Anilios fossor, Shea, Glenn M., 2015
|
publication ID |
https://doi.org/ 10.11646/zootaxa.4033.1.5 |
|
publication LSID |
lsid:zoobank.org:pub:D3D9BFAA-F967-4342-ABFC-BA3EAEA40E43 |
|
DOI |
https://doi.org/10.5281/zenodo.6112584 |
|
persistent identifier |
https://treatment.plazi.org/id/03CF800E-250F-3F0A-10B7-FE120E896CDB |
|
treatment provided by |
Plazi |
|
scientific name |
Anilios fossor |
| status |
sp. nov. |
Anilios fossor View in CoL sp. nov.
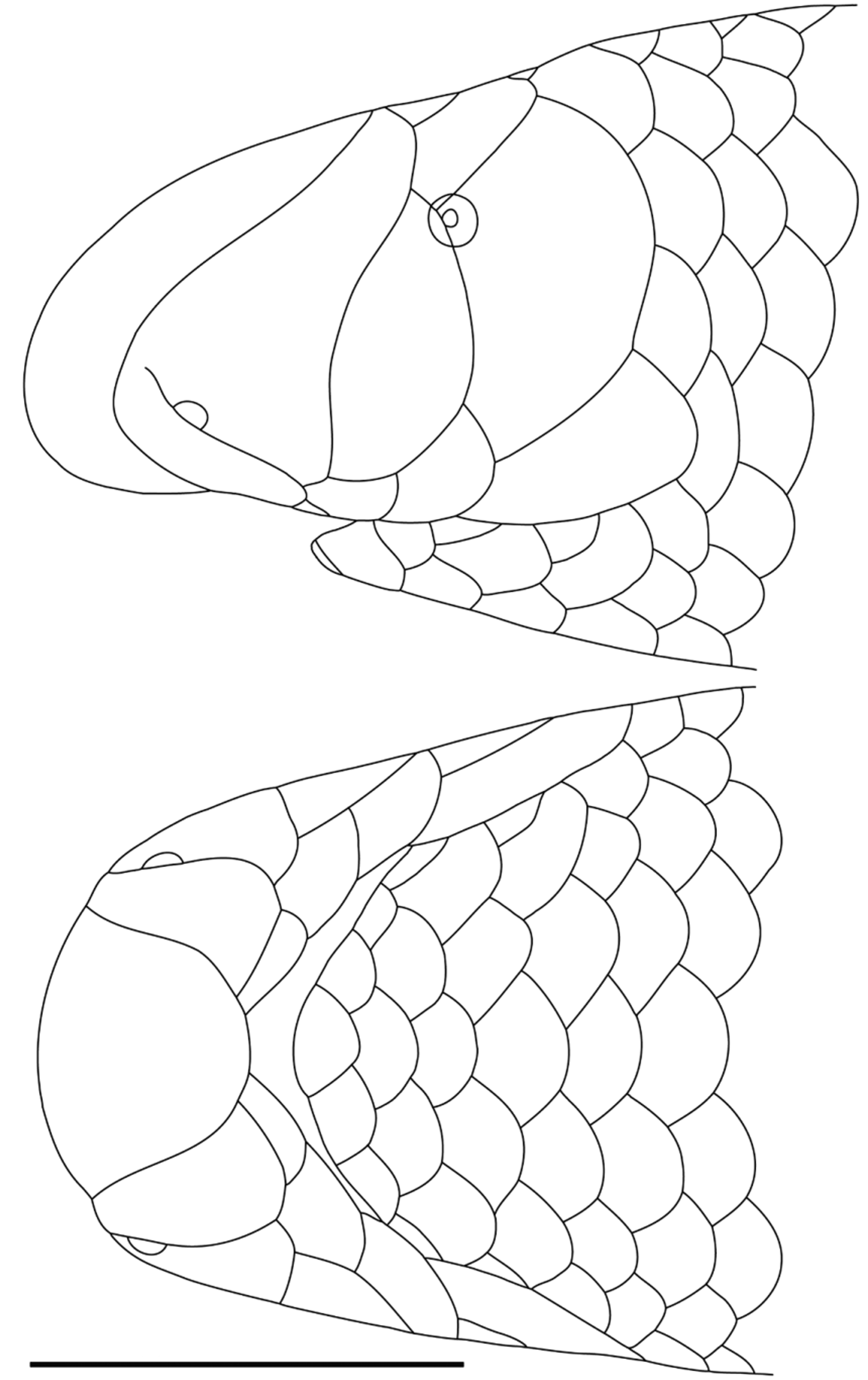
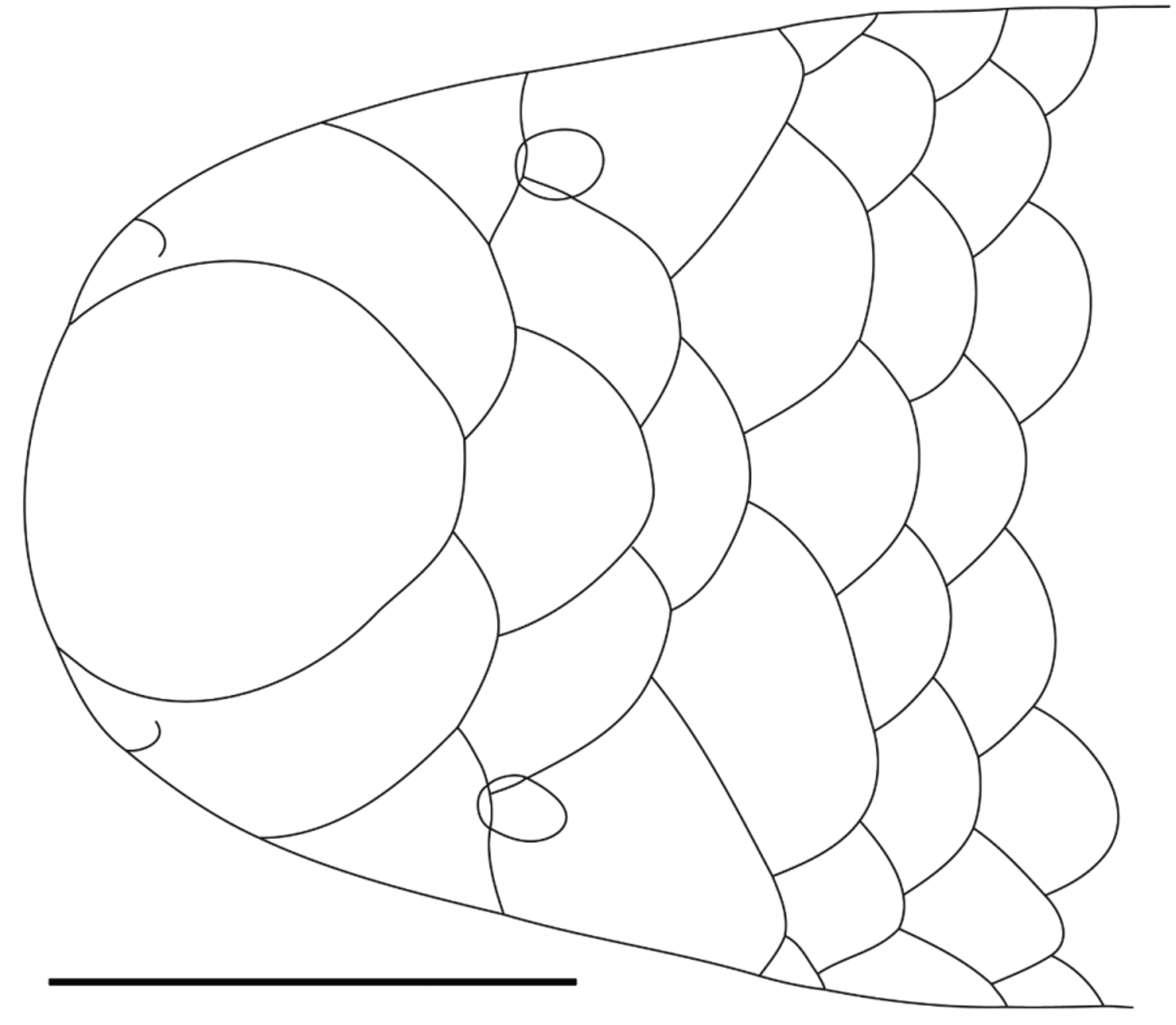
Figs. 1–3 View FIGURE 1 View FIGURE 2 View FIGURE 3
Holotype. NTM R14324 (field tag JCR30), Glen Annie, Ruby Gap Nature Park, Northern Territory, Australia, collected 15.x.1989 by J.R. Cole. The coordinates of the type locality are given in the NTM database as 23°28'S 135°00'E. However, from the map provided by the Gibson et al. (1992), the coordinates of Glen Annie are closer to 23°28'S 134°58'E, at an altitude of ca 500 m (from Google Earth).
Diagnosis. Anilios fossor is distinguished from all other described species in the genus (sensu Pyron & Wallach 2014) by the combination of 20 midbody scale rows, a rounded snout in lateral profile (lacking any rostroventral angulation), a nasal cleft contacting the second supralabial ventrally, but not extending to the dorsal surface of the head, and a large, round rostral scale. For more detailed comparisons with other species, see below.
Description of holotype (the only known specimen). Snout rounded in dorsal profile, with a very slight inflation of the nasal region providing a very weakly trilobed appearance. Rostral almost as broad as long, round with a slightly truncate posterior margin, maximum width 69% of head width at level of centre of eyes. Rostral overlaps nasals and frontal; nasals broadly separated by frontal; frontal wider than long, wider than postfrontal, overlapping supraoculars and postfrontal; supraoculars moderately separated by frontal-postfrontal suture; postfrontal overlaps parietals and interparietal; parietals moderately separated by postfrontal-interparietal suture, but more narrowly than separation between supraoculars. Dorsolaterally, supraoculars wider than long, extensively overlapping ocular, with narrower overlaps of postfrontal and parietal; parietal wider than long, of similar width to supraocular, and overlapping interparietal, upper postocular, and first scale of third (left side) or second and third (right side) longitudinal scale rows.
Snout evenly rounded in lateral profile, slightly depressed. Nasal overlaps frontal, supraocular and preocular; preocular about 2/3 width of nasal, posterior border evenly convex (no dimple to correspond to underlying eye) and overlapping supraocular and ocular; ocular wider than preocular, subequal in width to nasal, and overlapping parietal, two postoculars, and fourth supralabial. Eye moderate, pupil distinct, deep to (under) junction of supraocular, preocular and ocular scales, centre of eye just posterior to preocular.
Ventral rostral lobe moderately broad with rounded posterior margin. Nasal cleft short, from second supralabial, across anterior margin of nostril, then extending about 2/3 of the residual distance towards the rostral, not extending onto the dorsal surface of the head. First three supralabials of subequal length, first two narrow, third as tall as long. First supralabial overlapped by rostral lobe of nasal; second supralabial overlapped by rostral and caudal lobes of nasal, and preocular; third supralabial overlapped by preocular, but overlapping ocular (T-III pattern of Wallach 1993); fourth supralabial much longer than others, equal in height to third supralabial, and overlapped by ocular, but overlapping lower postocular. Postoculars two, equal in size to scales in succeeding longitudinal scale row.
Mental of similar size to adjacent infralabials and smaller than gulars; first three infralabials successively lower, fourth infralabial separated from third infralabial by first scale of third ventral longitudinal scale row. No visible marginal glands associated with head shields. Scale organs scattered over rostral and nasal scales, becoming coarser and much more sparsely distributed over supralabials, preocular, ocular and parietal. Longitudinal scale rows at midbody 20; total dorsal scale rows 514, body dorsal scale rows 503; subcaudals 11. SVL 240 mm (anterior body of holotype slightly concertinaed, so that measured SVL may be slightly underestimated); body width (BW) 4.7 mm (BW/SVL 0.0196); tail length (TL) 5 mm (TL/SVL 0.0208; TL/BW 1.06), terminal spine prominent. In preservative, pale yellow brown dorsally, yellow ventrally; no aggregations of pigment forming any stripes. Adult female, with thickened and obliquely pleated right oviduct; left oviduct absent.
Etymology. From the Latin fossor , a miner, in allusion to the fossorial habits of the genus and the type locality, where the numerous garnets in the bed of the Hale River, misidentified as rubies, sparked the Northern Territory's first mining rush ( Gibson et al. 1992). The species epithet is a noun in apposition.
Comparisons with other species. Anilios fossor keys to A. splendidus ( Aplin 1998) using the key provided by Cogger (2014), in having the nasal cleft contacting the second supralabial, 20 midbody scales, a head smoothly rounded in outline from above, ventral surface pale, the nasal cleft not extending onto the dorsum of the head or to the rostral to divide the nasal, and the snout rounded in profile (although splendidus has an angulate rostral in profile, contra the character state given in the key; Aplin 1998). However, it differs from A. splendidus in its much smaller size (mature female with SVL = 240 mm, vs SVL of only known specimen, also a mature female, 498 mm), paler coloration (pale yellow-brown dorsally vs dark grey-brown above, sharply demarcated from the pale venter), rounded snout in lateral profile (vs strongly angulate), larger eye, and much greater number of dorsal scales (dorsal scales to level of cloaca 503 vs 377) ( Aplin 1998 for comparative data). Additionally, A. splendidus is only known from North-West Cape, Western Australia, over half a continent away.
It shares the greatest overall similarity with A. wiedii ( Peters 1867a) , a species which is similar in size (SVL of mature females, the larger sex, 175–322 mm, mean = 232.5 mm, median = 231 mm, n = 65), coloration (pale yellow-brown in preservative) and number of midbody scales (20), and also has the nasal cleft contacting the second supralabial and a rounded snout in dorsal and lateral profile. It differs from A. wiedii ( Fig. 5 View FIGURE 5 ) in having a much larger, more rounded rostral scale, in the nasal cleft not extending to the dorsal surface of the head (vs extending well onto the dorsal surface, then extending medially towards the rostral to almost or completely divide the nasal), and in having more dorsal scales (514 vs 379–442 for males and 421–469 for females). The only individuals of A. wiedii with dorsal scales greater than 434 for males and 452 for females among the 135 individuals examined are from a single sample of 11 individuals from "Darling River Floods". This series was part of a major collection made by the Australian Museum collector Robert Helms between May and June 1890. Most of the registration entries for this collection do not specify where along the Darling River these collections were made. However, one series (AM R6457–67) does more specifically note that the collection was made between Bourke and Wilcannia, and a contemporary newspaper report (Anon 1890) notes that Helms spent nearly two weeks in June 1890 collecting amongst flood debris at Tallywaka (now Talyawalka, an overflow anabranch paralleling the Darling River near Wilcannia). This is the westernmost record of A. wiedii , but could represent individuals washed downstream from further east. Despite the somewhat higher dorsal scale counts of these specimens, which are geographically intermediate between the distribution of A. wiedii and A. fossor , in other respects this sample is typical of A. wiedii , and does not represent a morphological intermediate between the two species. The geographic distance between the only known locality for A. fossor , and A. wiedii at Talyawalka is 1250 km, with the Simpson and Strzelecki Deserts in the intervening area.
Five other species of Anilios have a distribution that reaches central Australia: A. bituberculatus ( Peters 1863) , A. centralis ( Storr 1984) , A. diversus ( Waite 1894) , A. endoterus ( Waite 1918) and A. grypus ( Waite 1918) , and hence could be sympatric with A. fossor . Anilios bituberculatus has a strongly trilobed snout in dorsal profile which is angulate in lateral profile, and has a much narrower rostral scale. Anilios centralis has a hooked snout in lateral profile, a narrower rostral scale (see Fig. 1 View FIGURE 1 in Storr 1984), more subcaudal scales (12–20, the lower end of the range presumably representing females) and slightly fewer dorsal scales (425–494) (data from Storr 1984; Pyron & Wallach 2014). Anilios diversus has a nasal cleft contacting the preocular scale, a very narrow rostral scale, and fewer transverse scale rows (dorsal scales 403–465 fide Pyron & Wallach 2014; ventral scales 389–457 fide Storr 1981; Aplin 1998 notes that ventral scales are roughly comparable to dorsal scales counted to the level of the cloaca). Anilios endoterus has 22 rows of scales at midbody, a nasal cleft joining the preocular, a snout that is angular in lateral profile, and fewer dorsal scale rows (422–463; Pyron & Wallach 2014; Shea et al. 2000). Central Australian populations of A. grypus have a strongly hooked snout in lateral profile, 18 midbody scales, a narrow rostral scale, and a dark head and tail.
An additional five species of Anilios from semiarid and mesic parts of Australia share with A. fossor the combination of 20 midbody scales, and the nasal cleft contacting the second supralabial: A. broomi ( Boulenger 1898) , A. leucoproctus ( Boulenger 1889) , A. pinguis ( Waite 1897) , A. sylvia ( Ingram & Covacevich 1993) and A. waitii ( Boulenger 1895a) . Of these, A. waitii of the semiarid and arid parts of Western Australia has a hooked rostral in lateral profile and a trilobed snout in dorsal view, and A. pinguis of south-western Australia is a much larger species (SVL up to 491 mm; Aplin 1998) with an angulate snout in lateral profile, rostral much longer than wide ("urn-shaped"; Storr, 1981), many fewer dorsal scales (to cloaca, 280–388, Aplin 1998) and a dark brown to black dorsal color. Anilios sylvia , a species confined to a small part of the southern Queensland coast, is a smaller species (maximum SVL 175 mm), with many fewer transverse scale rows (ventral scales 271–320), nasal cleft extending onto the head dorsum, smaller rostral scale (of similar size and shape to A. wiedii ) and a very dark dorsum ( Ingram & Covacevich 1993). Anilios leucoproctus , a species restricted to Cape York and the Torres Strait Islands, similarly has fewer dorsal scale rows (386–426 fide McDowell 1974; 377–394 fide Shea 1999), is darkly pigmented both dorsally and ventrally, has a narrower rostral scale, and has well-developed glands along the margins of the head shields ( McDowell 1974), while Anilios broomi , a species of the Wet Tropics of Queensland, from the Atherton Tableland to Cooktown, is strongly striped dorsally, has fewer dorsal scale rows (456–460 fide McDowell 1974; 445-510 fide Pyron & Wallach 2014), and a nasal cleft that extends onto the dorsal surface of the head to divide or nearly divide the nasal.
All other Anilios species either have fewer midbody scales (16: A. leptosoma ( Robb 1972) , A. longissimus ( Aplin 1998) , A. minimus ( Kinghorn 1929) , A. nema ( Shea & Horner 1997) ; 18: A. affinis ( Boulenger 1889) , A. aspina ( Couper et al. 1998) , A. chamodracaena ( Ingram & Covacevich 1993) , A. guentheri ( Peters 1865) , A. howi ( Storr 1983) , some A. leptosoma , A. margaretae ( Storr 1981) , A. micromma ( Storr 1981) , A. nigricauda ( Boulenger, 1895b) , A. nigroterminatus ( Parker, 1931) , A. yampiensis (Storr 1981)) or more midbody scales (22: A. australis Gray 1845 , A. bicolor ( Peters 1860) , A. hamatus ( Storr 1981) , A. kimberleyensis ( Storr 1981) , A. nigrescens Gray 1845 , A. pilbarensis ( Aplin & Donnellan 1993) , A. polygrammicus ( Schlegel 1839) , A. robertsi ( Couper et al. 1998) , A. torresianus ( Boulenger 1889) , A. troglodytes ( Storr 1981) ; 24: A. batillus ( Waite 1894) , A. ganei ( Aplin 1998) , A. ligatus ( Peters 1879) , A. unguirostris ( Peters 1867b) , A. yirrikalae (Kinghorn 1942)) , or if they have 20 midbody scales, they have the nasal cleft contacting either the first supralabial ( A. erycinus ( Werner 1901) , A. proximus (Waite 1893)) or the preocular ( A. ammodytes ( Montague 1914) , A. tovelli (Loveridge 1945)) .
Distribution. Only known from the type locality ( Fig. 4 View FIGURE 4 ), on the upper reaches of the Hale River and at the eastern extremity of the Central Australian Ranges, between the relatively low Amarata and Harts Ranges.
Due to their fossorial habits, typhlopids are generally difficult to target by normal collecting and short-term survey methods, but are instead most often collected opportunistically by long-term residents close to settled areas. This effect is enhanced in the arid zone of Australia, where the sparse settlement means that relatively few typhlopids are collected other than around towns and homesteads, with often large gaps between known localities. The majority of typhlopid specimens from the southern Northern Territory have been collected from the environs of Alice Springs, or near major tourist venues. Anilios fossor is not represented among the typhlopid material from Alice Springs in the central part of the Central Australian Ranges, and hence may not extend significantly west of the type locality, towards the McDonnell Ranges. The Hale River, which flows through the Ruby Gap Nature Park and could provide a dispersal route for the species, drains into the Simpson Desert, an extensive sandridge system representing a very different geomorphology to the type locality. Hence, it is possible that the species has a very restricted distribution.
However, I cannot exclude the alternative possibility that A. fossor has a more extensive distribution that is yet to be identified. Another species of arid Australian typhlopid, A. margaretae , was described in 1981 from a single specimen from Lake Throssel in Western Australia, and is generally reported as restricted to that region (e.g., Cogger 2014; Wilson & Swan 2013), but has recently been collected opportunistically at two localities in central South Australia, over 750 km to the south-east (M. Hutchinson, pers. comm.)— the intervening area has almost no permanent human settlement, and few roads.
Given the uncertainty about the distribution of the species, it is not possible to provide a conservation assessment, but there are no grounds to think there has been any significant decline in the species that may require a threatened conservation status, and the type locality is located in a remote nature reserve with little human impact (the locality is only accessible by four wheel drive vehicular traffic). However, further work is needed in the vicinity of the type locality to determine population density of this poorly known species.
Habitat. The holotype was collected during a fauna survey of the Ruby Gap Nature Park ( Gibson et al. 1992), at which time it was identified as Ramphotyphlops centralis . Two specimens identified as this species were collected and lodged in the Northern Territory Museum, the holotype of A. fossor and NTM 14316. The habitat notes for these two specimens were provided by Gibson et al. (1992) as "a low, open woodland of Eucalyptus thozetiana on stony plains, also a woodland of Eucalyptus camaldulensis on sandy loam soil near the Hale River". As the locality for NTM 14316, which is a specimen of A. centralis , is Thozet Box Camp, Loves Creek Station, a locality not on the Hale River, I presume that the second habitat description applies to A. fossor . The Eucalyptus camaldulensis vegetation association is described in the same report as a woodland with upper storey of E. camaldulensis , midstorey absent or with scattered Capparis spinosa and Acacia victoriae , and ground story of Cynodon dactylon , Brachyachne ciliaris , and ephemeral herbs and grasses on sand and sandy loam along major creeklines. This habitat is on Cainozoic alluvium, although the general surrounds are metamorphic quartzites, amphibolites, gneisses and tonalites of the Precambrian Heavitree Quartzites and Arunta Complex.
| NTM |
Northern Territory Museum of Arts and Sciences |
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.