Stumpffia analamaina, Klages, Johannes, Glaw, Frank, Köhler, Jörn, Müller, Johannes, Hipsley, Christy A. & Vences, Miguel, 2013
|
publication ID |
https://doi.org/ 10.11646/zootaxa.3717.2.8 |
|
publication LSID |
lsid:zoobank.org:pub:91AE5C97-8380-43B1-A97E-323CC64B9778 |
|
DOI |
https://doi.org/10.5281/zenodo.5695699 |
|
persistent identifier |
https://treatment.plazi.org/id/CED11ABE-2814-4B2F-8506-5F22E5BF6710 |
|
taxon LSID |
lsid:zoobank.org:act:CED11ABE-2814-4B2F-8506-5F22E5BF6710 |
|
treatment provided by |
Plazi |
|
scientific name |
Stumpffia analamaina |
| status |
sp. nov. |
Stumpffia analamaina View in CoL sp. nov.
( Figs. 3–5 View FIGURE 3 View FIGURE 4 View FIGURE 5 )
Holotype. Adult male ( Fig. 3 View FIGURE 3 ), ZSM 542/2009 (field number ZCMV 11428), collected at a bridge located at km 27 on the national road from Antsohihy to Mandritsara, 15°03'11.6''S, 48°12'23.1''E, 140 m above sea level, on 18 June 2009 by M. Vences, D. R. Vieites, F. Ratsoavina, R. D. Randrianiaina, E. Rajeriarison, T. Rajoafiarison, and J. Patton.
Paratypes. Three specimens, ZSM 2829/2010 (ZCMV 10034), ZSM 2830/2010 (ZCMV 10036) and ZSM 2831/2010 (ZCMV 10037), collected at the same locality as the holotype, on 29 June 2010 by M. Vences, E. Rajeriarison, T. Rajoafiarison, A. Rakotoarison, S. Rasamison, F. Ratsoavina, and D. R. Vieites.
Diagnosis. The new species is assigned to the genus Stumpffia on the basis of its very small size, absence of vomerine and maxillary teeth, ossified part of clavicles reduced to thin rod-like elements, sternum and omosternum not ossified, similarities in external body shape to other species in the genus, and molecular phylogenetic relationships. Within Stumpffia , it is uniquely characterized by the following combination of characters: (1) small body size, SVL of males and females 10–12 mm; (2) dorsum smooth or slightly tubercular; (3) absence of distinct terminal discs on fingers and toes; (4) advertisement calls consisting of a series of short melodious chirps; (5) greyish or brownish coloration with variable but typically indistinct pattern and without contrasted ventral coloration, red color elements on ventral side, or sharp color border between dorsum and flanks; (6) manus with four fingers (first finger slightly reduced in size) and pes with five toes (first toe slightly reduced in size); (7) third toe slightly to distinctly longer than fifth toe; (8) relative hand length, HAL/SVL 0.22–0.26, relative foot + tarsus length FOTL/SVL 0.68–0.78.
In comparison to the other nominal species of Stumpffia , the new species differs from S. helenae by absence of terminal discs on toes and fingers (vs. presence) and slightly smaller body size (SVL of males and females 10–12 vs. 14–15 mm); from S. be by distinctly smaller body size (SVL 10–12 mm vs. 25 mm), absence of terminal discs on fingers and toes (vs. presence); from S. hara by distinctly smaller body size (SVL 10–12 mm vs. 22–25 mm), absence of terminal discs on fingers and toes (vs. presence); from S. megsoni by distinctly smaller body size (SVL 10–12 mm vs. 21–22 mm), absence of terminal discs on fingers and toes (vs. presence); from S. staffordi by distinctly smaller body size (SVL 10–12 mm vs. 27–28 mm), absence of terminal discs on fingers and toes (vs. presence); from S. grandis by smaller body size (SVL 10–12 vs. 19–24 mm), and a different coloration (indistinct brown-greyish pattern vs. strongly contrasted pattern of brown patches and bluish venter with black markings); from S. roseifemoralis by smaller body size (10–12 mm vs. 18–19 mm) and different coloration (lack of reddish color ventrally on limbs vs. presence); from S. psologlosssa by slightly smaller body size (SVL 10–12 mm vs. 14– 16 mm), a more strongly reduced first toe (vs. slightly reduced), and a different structure of advertisement call (a series of short chirps vs. short trills); from S. tridactyla by presence of four externally recognizable fingers and five toes (vs. a single triangular finger and three toes); from S. tetradactyla by presence of five recognizable toes albeit first toe very small (vs. first toe almost completely reduced, only visible as a small tubercle); from S. gimmeli by smaller body size (SVL 10–12 mm vs. 15–18 mm), dorsal skin smooth or only slightly tubercular in life (vs. typically distinctly tubercular), first finger and first toe slightly reduced (vs. well developed), absence of terminal discs on fingers and toes (vs. typical presence of at least weakly developed discs, especially in larger specimens).
The new species is morphologically most similar to S. pygmaea and S. madagascariensis . It differs from S. madagascariensis by the lack of a sharp border between dark flanks and light dorsum (vs. presence; figure in Köhler et al. 2010). Furthermore, based on the limited morphometric data available to date (see Vences & Glaw 1991 and Table 1 View TABLE 1 ), it appears that the new species has longer hands and feet than S. pygmaea and S. madagascariensis : values for relative hand length and relative foot + tarsus length of S. analamaina (HAL/SVL, 0.22–0.26; FOTL/SVL, 0.68–0.81) are in the range known for S. psologlossa (HAL/SVL, 0.18–0.23; FOTL/SVL, 0.63–0.71) and S. gimmeli (HAL/SVL, 0.21–0.23; FOTL/SVL, 0.68–0.75) but larger than in S. pygmaea (HAL/ SVL, 0.16–0.18; FOTL/SVL 0.53–0.63) and S. madagascariensis (HAL/SVL, 0.16; FOTL/SVL 0.61).
The new species differs from all nominal species of Stumpffia by strong mitochondrial differentiation (>10% uncorrected pairwise distance in the 16S rRNA gene) and apparently exclusive haplotypes in the nuclear RAG1 gene (4 specimens sequenced for the new species).
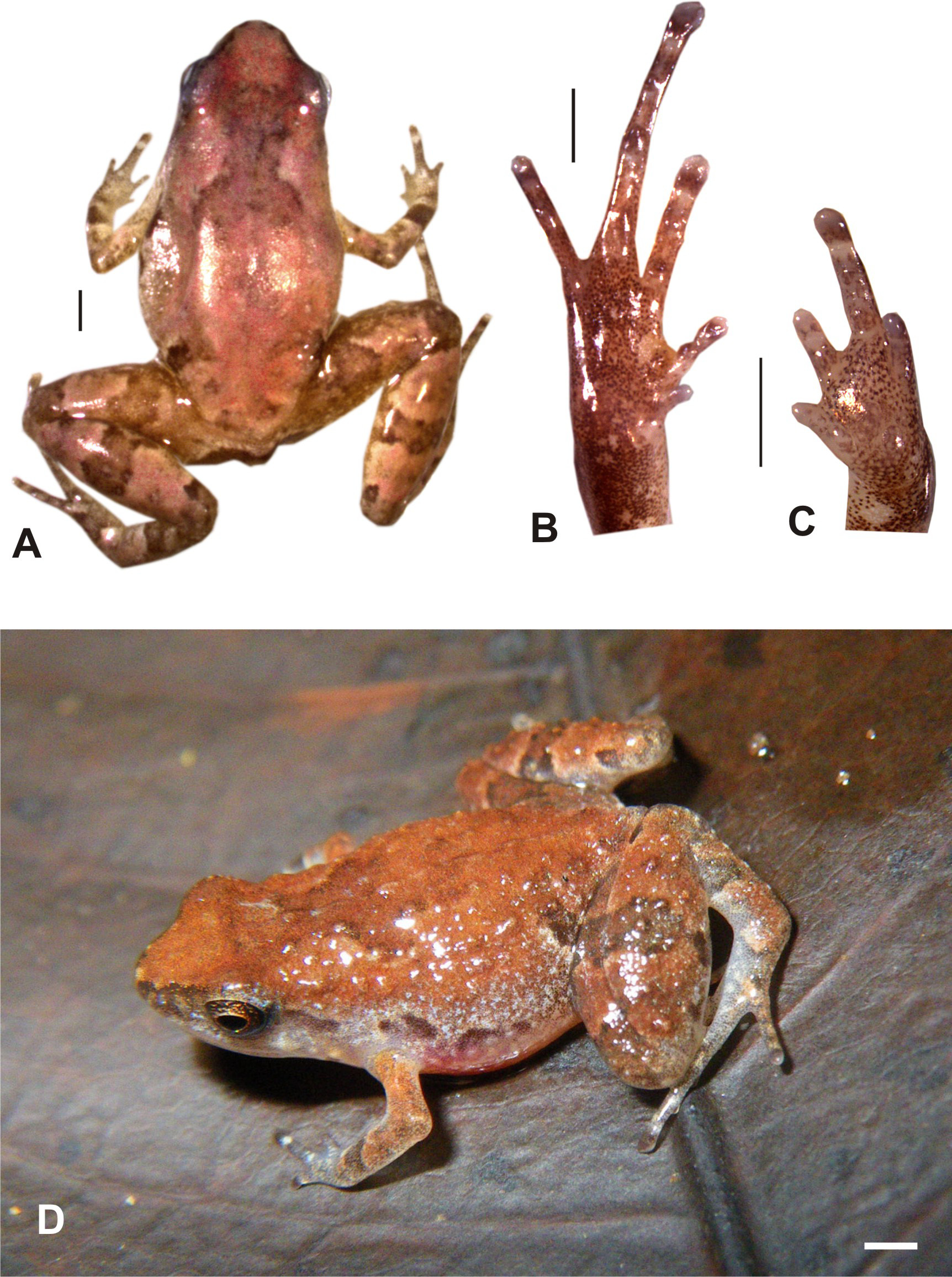
Description of the holotype. Adult male specimen in good state of preservation ( Fig. 3 View FIGURE 3 ); tissue removed from ventral part of right thigh and venter cut open for gonad inspection. SVL 11.1 mm; body slender; head slightly longer (3.7 mm) than wide (3.3 mm), head less wide than the body; snout rounded to slightly pointed in dorsal view, protruding and slightly pointed in profile; nostrils directed laterally, slightly protuberant, about equidistant to the tip of the snout (0.8 mm) and to the eye (0.8 mm); eye relatively large (1.4 mm diameter), pupil round; internarial distance (1.3 mm) distinctly smaller than interorbital distance (2.2 mm); canthus rostralis weakly marked and rounded; loreal region straight to slightly convex; tympanum not recognizable; supratympanic fold indistinct; tongue long and narrow, tip broadly rounded; maxillary teeth absent; vomerine teeth absent; choanae small and round. Arms slender; arm length from axilla to tip of longest finger 5.6 mm; hand length to tip of finger III: 2.6 mm; finger II with one and finger III with two small, flat, round, subarticular tubercles; three weakly marked, flat palmar tubercles, of which the inner tubercle is the largest and round; fingers without webbing; relative length of fingers I<IV<II<III, second toe slightly but distinctly longer than fourth toe; finger I reduced in length, all fingers relatively short; finger discs not enlarged, broadly rounded; prepollex absent; nuptial pads absent; tibiotarsal articulation reaches the nostril when hindlimb is adpressed along the body; hindlimbs slender; tibia length 5.8 mm, foot length 5.9 mm; lateral metatarsalia strongly connected; inner metatarsal tubercle indistinct, flat, ovoid; outer metatarsal tubercle absent; toe tips slightly broader than rest of toe without forming discs except on toe IV where a small disc is recognizable; toe tips with circummarginal grooves; distinct small, round subarticular tubercles; relative length of toes I<II<III=V<IV, fifth toe same length as third toe, first toe strongly reduced in length but still clearly visible; no pedal webbing. Skin on dorsum and venter smooth in preservative.
In life, the ground color of dorsum and dorsal sides of head and limbs was reddish brown, with an indistinct darker X-shaped marking of brownish color, of which the anterior branches reached above the eyes and the lower two branches faded at the level of the forelimb insertions. A dark patch was present in the inguinal region, and well-delimited although poorly contrasted brown stripes and patches were also present on the limbs: one crossband on each lower arm, a brown area around the knee, one broad crossband centrally on each thigh, shank, and tarsus. The flanks were densely covered with small silvery-whitish spots, without a sharp border between dorsal and lateral color. A number of brown patches were aligned along the flanks, and brown color was also present along the supratympanic fold and between eye and snout tip, including the nostril area. The iris was copper brown. After two years in preservative, the color remains largely similar, with a slightly stronger contrast between the brown dorsal pattern and the red-brown ground color.
Variation. Numerous specimens of S. analamaina from the type locality were observed alive but not preserved and therefore are not included in the paratype series. In general, all examined paratypes agreed with the holotype in morphology ( Fig. 4 View FIGURE 4 ; see Table 1 View TABLE 1 for selected measurements of paratypes). While the red-brown dorsal color characterized several specimens ( Fig. 4 View FIGURE 4 A), others had a more beige ground color ( Fig. 4 View FIGURE 4 F). The dark dorsal Xshaped pattern in several specimens was extended to form an indistinct "gummi-bear" marking as is typical for other Stumpffia species, especially S. psologlossa (photos in Glaw & Vences 2007).
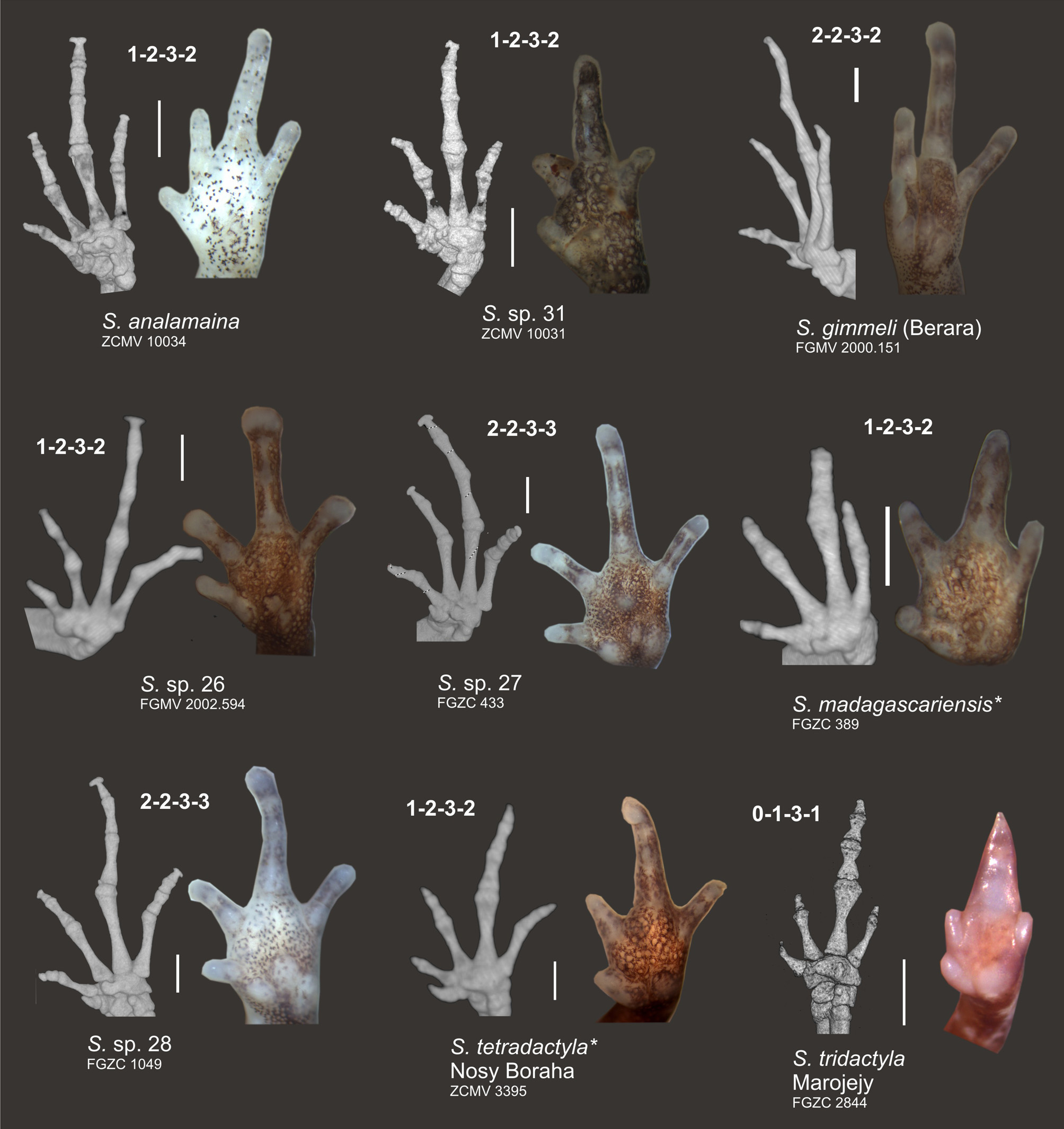
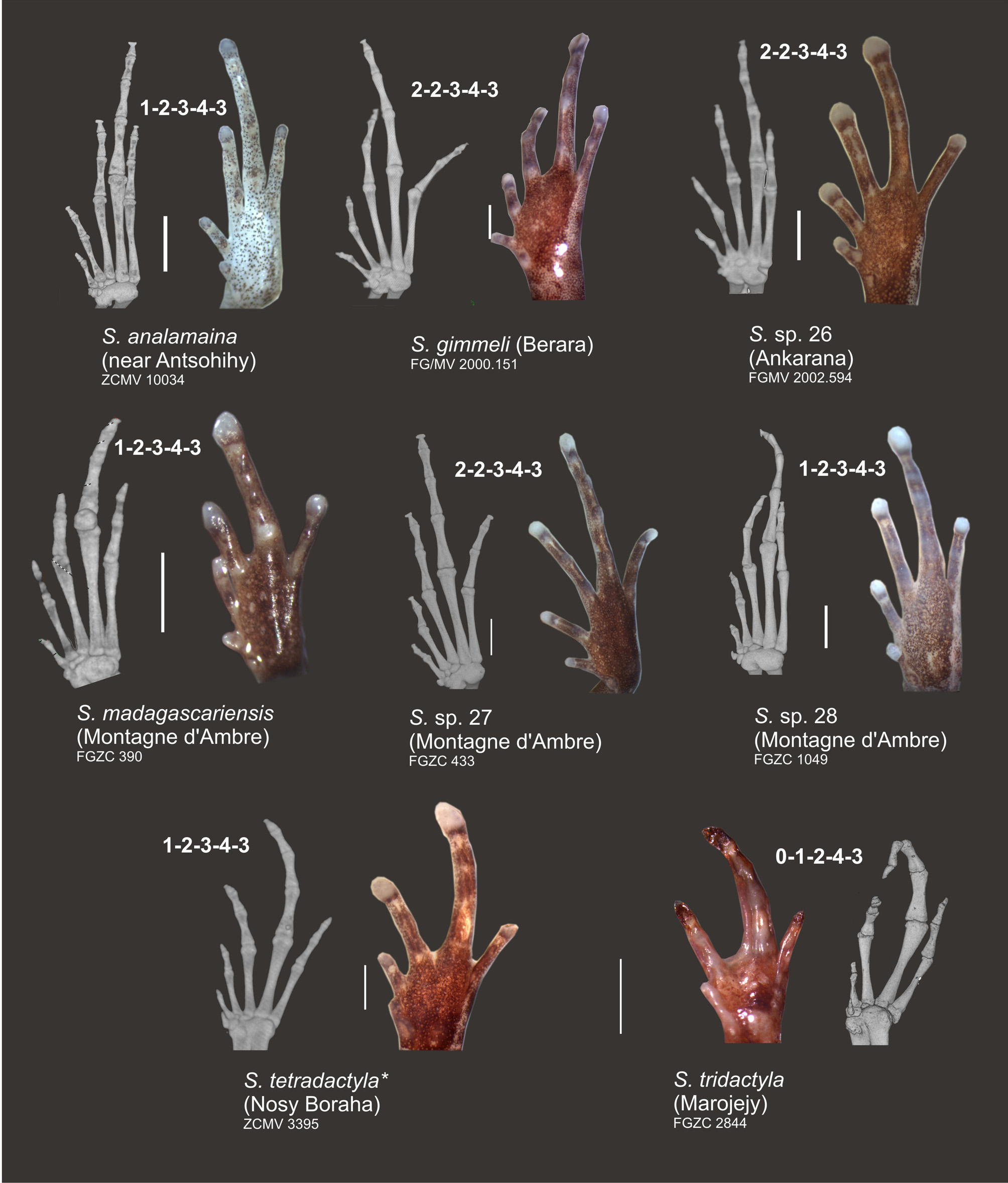
Osteology. Osteological features are described on the basis of CT scans of specimen ZCMV 10034 ( Fig. 5–7 View FIGURE 5 View FIGURE 6 View FIGURE 7 ). Vertebral column largely in agreement with description of Guibé (1978) for three other species of Stumpffia ; seven presacral vertebrae (first and second incompletely fused), wider than long, with relatively long diapophyses on the three anterior vertebrae and shorter on the four posterior ones. Sacrum with relatively broad diapophyses. No traces of teeth on premaxilla or maxilla. Nasals not in contact. Squamosal with a small zygomatic (anterior) process (much shorter than otic process). Coracoid well ossified, but clavicula only ossified as a thin rod; sternum and omosternum not visible in the CT scan, hence not calcified. Terminal phalanges of fingers and toes with a weakly expressed T-shaped expansion. Three free distal tarsals recognizable. Phalangeal formula 1–2–3– 2 in the manus and 1–2–3–4– 3 in the pes.
The degree of reduction of various digits in the manus and pes has served as an important diagnostic character to describe various new species such as S. tetradactyla which has its first toe almost completely reduced, or S. pygmaea , a highly miniaturized species with only slight digital reduction (Vences & Glaw 1991). While in S. tridactyla the bony anatomy of manus and pes was also found to be affected by reductions of certain phalangeal elements (Guibé 1978), this has so far remained unstudied in most other species. We here provide a first survey of hand and foot skeletons of S. analamaina and a set of superficially similar and related species, mainly belonging to the north/northwestern clade of Stumpffia ( Figs. 6–7 View FIGURE 6 View FIGURE 7 ). The phalangeal formula for the manus of the studied specimens of S. analamaina (ZCMV 10034), S. sp. 31 (ZCMV 10031; terminal phalanx of finger IV possibly very small), S. madagascariensis (FGZC 389), S. sp. 26 (FGMV 2002.594; phalangeal formula poorly recognizable), and S. tetradactyla (ZCMV 3395) was determined as 1–2–3–2 ( Fig. 6 View FIGURE 6 ). A deviant phalangeal formula of 2–2–3–3 was present in the two sister species, S. sp. 27 (FGZC 433) and S. sp. 28 (FGZC 1049), and a third combination of 2–2–3– 2 in S. gimmeli (FGMV 2000.151) ( Fig. 6 View FIGURE 6 ). A reduced number of phalanges was observed in S. tridactyla (FGZC 2844) as already reported by Guibé (1978): 0–1–3–1 (or 1–1–3–1 if the tiny terminal knob of the first finger is counted as rudiment of a phalanx) ( Fig. 6 View FIGURE 6 ).
In the pes ( Fig. 7 View FIGURE 7 ), we observed a phalangeal formula of 1–2–3–4– 3 in S. analamaina (ZCMV 10034), S. sp. 28 (FGZC 1049), S. tetradactyla (ZCMV 3395), and probably in S. madagascariensis (FGZC 389). The generalized, plesiomorphic anuran phalangeal formula of 2–2–3–4–3 (Alberch & Gale 1985) without losses was present in S. sp. 26 (FGMV 2002.594), S. sp. 27 (FGZC 433), and S. gimmeli (FGMV 2000.151) ( Fig. 7 View FIGURE 7 ). Again, a stronger phalangeal reduction is apparent in S. tridactyla : 0–1–2–4–3 ( Fig. 7 View FIGURE 7 ).
Etymology. The species epithet is composed by the Malagasy words ala (forest; an ala meaning "of the forest") and maina meaning dry, making reference to the occurrence of the new species in dry forest habitat in northwestern Madagascar. The species epithet is used as noun in apposition.
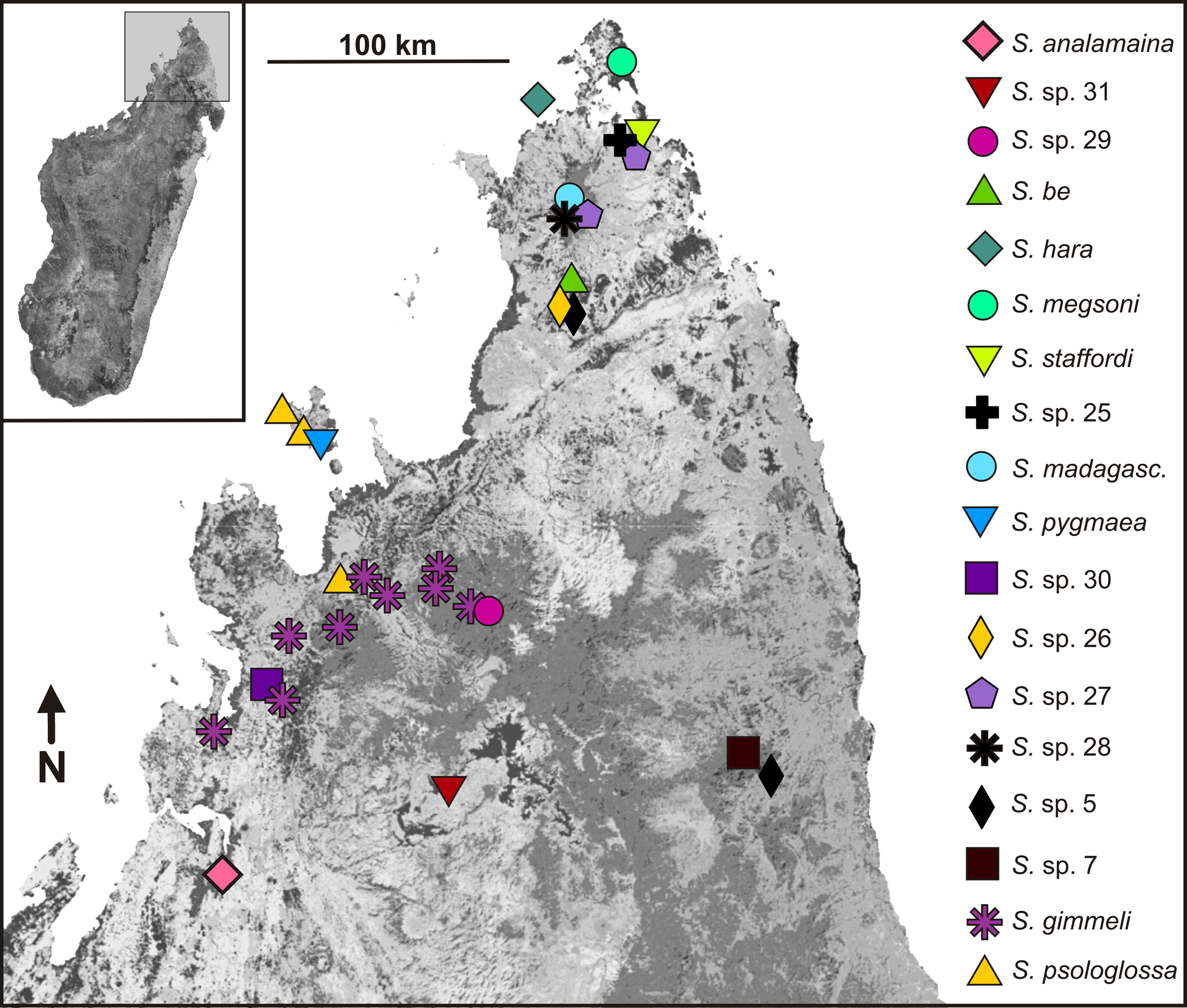
Molecular differentiation. The mtDNA tree ( Fig. 1 View FIGURE 1 ) places S. analamaina with maximum support into a clade with S. sp. 29 and S. sp. 31. Differentiation from these two candidate species in the 16S gene is 11.5% and 10.5% (uncorrected p-distance). Among the eight specimens of S. analamaina sp. nov. sequenced for 16S, two specimens differed by two substitutions (maximum sequence divergence 0.4%). In the RAG1 haplotype network ( Fig. 2 View FIGURE 2 ), S. analamaina sp. nov. and the two candidate species form three distinct clusters, with a minimum of seven and nine mutational steps between them and with a maximum differentiation of three mutational steps between haplotypes within S. analamaina .
Natural habitat and observations in captivity. Specimens of Stumpffia analamaina were collected in June 2009 and 2010 in the leaf litter directly adjacent to a small stream in a fragment of dry deciduous forest where they occurred in high densities. The forest of the area was heavily degraded and the forest fragment was approximately 10–20 m wide on each side along the stream ( Fig. 4 View FIGURE 4 C–D). The time of collection was during the dry austral winter and therefore the whole area except the immediate stream surroundings was characterized by very low humidity, probably causing the specimens to gather along the stream. Additional frogs found at this site were Mantidactylus ulcerosus , Mantella ebenaui , Boophis tampoka , and B. brachychir .
Specimens were kept over several months in captivity, 3– 6 adults in a terrarium of 30 x 30 x 30 cm, at temperatures between 20 and 25°C and air humidity of 63–93%, on a substrate of turf with leaf litter ( Terminalia catappa leaves) and with plastic plates as shallow water reservoirs. They were fed collembolans, mites, and fruit flies. Activity was mainly nocturnal, the individuals typical moved slowly and only jumped when disturbed (up to 60 cm jumping distance was recorded outside of the terrarium). Calls were emitted from concealed positions under dead leaves. In one case, during a period of high calling intensity in one terrarium, a foam nest was discovered within one of the plastic plates, with an estimated diameter of 50 mm ( Fig. 4 View FIGURE 4 E). It was guarded during several days by one individual. No eggs were laid in the foam nest, and no further reproductive activity was observed.
During prey capture we observed specimens exhibiting lateral tongue protrusion ( Fig. 4 View FIGURE 4 B), which is typical for microhylids (Meyers et al. 2004) but so far has not been reported for any representative of the Cophylinae .
Advertisement call. The following description is based on several series of notes emitted by a male in captivity, from a glass terrarium at an air temperature of 23°C. Vocalizations were arranged in series of 3–8 notes, although we suspect that similar to all other Stumpffia for which calls have been described, S. analamaina might be able to emit notes in long series of an undetermined number of notes, potentially enduring for many minutes. In the five recorded note series, and several other series heard from the terrarium, the notes were emitted at regular intervals and sounded very similar to each other; we are therefore confident that the call described here corresponds to the true advertisement call. However, due to the recording conditions, we cannot exclude that the note duration measured may be longer than actually emitted due to echo effects of the terrarium. Nevertheless, as the measured notes altogether are shorter in duration rather than longer compared to calls of most other Stumpffia , this should not influence the value of the call as a taxonomic character in the context used here.
The notes are small chirps of a rather noisy appearance ( Fig. 8 View FIGURE 8 ), less tonal than in most other Stumpffia . In ten notes from two series, all recorded from the same individual male, note duration was 168–228 ms (201 ± 22 ms) and duration of inter-note intervals was 2045–2570 ms (2315 ± 187 ms), which results in a note repetition rate of 21–27 notes per minute. The calls show frequency bands between 4–8 kHz, but we cannot exclude that this wide frequency range is an artefact due to the suboptimal recording conditions. Maximum call energy is distributed at 6.0–6.4 kHz.
In general, the call variables of Stumpffia analamaina are very similar to those of S. pygmaea . This species, as described by Vences & Glaw (1991), emits series of tonal notes of ca. 200 ms duration at a rate of 22–23 notes per minute and at a spectral frequency of 5.8–6.0 kHz. However, other species of the genus (e.g., S. tetradactyla ) also emit structurally similar calls (Vences & Glaw 1991; Vences et al. 2006), which can be differentiated from each other only by subtle differences (see discussion in Ndriantsoa et al. 2013). Therefore, and because of the suboptimal recording conditions, we prefer not to perform any more detailed comparison of the call of S. analamaina at this time. Clearly, recordings of highly motivated males under natural conditions are necessary to fully describe the temporal and spectral characteristics of the vocal repertoire of this species.
Distribution. The new species is so far only known from its type locality ( Fig. 9 View FIGURE 9 ). It is likely that it is more widespread within a small range, in remaining fragments of dry deciduous forests north and northeast of Antsohihy. Records of Stumpffia also exist from Ankarafantsika National Park, considerably south of Antsohihy (e.g., Blommers Schlösser & Blanc 1991, and Ramanamanjato & Rabibisoa 2002, as S. psologlossa and S. sp. respectively), and these populations have so far not been studied from a molecular perspective. Two mitochondrial lineages, here named S. sp. 29 and S. sp. 31, were placed together with S. analamaina in a clade with high support by the mtDNA data ( Fig. 1 View FIGURE 1 ). These two lineages occur at sites not too distant from the S. analamaina type locality; especially S. sp. 31 which occurs less than 100 km northeast of it ( Fig. 9 View FIGURE 9 ). No bioacoustic data on those populations are available, and the limited morphological data of S. sp. 31 ( Table 1 View TABLE 1 ) do not indicate morphological differentiation; however, these mtDNA lineages did not share haplotypes in RAG1 ( Fig. 2 View FIGURE 2 ), suggesting a consistent genetic differentiation across markers. We therefore do not consider these populations as conspecific with S. analamaina although as with all candidate species—their status remains pending a thorough integrative revision.
TABLE 1. Morphometric data (in mm) of selected specimens of S. gimmeli, S. madagascariensis, S. pygmaea, S. sp. 31, and S. analamaina sp. nov. SVL, snout-vent length; HAL, hand length; FOTL, foot and tarsus length; FOL, foot length; TIBL, tibia length; HT, holotype; PT, paratype.
| S. pygmaea | ZSM 557/1999 | ZFMK 52543 11.2 1.9 | 6.7 | 4.3 | 4.3 | 0.60 | 0.17 |
|---|---|---|---|---|---|---|---|
| S. analamaina sp. nov. (PT) | ZSM 2829/2010 | ZCMV 10034 9.6 2.3 | 7.5 | 5.1 | 5.4 | 0.78 | 0.24 |
| S. analamaina sp. nov. (PT) | ZSM 2830/2010 | ZCMV 10036 10.5 2.3 | 7.1 | 4.9 | 4.6 | 0.68 | 0.22 |
| S. analamaina sp. nov. (PT) | ZSM 2831/2010 | ZCMV 10037 10.8 2.8 | 8.0 | 5.3 | 5.2 | 0.74 | 0.26 |
| S. analamaina sp. nov. (HT) | ZSM 542/2009 | ZCMV 11428 11.1 2.6 | 9.0 | 5.9 | 5.8 | 0.81 | 0.23 |
| S. sp. 31 | ZSM 1825/2010 | ZCMV 12600 10.0 2.1 | 6.9 | 4.3 | 4.3 | 0.69 | 0.21 |
| S. sp. 31 | uncatalogued | DRV 6413 10.4 2.1 | 7.1 | 4.6 | 4.7 | 0.68 | 0.20 |
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.