Astylosternus occidentalis Parker, 1931
|
publication ID |
https://doi.org/ 10.5281/zenodo.280506 |
|
DOI |
https://doi.org/10.5281/zenodo.5698116 |
|
persistent identifier |
https://treatment.plazi.org/id/03C487E4-FF97-D677-56EA-DFCB2096FE5C |
|
treatment provided by |
Plazi |
|
scientific name |
Astylosternus occidentalis Parker, 1931 |
| status |
|
Astylosternus occidentalis Parker, 1931 View in CoL
Figs. 3–7 View FIGURE 3 View FIGURE 4 View FIGURE 5 View FIGURE 6 View FIGURE 7
Astylosternus occidentalis Parker, 1931 View in CoL , Ann. Mag. Nat. Hist., Ser. 10, 7: 492. Holotype: BMNH 1947.2.5.48. Type locality: "Sandaru, E. Sierra Leone " collected on 24 June 1930 by G.L. Bates.
Hylambates yalense Angel, 1944 , Bull. Mus. Natl. Hist. Nat., Ser. 2, 16: 420. Holotype: MNHNP 1944.128; female, 64 mm. Type locality: French Upper Guinea, Yale; in secondary forest, Mont Nimba region, Guinea. Synonymy with Astylosternus diadematus View in CoL by Guibé, 1950 "1948", Cat. Types Amph. Mus. Natl. Hist. Nat.: 58. Synonymy with Astylosternus occidentalis View in CoL by Lamotte, in Schiøtz, 1967, Spolia Zool. Mus. Haun., 25: 67.
Astylosternus diadematus sensu Lamotte & Zuber-Vogel 1954 View in CoL , Bull. Inst. fond. Afr. noire, Sér. A, 16, 1222. Astylosternus occidentalis sensu Guibé & Lamotte 1958 View in CoL , Mém. Inst. fond. Afr. noire, 53, 261.
Material studied. See Appendix 1.
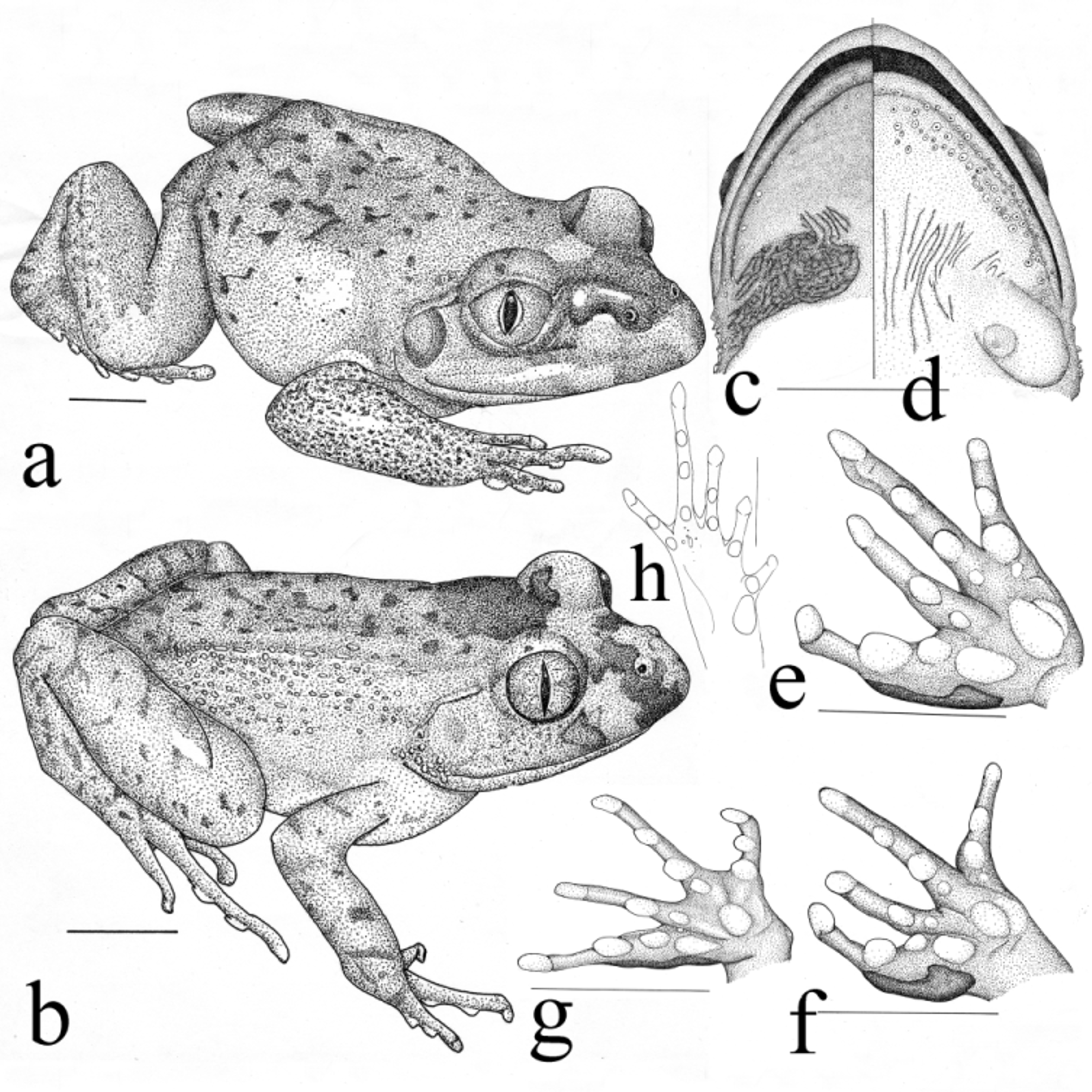
Re-description of the holotype [ BMNH 1947.2.5.48; Fig. 3 View FIGURE 3 ; measures in mm]. The adult male has a snoutvent length of 59.3 (63 according to original description; Parker 1931); snout rounded in dorsal view, obtuse in lateral view, longer than eye diameter; head width 22.3, head as long as broad; interorbital distance (8) narrower than length of upper eyelid; large protruding eyes, diameter 7.6; pupil vertically elliptic; tympanum large, distinct and vertically elliptic, diameter 4.5, 2/3 of eye diameter; distance eye-nose only slightly larger (5.3), than distance nose-snout tip (4.8), internarial distance 4.7; canthus rostralis distinct and curved, slightly bulging; loreal region concave; vomerine teeth in two short transverse rows between choanae; supratympanal fold distinct, bending from posterior corner of eyelid ventrad to a position in-between angle of mouth and forearm base; hypertrophied forearm; first finger 8.8, longer than second, 6.3; relative finger length: III>I>II≥IV; no digital webbing; femur length 23.7, shorter than tibia 28.2; foot with longest toe 36.8; finger and toe tips slightly broadened without forming discs; sub-articular tubercles very prominent; relative toe length: IV>III>V>II>I; distinct rudiments of webbing on toe bases; no skin fringes along toes; inner metatarsal tubercle (3.0) almost as long as shortest toe; outer metatarsal tubercle absent; skin on back “smooth” (very small spines present everywhere, only visible with higher magnification), i.e. no warts or ridges; paired vocal sac mediad to angle of mouth; vocal sac skin granular and coarsely folded; spines on lower mandible, arranged in 2–3 rows parallel to lower lip; spines conical with dark tip; large nuptial pads on thumb; scattered small spines along lateral edges of other fingers and beneath tympanum; conical spines on belly (dark in original description; possibly fainted); skin on outer part of thighs granular; skin on dorsal parts of body light brown, irregularly beset with small, roundish, dark brown spots; snout tip darker; light inter-ocular bar, posteriorly bordered by dark marking; iris uniform grey; lateral aspects of head beige, tympanum darker, upper lip with two dark bars; brown on flanks towards belly gradually fainting; limbs with indistinct brown transverse, partly interrupted bars; outer parts of thighs and sole of foot darker; ventral parts of skin yellowish white; vocal sac white like belly.
Additional characters not assessed in holotype and variation. Mandible with single, small, tooth-like process in front of lower jaw, with socket in between pre-maxillae; upper pre-maxillae and maxillae with numerous teeth; vomerine teeth in form of two hemispherical odontophores, perpendicular to body axis, not in contact to each other medially, each odontophore with row of teeth like tips; distance from odontophores to elliptical choanae ranging from slightly larger to slightly smaller than maximum length of odontophores; choanae smaller than odontophores; tongue broadly heart shaped, deeply notched anteriorly, densely beset with small papillae, extends along entire length of lower jaw; posterior 2/3 of tongue free.
Females are larger than males; otherwise measures and body ratios do not differ substantially between sexes (summarised in Tables 1 View TABLE 1 & 2). Males have external vocal sacs, which can be white to deep black; dark brown to black nuptial pads (see below), slightly hypertrophied forearms and skin along canthus rostralis more prominent, slightly swollen; the latter region in females much less distinct. Breeding males from Guinea with black spines in pectoral region ( Fig. 5 View FIGURE 5 b; not seen in males from Ivory Coast, Liberia and Sierra Leone; spines exhibiting same colour as rest of belly, but see above for holotype); spiny ventral area slightly converging towards and almost reaching vent; caudal half of ventral surface of shanks with honeycomb-like structure, in each comb a black roundish spine; respective surfaces of females smooth or slightly granular (caudad part of shanks). Whereas spines on breast and belly in breeding males (black nuptial pads on thumb) of all populations are almost always present, spines on shanks may be absent ( ZMB 75469, 75468, both males with black nuptial pads on thumb and black spines on throat) or only weakly developed. Spiny pectoral area with loose black skin on otherwise white venter in males from western Guinea ( Fig. 5 View FIGURE 5 a), skin loosens or even disappears in preservation; in these males the throat along the lower mandible is almost black and males from Boffa have distinct spines in the angle of the mouth (less distinct or absent in others). No or traces of pedal webbing ( Fig. 4 View FIGURE 4 h).
The dorsal colour varies significantly between and within populations ( Fig. 6 View FIGURE 6 a–p) and ranges from almost uniform coloured dorsal surfaces in grey, beige, yellow, orange, light and dark brown; to animals with regular or irregular dark spots on back; spots or bars on extremities; unmarked flanks and flanks with darker spots. A clear intraocular band is almost always visible, as is a dark barred upper lip. The most common colour patterns are shown in Fig. 6 View FIGURE 6 a, b and d, respectively. Rarely animals with a chocolate brown back and an irregular pattern of reddish spots have been found (e.g. two females: ZMB 75375 from Loma mountains; ZMB 75361 from the Western Area Peninsula Forest Reserve, Fig. 6 View FIGURE 6 i, both Sierra Leone). On Simandou we found one frog with olive spots on the back, the edges of these spots beset with black points ( Fig. 6 View FIGURE 6 h). ZMB 75473 shows an especially pronounced dotted back pattern, being light beige with black spots where the edges of spots are darker than their interior part. In contrast, ZMB 75479 ( Fig. 6 View FIGURE 6 e) is more or less greyish, exhibiting a “dirty” pattern without distinct black spots or dots. Animals from Ivory Coast, i.e. the region of Taï National Park, most often were yellow with small black points ( Fig. 4 View FIGURE 4 b). Whereas some specimens have distinct black bars on fore and hind limbs ( Fig. 6 View FIGURE 6 a, k, n); others have uniform ( Fig. 6 View FIGURE 6 c, d, f) or a mottled limb pattern ( Fig. 6 View FIGURE 6 g). The iris colour of all specimens was uniform grey. Throat and belly white to flesh coloured (exception some breeding males, see above), throats may be also speckled with dark brown patches, in some individuals (e.g. ZMB 75479) belly almost translucent; lower side of extremities grey to fleshy pinkish. Overall males seem to exhibit more often darker colours with less distinct pattern than females. These colour patterns all faint in preservation, usually resulting in specimens with beige to dark brown backs, black points and dots usually remaining discernible.
The dorsal skin texture ranges from almost smooth ( Fig. 6 View FIGURE 6 e), to slightly granular ( Fig. 6 View FIGURE 6 g, i), irregularly best with longish flat warts ( Fig. 6 View FIGURE 6 f), to animals which exhibit a more or less smooth back skin and flanks with discontinuous longitudinal rows of narrow ridges ( Fig. 6 View FIGURE 6 a). We could not detect a consistent pattern of skin texture correlated with sex, age or season (e.g. breeding versus non-breeding animals).
Advertisement call. Described by Schiøtz (1964b) based on a specimen ( ZMUC R 074932) from Kassewe, Sierra Leone. The call consists of two parts. The first part seems to be very similar to a call which we heard from Astylosternus from Mont Péko and which resembled the deep rattling call of some Ptychadena e.g. Ptychadena cf. schillukorum ( Schiøtz, A. 1964b as Abrana floweri ; M.- O. Rödel, unpubl. data). The second, buzzing note was often heard alone, the first note was always followed by the second. Astylosternus occidentalis from Taï National Park, Ivory Coast, uttered a buzzing call only.
Tadpole [description based on Lamotte & Zuber-Vogeli 1954 and tadpoles stored in MNHN and ZMB, see Appendix 1]. Exotrophic, lentic tadpoles; Gosner Stage 25– 35 larvae with: body elongate almost rectangular in dorsal, slightly ovoid in lateral view ( Fig. 7 View FIGURE 7 a), sides of body almost parallel; large lateral sacs originating posterior to eye run along flanks, less distinct in smaller than in large larvae; snout in dorsal view broadly rounded, a bit more pointed in lateral view; small eyes, positioned dorsolaterally; nares small, positioned dorsolaterally, closer to snout tip than to eyes; oral apparatus in anteroventral position; dorsal lip wide and smooth, with large anterior gap between marginal papillae; lateral papillae in multiple rows; ventral lip with large, uni- or biserial marginal papillae; upper jaw sheath massif, broad U-shaped, with strongly serrated margin; lower jaw sheath massif, V-shaped, margin strongly serrated; Stage 25 tadpoles and older have a labial tooth-row formula of 1:2+2/2+2:1 or 1:1+1/ 2+2:1; all keratodont rows on skin sheaths; kertodonts set very dense to each other; labial keratodonts unidenticulate, connected by hyaline skin reaching almost tips of keratodonts; vent tube dextral; spiracle sinistral; very long tail axis (approx. 2.5 times body length); tail axis height exceeds height of dorsal and most parts of ventral fin; dorsal fin originates slightly posterior to tail body junction; dorsal fin almost parallel to tail axis up to tail tip; ventral fin mostly narrower than tail axis, only in last third broader than tail axis; ventral fin almost parallel to tail axis; tail tip rounded; lines of pores (probably neuromast canals making up a side line system) starting on snout-tip; extending dorsally between eyes in two parallel rows to insertion of dorsal tail fin, below eyes dorsal from spiracle to about mid-body on flanks; pore rows circumventing eyes; visibility of pore rows better in younger stages; body more or less uniform dark brown to almost black, tail fin dark brown to almost black in last third of tail.
The largest tadpole, Gosner Stage 37, measured 29.2/94.3 (body length/total length). Metamorphosing froglets (with rests of tail) measured 27.5–35.7 mm SVL (x ± sd: 32.3 ± 2.8; N = 10). Parker (1936) reported tadpoles collected in Liberia, the largest larvae with fully grown hind limbs measuring 88 mm (61 mm to the tail); a freshly metamorphosed froglet had a SVL of 30 mm. Lamotte & Zuber-Vogeli (1954) hint on the delayed appearance of the extremities, an adaptation to maintain, as long as possible, a good swimming performance in fast flowing habitats. Guibé & Lamotte (1958) mention tadpole sizes of close to 10 cm shortly before metamorphosis. Parker (1936) hints on the similarity of his tadpoles (body shape, proportions and dentition) with Angel’s (1930) description of Gampsosteonyx batesi (tadpoles from Foumban, Cameroon; thus most likely being tadpoles of A. diadematus ).
Natural history. Astylosternus occidentalis occurs along swift to fast-flowing creeks and streams in dense forest from almost sea level (130 m) to about 1300 m a.s.l. ( Guibé & Lamotte 1958; Böhme 1994; this study). Its habitats may consist of primary as well as degraded or fragmented forests within the rainforest zone or dense gallery forest in the southern part of the humid Guinea savanna zone ( Rödel & Branch 2002; Ernst & Rödel 2006; Ernst et al. 2006; Hillers & Rödel 2007; Hillers et al. 2008a; this study). The species is usually not abundant and patchily distributed, similar to other anuran species associated with rivers in forests of hilly or mountainous areas ( Lamotte 1966). In Mont Sangbé National Park individuals were observed within the forest in a steep valley (Rödel 2003). Whereas the vegetation on the valley ground and slopes consisted of rainforest trees, the hill tops carried savanna vegetation. One A. occidentalis male was found still within forest but only a few meters from true Guinea treesavanna (Rödel 2003). Similarly A. occidentalis has been recorded in gallery forest in the savanna zone of southeastern and western Guinea (this study). Guinean specimens in particular have been regularly encountered in some distance to water. Most populations, however, were recorded within forest close to flowing water. Males are calling from the forest floor close to but not in water ( Schiøtz 1964b). In contrast to Central African Astylosternus , we never observed West African frogs of the genus using the terminal phalanges of their hind feet in defence (see Blackburn et al. 2008). However, phalanges of western and central African Astylosternus species are anatomically indistinguishable ( Barej et al. 2010).
The reproduction behaviour is unknown. Tadpoles can usually be observed at night on the ground of slow or almost stagnant parts of forest creeks and rivers. Larger rivers as well as very small tributaries are inhabited. When disturbed tadpoles flee immediately into deeper and fast flowing water or burrow themselves into loose sediment of the shallow parts. Other anuran species which often occur in syntopy with A. occidentalis are Amietophrynus togoensis (Ahl, 1924) , Cardioglossa occidentalis Blackburn, Kosuch, Schmitz, Burger, Wagner, Gonwouo, Hillers , & Rödel, 2008, Leptopelis macrotis Schiøtz, 1967 , Phrynobatrachus liberiensis Barbour & Loveridge, 1927 , Petropedetes natator Boulenger, 1905 , Conraua spp., and Hyperolius chlorosteus (Boulenger, 1915) .
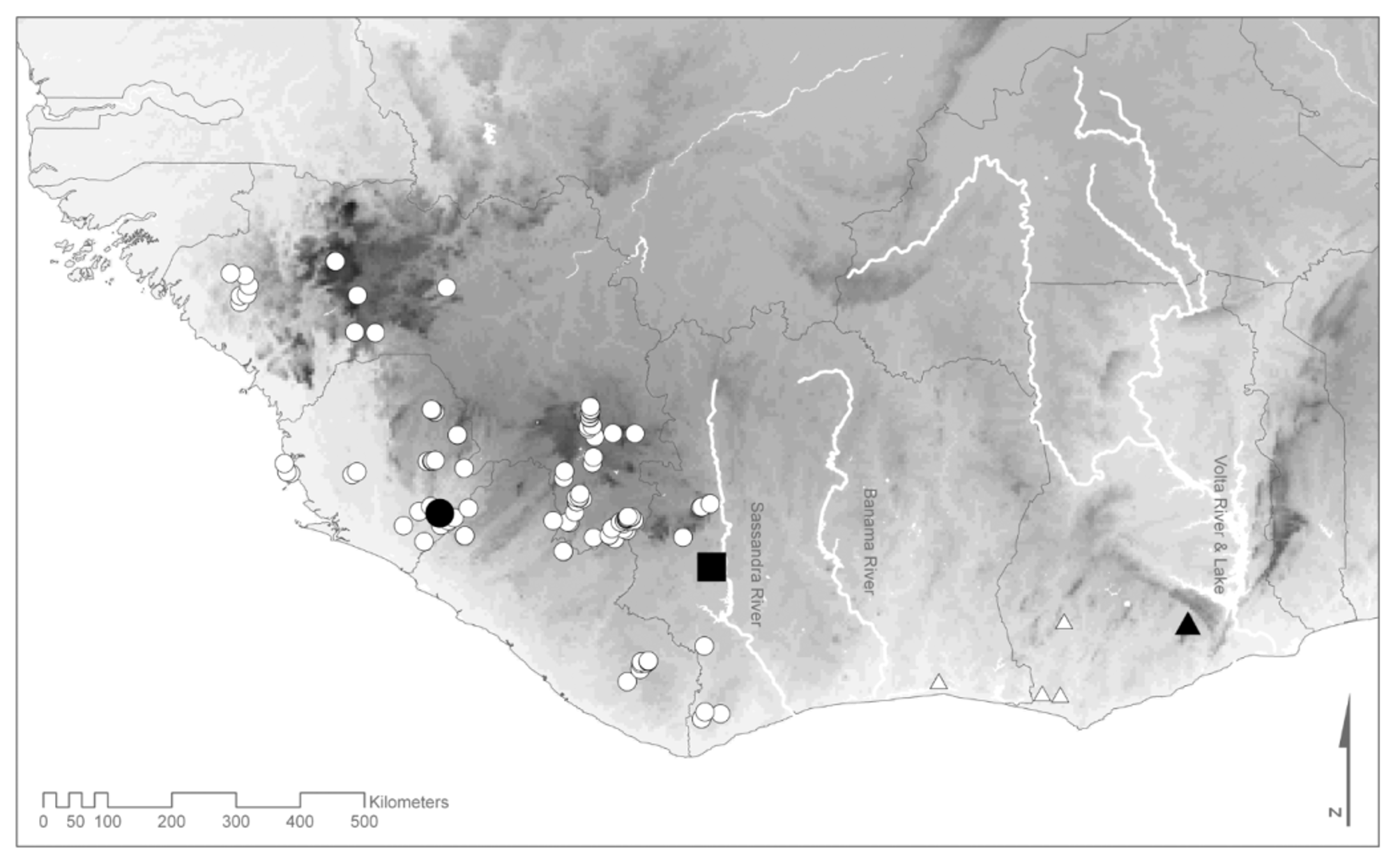
On Mont Péko, Ivory Coast ( Fig. 8 View FIGURE 8 ) the local Astylosternus , in particular their larvae, behaved differently. We collected two males and tadpoles (see taxonomic remarks) close to the summit at about 1000 m a.s.l. at the border of a heavily overgrown shallow creek (Rödel & Ernst 2003), flowing over massive granite underground. These males were calling at night, well concealed in small cavities under stones close to the creek’s bank. Several more males were heard but could not be exactly localised. The tadpoles were caught in shallow, slow flowing parts of the same creek and in very shallow (<1 cm water height) flooded parts in the open moorlands (see fig. 2 in Rödel & Ernst 2003). Other frogs observed close to this site were Ptychadena cf. schillukorum (Werner, 1908) and Hyperolius lamottei Laurent, 1958 (Rödel & Ernst 2003) .
Distribution. Astylosternus occidentalis is restricted to the western part of the Upper Guinean forests ( Fig. 8 View FIGURE 8 ). Records have been published from Sierra Leone ( Parker 1931; Schiøtz 1964a); Liberia ( Parker 1936; Hillers & Rödel 2007); Guinea ( Guibé & Lamotte 1958; Böhme 1994; Rödel & Bangoura 2004; Rödel et al. 2004; Hillers et al. 2006, 2008a); Ivory Coast ( Rödel & Branch 2002; Rödel 2003; Ernst & Rödel 2006; Ernst et al. 2006; Hillers et al. 2008b). The type specimen was collected at Sandaru in Sierra Leone. We tried to localise this locality and identified two places with this name at 7.8 / -11.0 and 8.4 / -10.7, respectively. In the map ( Fig. 8 View FIGURE 8 ) we have used the first coordinates to plot the “ type locality”, although, we failed to identify any objective criteria on which we could decide whether the one or the other Sandaru was meant by Parker (1931). Both localities, however, are comparatively close to each other and well within the range of A. occidentalis . Records from Ghana ( Hughes 1988) most likely are based on misidentifications of the species which is described below.
Taxonomic remarks. Hylambates yalense has been described from Mount Nimba, Guinea ( Angel 1944; not 1940 as cited in Frost 2010). According to A. Ohler (pers. comm. February 2005) the type of H. yalense had been donated to the collection of the Institute Fondamental d’Afrique Noire in Dakar, Senegal. However, there it could not be traced (A. Seck, pers. comm. May 2005). According to the original description ( Angel 1944) the type, a female of 64 mm SVL, would deviate from the A. occidentalis description given above by the following characters: nares in equal distance to eye and snout tip (instead of nares closer to snout); internarial distance equal to interorbital distance (instead of interobital distance larger). All other characters listed by Angel (1944) are within the range of A. occidentalis . As the two differences might be simply due to different ways in taking these measures and as we had a large number of A. occidentalis vouchers and tissue samples available from Mount Nimba and surroundings (see Appendix 1), all of which were identical to other A. occidentalis populations, we follow Lamotte (in Schiøtz 1967) in regarding Hylambates yalense as a junior synonym of A. occidentalis .
The Astylosternus from Mont Péko National Park, Ivory Coast ( Figs. 4 View FIGURE 4 a, c, d; SMNS 9615, male, 66.5 mm; 67.6 mm in life, SMNS 9616, male, 51.8 mm; 55.6 mm in life, 7.0 / -7.2, 1 September 2000, densely overgrown creek on granite mountain,> 1000 m a.s.l.; coll. R. Ernst & M.- O. Rödel) appeared to differ from other A. occidentalis populations (compare Rödel & Ernst 2003). Their head width is in the range of the new species described below ( Table 2), thus wider than in typical A. occidentalis . One male (SMNS 9615) was larger than any other Astylosternus from the western Upper Guinea forests ( Fig. 1 View FIGURE 1 ). They seemed to be more massive and had a smooth back skin (in life A. occidentalis granular skin, warts or ridges are usually discernible). For measures of both males see Tabs. 1 View TABLE 1 and 2. At the same locality we collected tadpoles (ZMB 77194, Fig. 7 View FIGURE 7 b, c; same collection details as SMNS 9615 and 9616). The larval habitat was different to that of other known Astylosternus populations (see above). A species endemic to the Mont Péko region in Ivory Coast would be in line with recent findings in other frog genera of similar ecological requirements ( Conraua and Petropedetes ; M.F. Barej & M.- O. Rödel unpubl. data). However, given the range of variation within the various A. occidentalis populations, further vouchers and genetic samples are needed to clarify the taxonomic status of the Mont Péko frogs.
Conservation status. Given the rather wide distribution of the species, including various protected areas (i.e. Taï National Park, Sapo National Park, Mount Nimba Biosphere Reserve) and its apparent potential to survive in altered forest habitats, the current IUCN RedList classification of “Least Concern” should be kept. If new findings would proof the Mont Péko frogs being a separate species; this taxon would be highly threatened due to a restricted range of occurrence and very intense pressure of logging and other anthropogenic activities.
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |
Astylosternus occidentalis Parker, 1931
| Rödel, Mark-Oliver, Barej, Michael F., Hillers, Annika, Leaché, Adam D., Kouamé, N’Goran G, Ofori-Boateng, Caleb, Assemian, Emmanuel, Tohé, Blayda, Penner, Johannes, Hirschfeld, Mareike, Doumbia, Joseph, Gonwouo, Legrand Nono, Nopper, Joachim, Brede, Christian, Diaz, Raul, Fujita, Matthew K., Gil, Marlon & H, Gabriel 2012 |
Astylosternus occidentalis sensu Guibé & Lamotte 1958
| Guibe & Lamotte 1958 |
Astylosternus diadematus sensu
| Lamotte & Zuber-Vogel 1954 |
Hylambates yalense
| Angel 1944 |
Astylosternus occidentalis
| Parker 1931 |