Rubiogethes Audisio & Cline, 2009
|
publication ID |
https://doi.org/10.5281/zenodo.5319334 |
|
persistent identifier |
https://treatment.plazi.org/id/03BE87CC-F627-FFD5-BA62-FB57FD0FFE41 |
|
treatment provided by |
Felipe (2021-08-28 07:26:47, last updated by Plazi 2023-11-05 05:53:56) |
|
scientific name |
Rubiogethes Audisio & Cline |
| status |
gen. nov. |
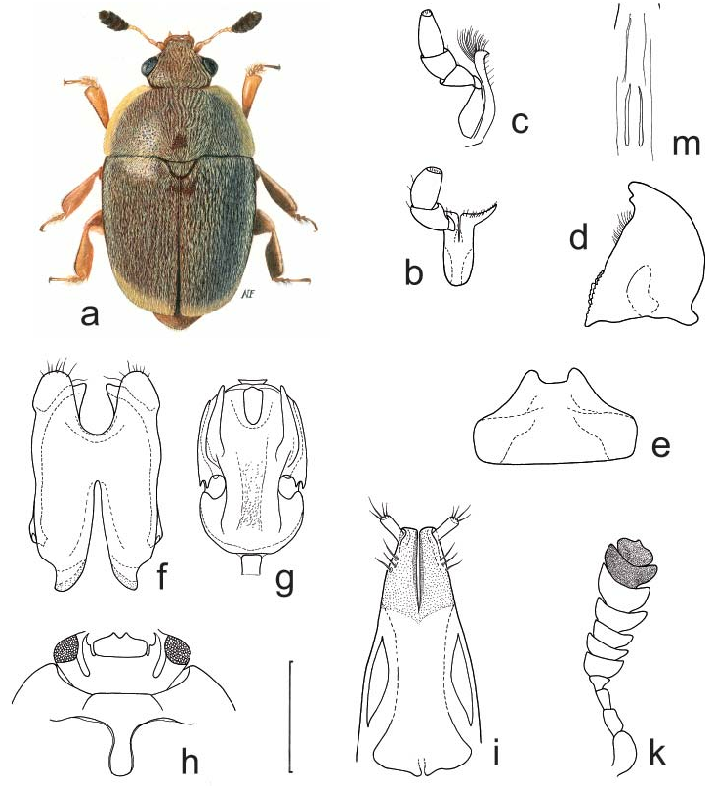
22. Rubiogethes Audisio & Cline , gen. nov.
( Figs. 22 a–k View Fig )
Type species. Meligethes newtoni Kirejtshuk, 1990: 89 (by present designation) [= Rubiogethes newtoni ( Kirejtshuk, 1990) comb. nov.].
Generic description and diagnosis. Inclusive species vary moderately in size (1.6–2.4 mm length), and share the following combination of characters.
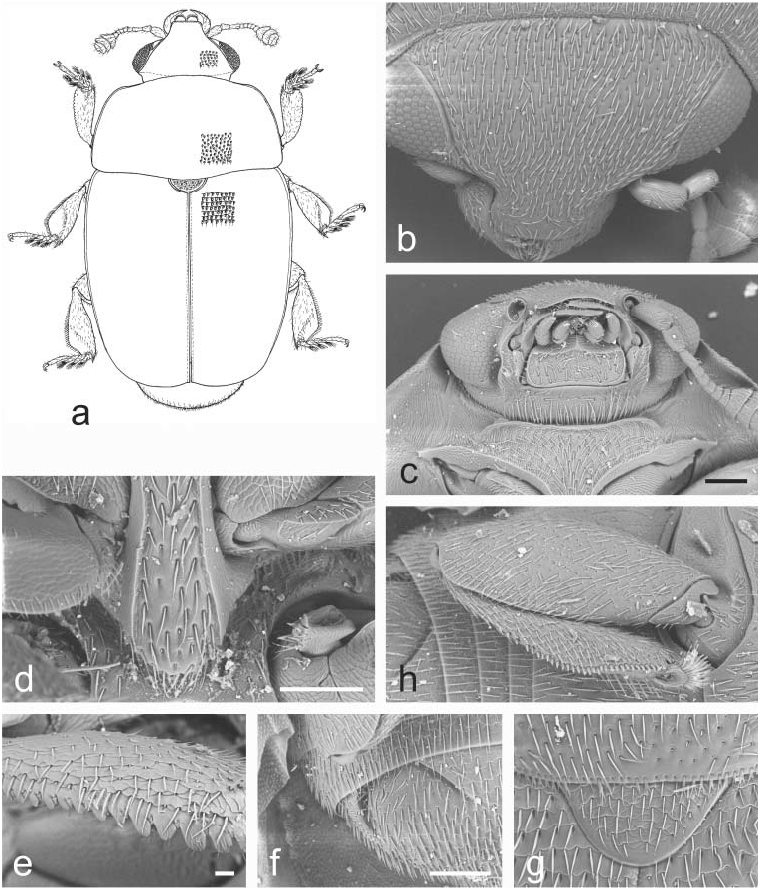
Body color and pubescence: pubescence silvery-whitish, fine, scarcely developed, recumbent, never obscuring the usually brown or blackish-brown dorsal body surface; pronotal and elytral sides narrowly flattened, typically same color as disc. Lateral margin of pronutum and elytra with a series of faintly distinct, small and short setae, each seta 0.3–0.5× as long as those on elytral disc; posterior margin of pronotum with long, usually distally bifid microsetae, absent (or nearly so) on short middle portion anterior to scutellum ( Fig. 22d View Fig ).
Dorsal habitus: body moderately strongly convex, long and slender and relatively parallelsided ( Fig. 22a View Fig ), or shortly oval; dorsal punctures on discal portion of pronotum larger than eye facet, usually deeply impressed and densely distributed ( Fig. 22b View Fig ); anterior margin of clypeus slightly sinuate medially, faintly bordered, lateral angles blunt ( Fig. 22b View Fig ), without small, faintly distinct, medial bulge; circum-ocular furrows (occipital sulci) on dorsal side of head narrow, shallowly impressed, and slightly obliterated posteriorly, incomplete ( Fig. 22b View Fig ); eyes large and usually moderately projecting laterally ( Figs. 22a, b View Fig ); pronotum with obtusely distinct posterior angles, never directed posteriorly ( Fig. 22a View Fig ); scutellum minutely punctured on posterior half of exposed portion ( Fig. 22d View Fig ); elytra always more or less strongly transversely strigose ( Fig. 22d View Fig ); elytral humeral striae indistinct; elytral pre-sutural striae faintly distinct, originating posteriorly to scutellar vertex, terminating close to elytral apex, delimiting on each elytron a flat and narrow sutural border, narrower than proximal portion of 3 rd antennomere; elytral apices truncately rounded in both sexes ( Fig. 22a View Fig ); pygidium partially exposed, moderately convex, apically rounded in both sexes ( Fig. 22a View Fig ).
Ventral habitus: antennal furrows markedly delimited, nearly parallel-sided, slightly sinuate, slightly divergent posteriorly; mentum subpentagonal ( Fig. 22c View Fig ), strongly transverse, trapezoidal; prosternal antennal furrows on anterior margin of prosternum strongly raised and relatively long ( Figs. 22c, e View Fig ); prosternal process usually moderately wide, subapical dilated portion 2.6–2.9× as wide as maximum width of 1 st antennomere, with apex blunt and microscopically crenulate on posterior margin ( Fig. 22e View Fig ); lateral borders of prosternal process delimiting shallowly impressed but wide and distinct furrows, distally terminating over predistal lateral expansions ( Fig. 22e View Fig ); posterior margin of mesoventrite never medially incised, slightly arcuately convex posteriorly ( Fig. 22e View Fig ); male impressions on metaventrite moderately developed; first two visible abdominal ventrites simple in both sexes, without tufts of setae; caudal marginal lines of metacoxal cavities simple, parallel and contiguous to posterior margin of metacoxal cavities, comprising moderately deep arched impression of outer ‘axillary’ line ( Fig. 22g View Fig ); ‘axillary’ space on first abdominal ventrite well developed, ‘axillary’ angle obtuse ( Fig. 22g View Fig ); large, long, and deeply impressed arched impressions on basal portion of last visible abdominal ventrite, rarely partially covered by distal portion of penultimate visible abdominal ventrite ( Fig. 22f View Fig ).
Appendages: male 1 st antennomere 0.8–0.9× as long as width of protibiae excluding distal teeth ( Fig. 22c View Fig ); 3 rd antennomere in both sexes usually moderately short, 2.1–2.2× as long as wide, 0.7–0.8× as long as but distinctly thinner than 2 nd antennomere ( Fig. 22c View Fig ); 4 th and 5 th antennomeres subequal in both sexes, short, nearly as long as wide; antennal club compact, small, simple, comprising last 3 antennomeres in both sexes (8 th antennomere scarcely widened, 0.4–0.5× as wide as 9 th antennomere) ( Fig. 22c View Fig ), narrower than width of protibiae, sexual dimorphism absent; labial palpi relatively short in both sexes ( Fig. 22c View Fig ), terminal segment 1.4–1.5× as long as wide; maxillary palpi moderately long and slender in both sexes ( Fig. 22c View Fig ), terminal segment 2.1–2.2× as long as wide; mandible small- to mid-sized ( Fig. 22c View Fig ), apex moderately acuminate, no sexual dimorphism; tarsal claws simple, not toothed at base ( Fig. 22h View Fig ); tarsi of normal size and shape, 0.6–0.7× as long as corresponding tibiae ( Fig. 22h View Fig ); protibiae with a series of variable, uneven, large and sharp teeth on distal portion of lateral margin ( Fig. 22k View Fig ); lateral margin of meso- and metatibiae bearing a single and usually even row of long and robust pegs ( Fig. 22h View Fig ), without U-shaped sinuosity at distal third; meso- and metatibiae moderately slender and relatively narrow ( Fig. 22h View Fig ), never subtrapezoidal or axe-shaped; meso- and metatibiae with scarce sexual dimorphism; tarsal plates of prolegs distinctly wider in males; posterior margin of metafemora simple in both sexes, without tubercles or projections.
Male genitalia: processes along inner side of parameres absent ( Figs. 26–27 View Fig View Fig in EASTON 1959b; Figs. 44–45, 50– 53 in EASTON 1960; Figs. 19–20 View Fig View Fig in AUDISIO 1996), distal margin variably incised, without deep median longitudinal desclerotization from proximal portion of tegmen extending to medial distal V-shaped excision; median lobe of aedeagus variably shaped, some species exhibit a slight subdistal lateral emargination bearing a short, flat distally projecting lobule, narrowed and obtuse distally, without distal marked excision or emargination.
Female genitalia (ovipositor): small, slender; styli usually long and pigmented, cylindrical, inserted at apex of contiguous gonostyloids (Fig. 59 in EASTON 1959b; Fig. 103 in EASTON 1960; Fig. 42 View Fig in EASTON 1964b; Fig. 23 View Fig in AUDISIO 1996); each gonostyloid lightly sclerotized and typically darkly pigmented distally, with a simple, never indentate outer portion of narrow basicoxites, and a single, narrow, lightly pigmented and sclerotized arcuate area along outer subdistal portion of gonostyloids. ‘Central point’ of ovipositor centrally located, without proximad directed spicule.
Etymology. The generic name is derived from the host-plant family of all inclusive species, i.e. Rubiaceae , and from ‘- gethes ’, to emphasize its phylogenetic relationship with Meligethes . Gender masculine.
Biology. All species are likely to be strictly associated for larval development with flowers of herbaceous Rubiaceae , in particular Pentas Benth. , Pentanisia Harv. , and allied genera ( EASTON 1960, AUDISIO unpublished data). The presumed association of several adult specimens of Meligethes newtoni ( Kirejtshuk, 1990) with Striga Lour. (Scrophulariaceae) in South Africa (see KIREJTSHUK 1990) was probably due to an occasional availability of flowers of this plant genus (adult Meligethinae are usually polyphagous when their true host plants are not in flower), or to a misidentification of the plants; in fact, this southern African species is strictly associated as larvae with Pentanisia spp. , specifically the widespread P. prunelloides (Klotzsch ex Eckl. & Zeyh.) Walp. (AUDISIO unpublished data).
Phylogenetic position. Available morphological datasets provide evidence for a monophyletic clade including Rubiogethes gen. nov. and Lamiogethes gen. nov., both of which are marginally related to Paleogethes gen. nov., Jelinekigethes gen. nov., and Astylogethes . However, phylogenetic relationships between these taxa remain unclear, and are only partially supported with molecular data ( TRIZZINO et al. 2009).
Taxonomy and geographic distribution. Rubiogethes gen. nov. includes eight described Afrotropical species ( EASTON 1959b, 1960, 1964b; KIREJTSHUK 1990; AUDISIO unpublished data). EASTON (1959b, 1960) first identified an isolated ‘ Meligethes spissus ’ species-group, correctly hypothesizing larval relationships of inclusive species with Rubiaceae , but later were attributed other species to this group that were actually members of Lamiogethes gen. nov. These species exhibited superficial parallelisms with Rubiogethes gen. nov., which were the cause for the misplacement. On the contrary, Meligethes impexus ( Kirejtshuk & Viklund, 2002) was originally included in the M. ruficollis species-group (a group now included in Lamiogethes gen. nov.), but is probably a member of Rubiogethes gen. nov. The true taxonomic placement of Meligethes culminis Easton, 1959 from ‘Abyssinia’ (not recently re-examined by the authors) requires further analysis to definitively confirm placement in Rubiogethes gen. nov., but within which it is currently placed.
Rubiogethes culminis (Easton, 1959) comb. nov. Ethiopia
Rubiogethes impexus ( Kirejtshuk & Viklund, 2002) Kenya comb. nov.
Rubiogethes kulalensis ( Easton, 1960) comb. nov. Kenya
Rubiogethes newtoni ( Kirejtshuk, 1990) comb. nov. South Africa: Limpopo, KwaZulu-Natal, Mpumalanga
Rubiogethes pentasi ( Easton, 1960) comb. nov. Tanzania
Rubiogethes spissus (Grouvelle, 1908) comb. nov. Ethiopia, Kenya, Tanzania
Rubiogethes suffuscus (Easton, 1964) comb. nov. Congo
Rubiogethes undosus (Easton, 1964) comb. nov. Congo
Rubiogethes varus ( Easton, 1960) comb. nov. Kenya
AUDISIO P. 1996: New and little-known South African Meligethes Stephens of the M. convexus group (Coleoptera: Nitidulidae: Meligethinae). African Entomology 4: 213 - 230.
EASTON A. M. 1959 b: The Meligethes of Abyssinia (Col.; Nitid.). Transactions of the Royal Entomological Society of London 111: 367 - 403.
EASTON A. M. 1960: The Meligethes of East Africa (Coleoptera: Nitidulidae). Transactions of the Royal Entomological Society of London 112: 263 - 318.
EASTON A. M. 1964 b: Genus Meligethes Stephens (Coleoptera, Nitidulidae). Exploration du Parc National de l'Upemba, Mission G. F. de Witte 68: 29 - 59.
KIREJTSHUK A. G. 1990: Novye taksony zhukov-blestyanok vostochnogo polushariya. Chast' 4. [New taxa of the Nitidulidae (Coleoptera) of the Eastern Hemisphere. Part 4]. Trudy Zoologicheskogo Instituta, Akademiya Nauk SSSR 211: 84 - 103 (in Russian).
KIREJTSHUK A. G. & VIKLUND B. 2002: Contribution to the knowledge on the subgenus Meligethes (Clypeogethes Scholtz, 1932) from Kenya (Coleptera, Nitidulidae). Annales Historico-Naturales Musei Nationalis Hungarici 94: 9 - 21.
TRIZZINO M., AUDISIO P., ANTONINI G., DE BIASE A. & MANCINI E. 2009: Comparative analysis of sequences and secondary structures of the rRNA internal transcribed spacer 2 (ITS 2) in pollen-beetles of the subfamily Meligethinae (Coleoptera, Nitidulidae): potential use of slippage-derived sequences in molecular systematics. Molecular Phylogenetics and Evolution 51: 215 - 226.
Fig. 22. Rubiogethes Audisio & Cline, gen. nov.: a–k – R. newtoni (Kirejtshuk, 1990). a – female habitus; b – dorsal view of head; c – ventral view of head and anterior portion of prosternum; d – scutellum and microsetae on middle of posterior margin of pronotum; e – prosternal process and mesoventrite; f – exposed portion of last visible abdominal ventrite; g – caudal marginal line of metacoxal cavity; h – middle leg with outer margin of mesotibia; k – protibia. Scale bars: Figs. a, b = 200 μm; Figs. c, d, e, f, g = 100 μm; Figs. h, k = 20 μm.
Fig. 26. Anthystrix Kirejtshuk, 1981: a, f–g, i – A.squamosa Kirejtshuk, 1981; b–e, h, k, m – A. longiclava Kirejtshuk & Easton, 1988. a – male habitus (length 2.5 mm); b – labium and right labial palpus; c – right maxilla and palpus; d – left mandible; e – labrum; f–g – male genitalia; h – ventral view of head and anterior portion of prosternum; i – distal portion of ovipositor; k – male antennal club; m – major sclerites of male endophallus. Drawings b–m: refer to AUDISIO et al. (2009a) for scale.
Fig. 27. Tarchonanthogethes Audisio & Cline, gen. nov.: a, c–i – T. rotundiclava (Kirejtshuk & Easton, 1988); b – T.sp.; k – T. martini (Grouvelle, 1899). a, b – male habitus (a – length 2.6 mm; b – length 2.0 mm); c–d – male genitalia (c – length 0. 43 mm; d – length 0.38 mm); e – major sclerites of male endophallus (length 0.20 mm); f – scutellum and microsetae on middle of posterior margin of pronotum; g – ventral view of body; h – male antenna (length 0.65 mm); i – dorsal view of head; k – distal portion of ovipositor (length 0.4 mm). Scale bars: Figs. f, i = 100 μm; Fig. g = 300 μm.
Fig. 19. Astylogethes Kirejtshuk, 1992: a–h – A. subrugosus (Gyllenhal, 1808). a – male habitus (length 2.4 mm); b – dorsal view of head; c – ventral view of head and anterior portion of prosternum; d – prosternal process and mesoventrite; e – outer margin of protibia; f – exposed portion of last visible abdominal ventrite; g – scutellum and microsetae on middle of posterior margin of pronotum; h – caudal marginal line of metacoxal cavity. Scale bars: Figs. c, d, f = 100 μm; Fig. e = 20 μm.
Fig. 20. Stachygethes Audisio & Cline, gen. nov.: a, c, f, k – S. ruficornis (Marsham, 1802); b – S. variolosus (Easton, 1964); d, e, g, h – S. assimilis (Sturm, 1845). a, b – male habitus (a – length 2.5 mm; b – length 2.5 mm); c – dorsal view of head; d – ventral view of head and anterior portion of prosternum; e – scutellum and microsetae on middle of posterior margin of pronotum; f – caudal marginal line of metacoxal cavity; g – prosternal process and mesoventrite; h – exposed portion of last visible abdominal ventrite; k – outer margin of mesotibia. Scale bars: Figs. d, f, g, h = 100 μm; Fig. e = 30 μm; Fig. k = 20 μm.
Fig. 42. Microporum C. O. Waterhouse, 1876: a–g – M. mordace Cooper, 1974; h–m – M. scotti (Grouvelle, 1913). a – male habitus; b – ventral view of body; c – male protibia; d, h – male antennae; e–f, k – male genitalia;g – ovipositor; m – dorsal view of male head and first three antennomeres. Drawings a–g modified from COOPER (1974); drawings h–m modified from ENDRÖDY-YOUNGA (1978). Refer to COOPER (1974) and ENDRÖDY-YOUNGA (1978) for scale.
Fig. 23. Lamiogethes Audisio & Cline, gen. nov.: a – L. paschalis (Spornraft, 1975); b – L. convexus (Boheman, 1851); c–e, g–h, m – L. ruficollis (Reitter, 1872); k, f, n – L. difficilis (Heer, 1841). a, b – male habitus (a – length 3.0 mm; b – length 2.7 mm); c – ventral view of head and of anterior portion of prosternum; d – dorsal view of head; e–f – scutellum and microsetae on middle of posterior margin of pronotum; g – ventral view of body; h–k – prosternal process and mesoventrite; m – last tarsomere of middle leg; n – outer margin of mesotibia. Scale bars: Figs. c, d, e, f, h, n = 100 μm; Fig. g = 1 mm; Fig. m = 20 μm.
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |