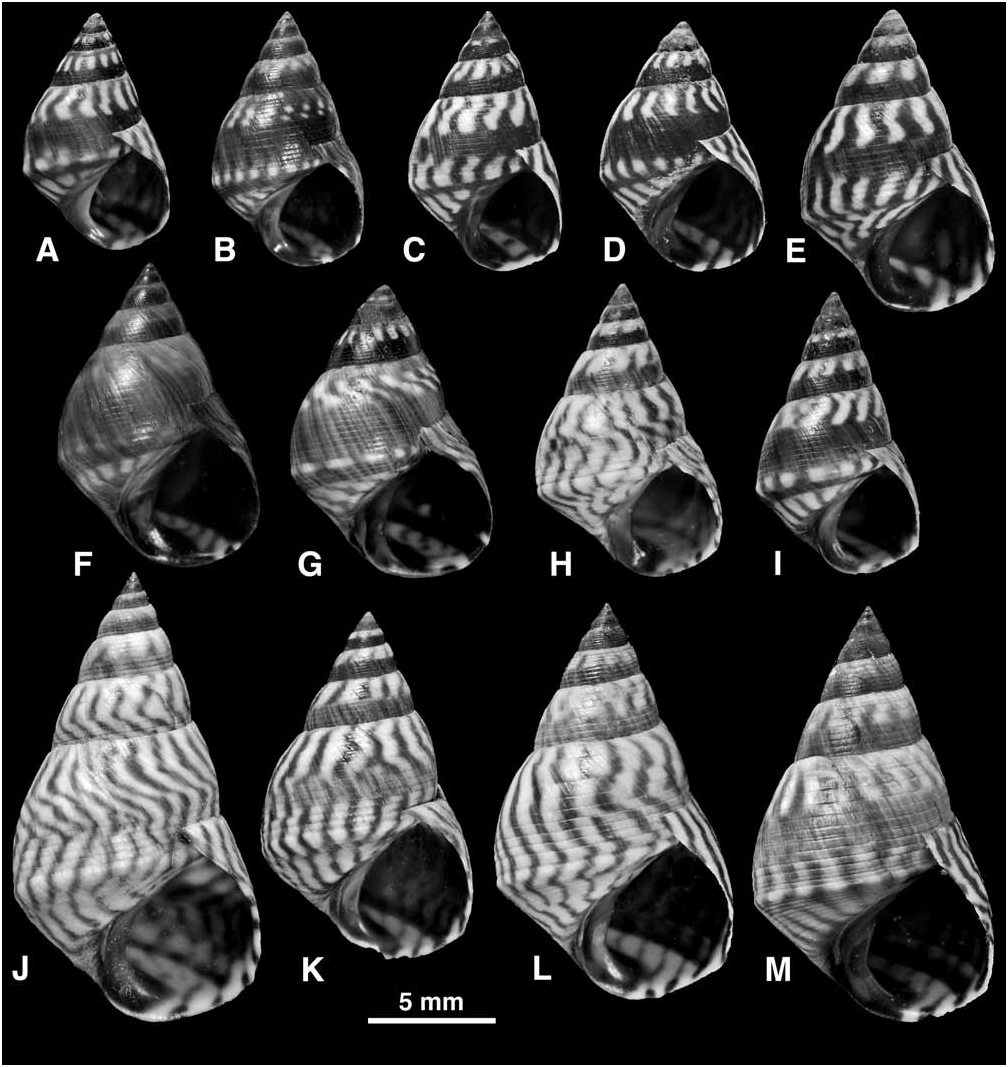
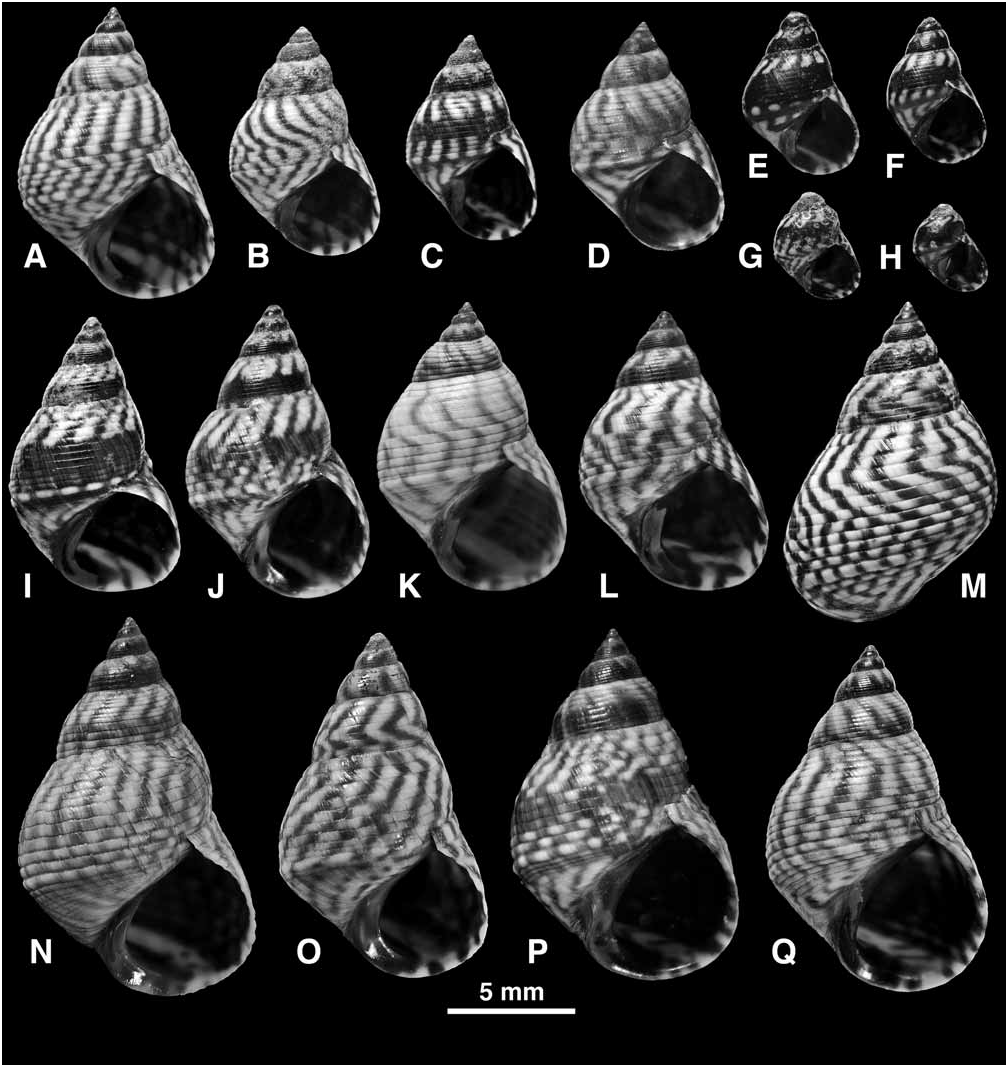
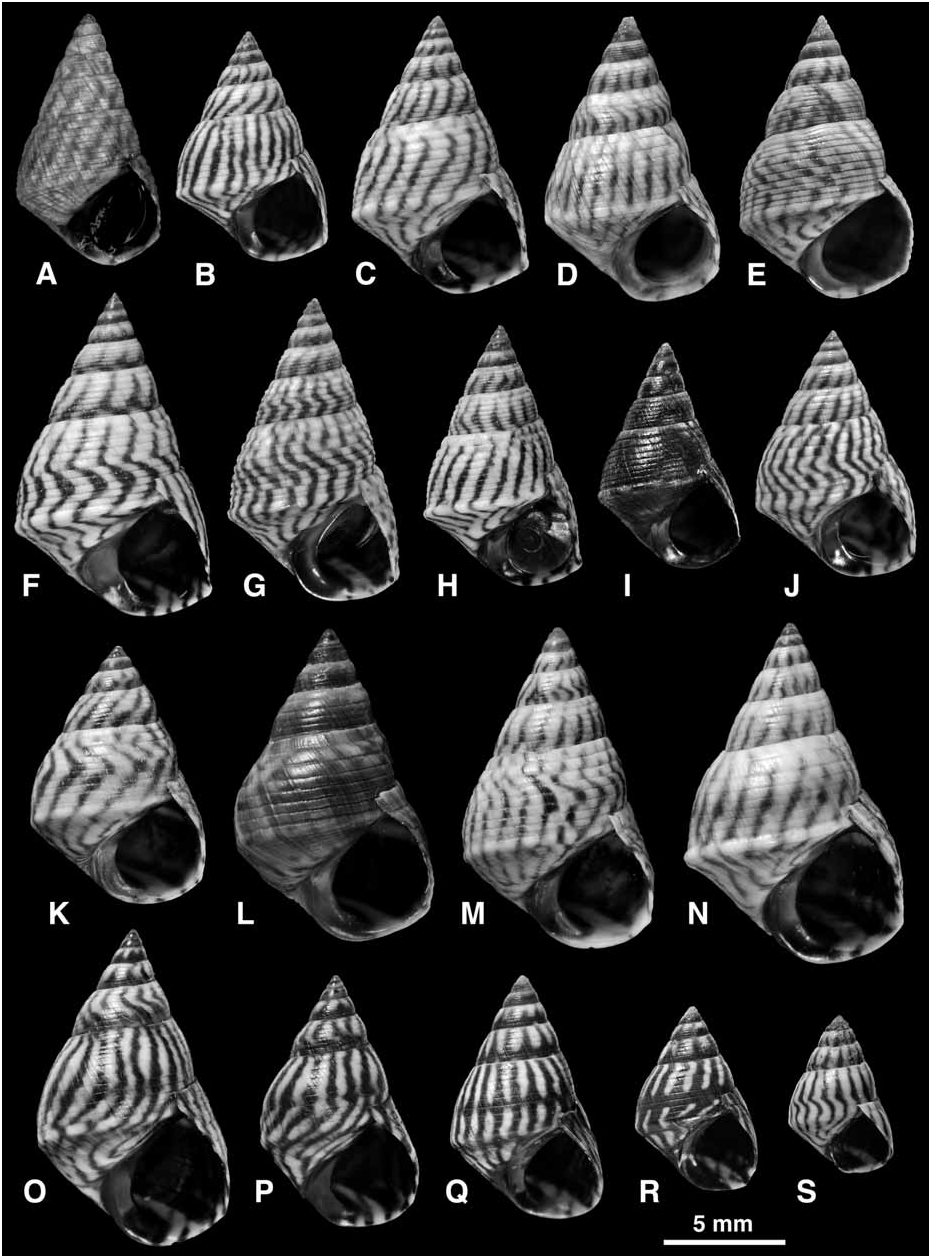
Shell ( Fig. 27 View FIGURE 27 ): Mature shell height 5.0–19.0 mm. Shape high turbinate to tall (H/B = 1.45–1.86, SH = 1.73–2.27); spire whorls flattened or sometimes gently rounded; suture distinct; spire profile straight; periphery of last whorl sharply angled; base may be slightly concave. Columella short, slightly hollowed and pinched at base; sometimes a slight pseudumbilical groove; no eroded parietal area. Sculpture of 6–7 primary spiral grooves on spire whorls; secondary grooves often absent (then 9–10 incised lines present above and 6–9 below periphery on last whorl); some secondary grooves may appear (by division of ribs) near suture, periphery and on base of last whorl (then up to 13, rarely 17, incised lines above and up to 14 below periphery; Fig. 27E–G View FIGURE 27 ); sculpture occasionally becomes obsolete on last whorl ( Fig. 27S View FIGURE 27 ); peripheral rib enlarged, twice as wide as those above it, sometimes carinate; spiral microstriae absent. Protoconch 0.26–0.27 mm diameter, 2.3 whorls. Ground colour white, a broad band above and another below periphery may be pale blue-grey; with narrow oblique brown to black stripes or zigzag lines; rarely a black band on lower half of spire whorls ( Fig. 27P, Q View FIGURE 27 ); darkest shells entirely blackish brown (present at a low frequency in many populations; Fig. 27I, L View FIGURE 27 ) or rarely reddish brown; aperture dark brown with pale band at base and at shoulder; columella purple brown.
Animal: Head black, no unpigmented stripe across snout; tentacle pale around eye, with two narrow longitudinal black lines (sometimes weak or absent) and black dot at tip; sides of foot black. Operculum ( Fig. 2A–C View FIGURE 2 ): opercular ratio 0.52–0.57 (exceptionally 0.45–0.74). Penis ( Fig. 28A–G View FIGURE 28 ): long, gradually tapering filament with no glandular thickening at base, 0.6–0.7 total length of penis, sperm groove ends terminally; mamilliform gland approximately same size as projecting lobe of penial glandular disc, borne together on projection of base; penis unpigmented. Euspermatozoa 61–82 µm; paraspermatozoa ( Fig. 28K–M View FIGURE 28 ) containing single parallel-sided rod-piece with rounded ends, sometimes with a collar-like structure at one end, 21–24 µm, rod-piece usually projecting slightly from cell, which is packed with large round granules. Pallial oviduct ( Fig. 28H View FIGURE 28 ): copulatory bursa separates at posterior end of straight section and extends back to albumen gland. Spawn ( Fig. 28I, J View FIGURE 28 ): an asymmetrically biconvex pelagic capsule 224–249 µm diameter by 143 µm high, with peripheral rim slightly overhanging base, dome-shaped upper side sculptured by 4–8 concentric rings, containing single ovum 80–90 µm diameter ( Lewis 1960: Barbados; T.V. Borkowski & M.R. Borkowski 1969: Florida; Borkowski 1971: Florida; pers. obs.: Florida).
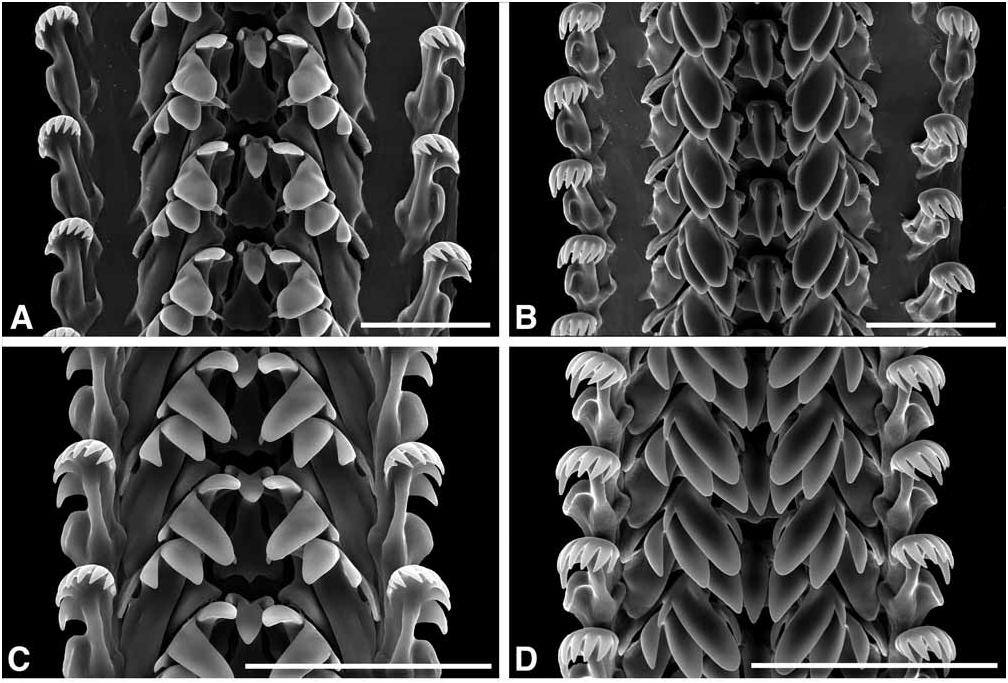
Radula ( Fig. 29 View FIGURE 29 ): Relative radula length 3.38–9.0. Rachidian: length/width 1.57–1.82; tip of major cusp pointed; may sometimes be narrowed ( Bandel 1984: fig. 13 illustrated a radula with length/width = 6.0). Lateral and inner marginal: 4 cusps, tip of major cusp rounded. Outer marginal: 7(8) cusps.
Range ( Fig. 30 View FIGURE 30 ): Throughout the Caribbean Sea, Bahamas, E Florida, Bermuda. Range limits: Eau Gallie, Florida ( USNM 638073 About USNM ) ; Bahia Honda Key , Florida ( BMNH; IRSNB) ; Key West, Florida ( USNM 862278 About USNM ) ; South Shore , Devonshire, Bermuda ( BMNH 20081036 ) ; San Salvador, Bahamas ( ZMA; USNM 749807 About USNM ) ; Silver Sands, Christ Church, Barbados ( BMNH 20081037 ) ; Cumana, Venezuela ( USNM 709111 About USNM ) ; Permé, NW Cape Tiburon, Panama ( USNM 862279 About USNM ) ; San Blas Archipelago, Panama ( USNM 734655 About USNM ) ; Colon, Panama ( USNM 759031 About USNM ; IRSNB; MNHN) ; Cahuita , 40 km S Puerto Limón, Costa Rica ( BMNH) ; Portete, Limón, Costa Rica ( USNM 706423 About USNM ) ; Isla Roatan, Honduras ( USNM 862257 About USNM ) ; South Water Cay , Belize ( BMNH 20081024 ) ; Bahía de la Ascención, Quintana Roo, Mexico ( USNM 736255 About USNM ) ; Isla Mujeres, Quintana Roo, Mexico ( USNM 662266 About USNM ) .
This species is common throughout its range in eastern Florida, the Bahamas, Bermuda, the islands of the Greater and Lesser Antilles, and Netherlands Antilles. However, on the mainland coast of Central America its occurrence is patchy, reflecting its preference for oceanic habitats. In this area it is present only on promontories, offshore islands and in regions of low primary productivity with clear, oceanic water such as the northeastern coast of the Yucatan Peninsula. In other parts of Central America it is replaced by its continental congener, E. interrupta . Thus, in Venezuela, Colombia, Panama and Costa Rica E. angustior is generally less common than E. interrupta , and has not been found at all on the mainland (and largely sedimentary) coasts of Nicaragua, Honduras and Belize. T.V. Borkowski & M.R. Borkowski (1969) noted that it was rare north of Sebastian Inlet in Florida. Echinolittorina angustior has not been definitely recorded from the Gulf of Mexico, but a few specimens may occasionally arrive in the southern Gulf ( Britton & Morton 1989).
Habitat: On limestone, beachrock and concrete, in littoral fringe, on sheltered and moderately exposed coasts. Mainly in oceanic settings with clear water.
This species occupies the middle and upper parts of the littorinid belt on Caribbean shores. At Santa Marta, Colombia it can be found in the algal zone of the upper eulittoral, but is typical of the littoral fringe, and is more common in more sheltered parts ( Brattström 1980). In Panama it extends to the same level as Cenchritis , the most nearly terrestrial of the local littorinids (Brattström 1986), and is typical of the black zone in the littoral fringe on limestone shores in the Bahamas ( Brattström 1999). In Jamaica it occurs with E. tuberculata at the highest levels on the shore, on both open surfaces and in pits and crevices, and on high and low-energy shores ( Vermeij 1973a; Lang et al. 1998; Minton & Gochfeld 2001). It occupies the lower littoral fringe in Venezuela and tolerates a narrow salinity range, 34–37 ppt, so it is the most stenohaline of the Echinolittorina on these shores ( Flores 1973b). It also inhabits shallow pools in the littoral fringe ( Vermeij 1973b). Animals survive emersion for at least 14 days ( Britton 1992) and show thermoregulation both above and below ambient temperature ( Lang et al. 1998). Erosive activity on a limestone platform in Jamaica has been measured by Dobson-Moore & Britton (2001).
In Florida males are mature for most of the year except March, while females spawn between April and December ( Borkowski 1971). In the field spawning occurs whenever the tide rises above MHWS, and not on a lunar rhythm; in the laboratory it was found to be induced by splash ( Borkowski 1971). Size-frequency data suggest settlement from January to March in Jamaica ( Lang et al. 1998), and growth rates have been measured in Florida ( Borkowski 1974).
Remarks: Shells that are tall, straight-sided, strongly keeled at the periphery, and show narrow oblique black lines and no spiral bands, are distinctive of E. angustior , and cannot be mistaken for any other. Nevertheless, in some populations the whorls are more rounded, the keel less developed, sculpture weaker ( Fig. 27O–S View FIGURE 27 ) and rarely a spiral band may appear ( Fig. 27Q, R View FIGURE 27 ), and these shells approach E. interrupta ( Fig. 22 View FIGURE 22 ) or E. placida ( Fig. 25 View FIGURE 25 ). Such shells occur in the Lesser Antilles and, although they show the anatomical characters of E. angustior , it is desirable to test their identification with molecular data.
The differences between the three similar species E. angustior , E. interrupta and E. placida are summarized in Table 1. The coiling of the operculum is a useful character, being more multispiral in E. angustior than in E. interrupta and E. placida . There is, however, some variation in the coiling and thickness of the operculum, apparently correlated with the shape of the aperture; in a sample from St Ann’s Bay, Jamaica, the opercular ratio ranged from 0.54 to 0.74 ( Fig. 2A–C View FIGURE 2 ).
In many populations throughout the range (23 out of 173 samples examined) a small proportion (estimated at 1–5%) of the shells are entirely dark brown to black ( Fig. 27I, L View FIGURE 27 ). This ‘melanic form’ was also noted in Florida by T.V. Borkowski & M.R. Borkowski (1969), and Janson (1985) found that black and normal forms showed identical allozyme patterns. Similar forms occur in other western Atlantic Echinolittorina ( E. ziczac , E. jamaicensis ), but are most common in the present species.
Variation in egg capsule form has been described in populations from Florida ( Borkowski 1971, 1975) and confirmed here ( Fig. 28I, J View FIGURE 28 ). The sculptured rings of the cupola may be concentric or a continuous spiral, and the number of rings ranges from 4 to 7 (pers. obs.; 5–8, Borkowski 1971). At hatching, the veliger larva emerges through a tubular opening or rupture in the slightly convex base (pers. obs.).
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |
Echinolittorina angustior ( Mörch, 1876 )
| Reid, David G. 2009 |
Echinolittorina angustior
| Williams, S. T. & Reid, D. G. 2004: 2227 |
| Williams, S. T. & Reid, D. G. & Littlewood, D. T. J. 2003: 60 |
Nodilittorina angustior
| Reid, D. G. 2002: 259 |
| Espinosa, J. & Ortea, J. 2001: 15 |
| Redfern, C. 2001: 28 |
| Britton, J. C. & Morton, B. 1989: 86 |
Nodilittorina (Echinolittorina) angustior
| Reid, D. G. 1989: 99 |
Littorina angustior
| Diaz, J. M. & Puyana, M. 1994: 124 |
| De Jong, K. M. & Coomans, H. E. 1988: 19 |
| Sterrer, W. 1986: 408 |
| Janson, K. 1985: 871 |
Nodilittorina (Nodilittorina) angustior
| Bandel, K. & Kadolsky, D. 1982: 25 |
Littorina (Austrolittorina) angustior
| Vokes, H. E. & Vokes, E. H. 1983: 14 |
| Abbott, R. T. 1974: 68 |
Littorina jamaicensis
| Bandel, K. 1974: 99 |
Littorina (Austrolittorina) lineata
| Rosewater, J. 1970: 423 |
Littorina lineata
| Borkowski, T. V. 1975: 369 |
| Flores, C. 1973: 14 |
| Borkowski, T. V. & Borkowski, M. R. 1969: 409 |
Littorina lineolata
| Abbott, R. T. 1968: 82 |
| Abbott, R. T. 1964: 65 |
Littorina (Melarhaphe) ziczac lineata
| Johnson, C. W. 1934: 102 |
Litorina (Melarhaphe) ziczac
| Dall, W. H. & Simpson, C. T. 1901: 429 |
Littorina (Melarhaphe) lineata
| Martens, E. von & Godman, F. D. & Salvin, O. 1900: 583 |
Littorina (Melarhaphe) ziczac
| Warmke, G. L. & Abbott, R. T. 1961: 52 |
| Tryon, G. W. 1887: 251 |
Litorina ziczac
| Weinkauff, H. C. 1883: 220 |
Litorina angustior
| Weinkauff, H. C. 1883: 220 |
Littorina (Melarhaphe) carinata
| Morch, O. A. L. 1876: 139 |
Littorina (Melarhaphe) angustior Mörch, 1876: 139
| Nevill, G. 1885: 140 |
| Morch, O. A. L. 1876: 139 |