Siren nettingi ( Goin, 1942 )
|
publication ID |
https://doi.org/10.11646/zootaxa.5258.4.1 |
|
publication LSID |
lsid:zoobank.org:pub:73F90E50-168F-4441-8B68-8DDFFF8E17D4 |
|
DOI |
https://doi.org/10.5281/zenodo.7786182 |
|
persistent identifier |
https://treatment.plazi.org/id/03BB87B1-FF9E-0F34-43D5-FDC9FD2DFD0B |
|
treatment provided by |
Plazi (2023-03-30 08:21:49, last updated 2024-11-28 04:35:20) |
|
scientific name |
Siren nettingi ( Goin, 1942 ) |
| status |
|
Siren nettingi ( Goin, 1942) View in CoL
( Figs. 1‒6 View FIGURE 1 View FIGURE 2 View FIGURE 3 View FIGURE 4 View FIGURE 5 View FIGURE 6 & 9 View FIGURE 9 )
Common name. Western Siren
Holotype. Carnegie Museum 7580. Adult female collected in May 1928 from Imboden , Lawrence Co., Arkansas, USA ( Goin 1942).
Paratypes. See Goin (1942).
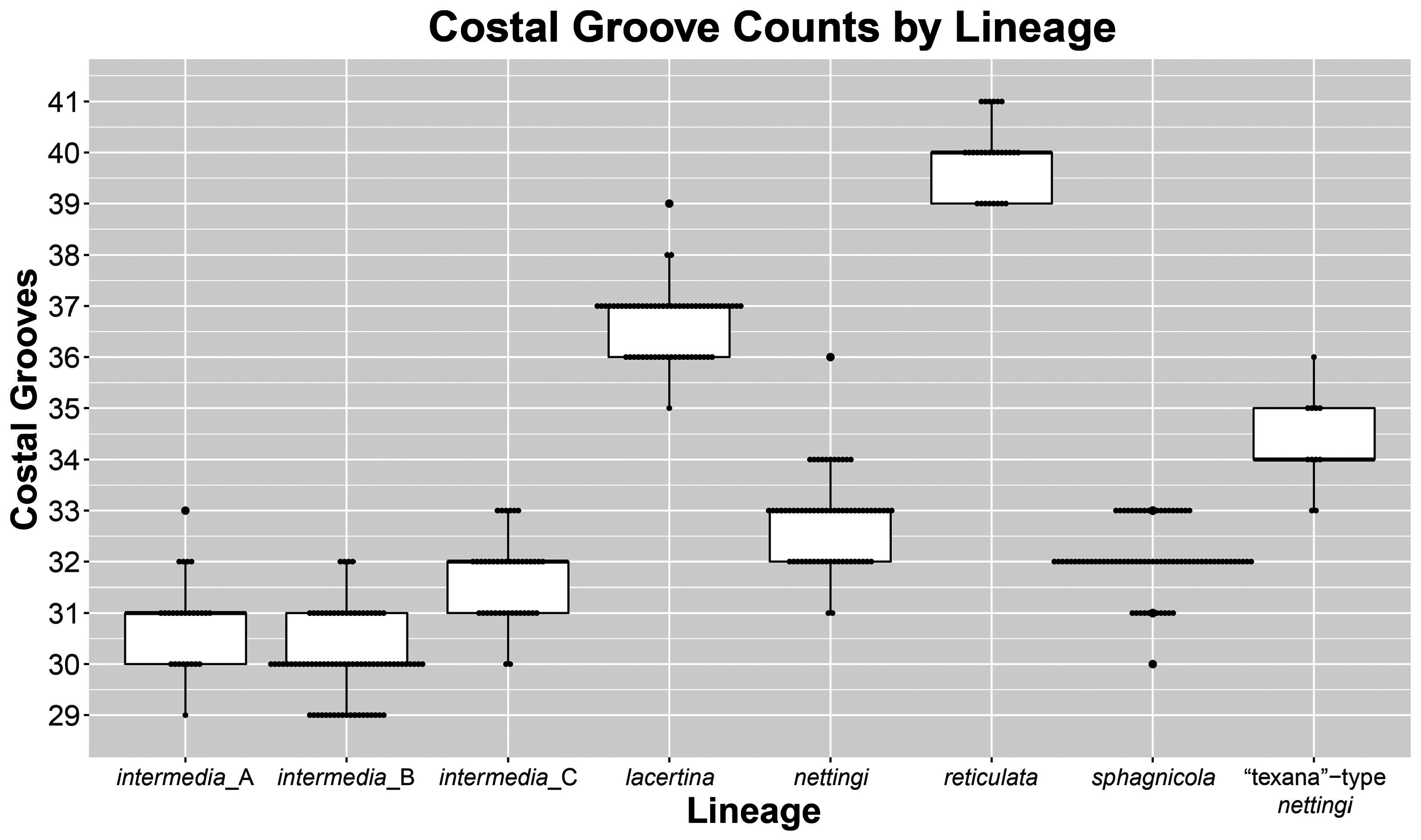
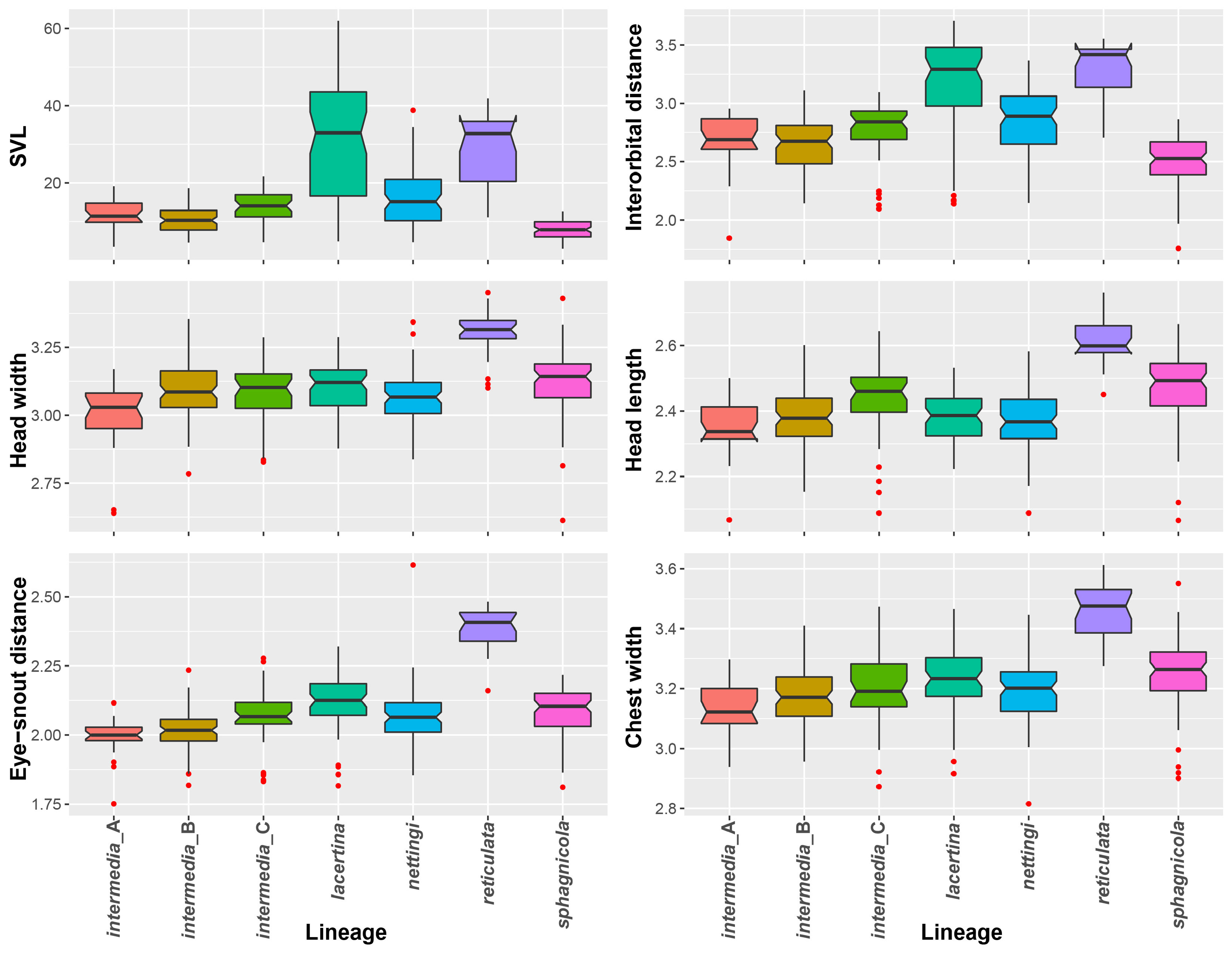
Diagnosis. Siren nettingi has typical Siren characteristics: external gills with three fimbriate gill stalks, three associated gill slits, four toes on the forelimbs, lack of pelvic girdle and hindlimbs, and a thin, pigment-bearing mucus layer that overlies the keratinized skin. Goin (1942) distinguished S. nettingi from S. intermedia by the presence of well-defined light spots on the sides and venter and also specifying that these were not the bar-like streaks found in S. lacertina . Additionally, Goin (1942, p. 212) stated that S. nettingi has “about two more costal grooves (usually 33 in intermedia , 35 in nettingi ),” a value slightly overlapping but less than the average for S. lacertina . See comments below for possible reasons for variation in data.
In areas of sympatry, the costal groove range of S. nettingi overlaps that of S. sphagnicola . Siren nettingi has 32‒34 costal grooves, with two outliers of 31 and 36 ( Fig. 4 View FIGURE 4 ). Goin (1942) gave a slightly higher range of 33‒37 costal grooves, but we infer that he included the axial groove or terminated counting grooves at a point posterior to our stopping point. Siren nettingi specimens similar in size to S. sphagnicola can be distinguished by the presence of a solid yellow labial stripe and rostral patch, whereas S. sphagnicola has, at most, several beige spots where the labial stripe occurs in other species. In addition, bodies of small S. nettingi often have a green or yellowish hue and gold flecking, both of with are lacking in S. sphagnicola .
Comments. This species occupies a large geographic area, and we analyzed relatively few specimens, mainly from Alabama and Louisiana ( Fig. 9 View FIGURE 9 ). Our information may not represent the full extent of variation found in this species. Reinhard et al. (2013) provided photographs of larvae and mature adults of this species.
After examining the holotype, we concluded that the well-defined, light spots on the venter referenced by Goin (1942) represent sensory pores rather than chromatophores. Chromatophores on the holotype are neither defined nor extensive in coverage. Regardless of this distinction, neither the distinct, light-colored sensory pores nor the yellow to yellow-green flecking of chromatophores is unique to S. nettingi . These pores are frequently obfuscated by the slime layer but become more visible when this is removed. This slime layer frequently sloughs off of poorly preserved or frozen specimens.
Validity of the Rio Grande Siren ( S. intermedia texana Goin, 1957 ) as a taxonomic unit distinct from S. nettingi has been questioned in recent decades. Our analyses suggest that S. nettingi and “texana” are either closely related sister taxa or ecomorphs of the same species adapted to habitats present in different regions. Further investigation of this topic is needed, but we treated “texana” as a distinct unit for morphological analyses. Furthermore, S. i. “texana” is no longer recognized by Highton et al. (2017), who accepted the erroneous designation by Flores-Villela and Brandon (1992) that the large siren from southern Texas and Mexico is S. lacertina ( LaFortune 2015) .
Size. Siren nettingi has a reported maximum TL of 686 mm for the larger “texana” form found in southern Texas and Mexico, whereas the smaller form found in the rest of its range has a reported maximum TL of 502 mm ( Martof 1973a). Flores-Villela and Brandon (1992) cited Goin (1957) for total lengths given by Martof (1973a and 1973b) and incorrectly reported the TL as the SVL for both the “texana” form of S. nettingi (then S. intermedia texana ) and S. lacertina .
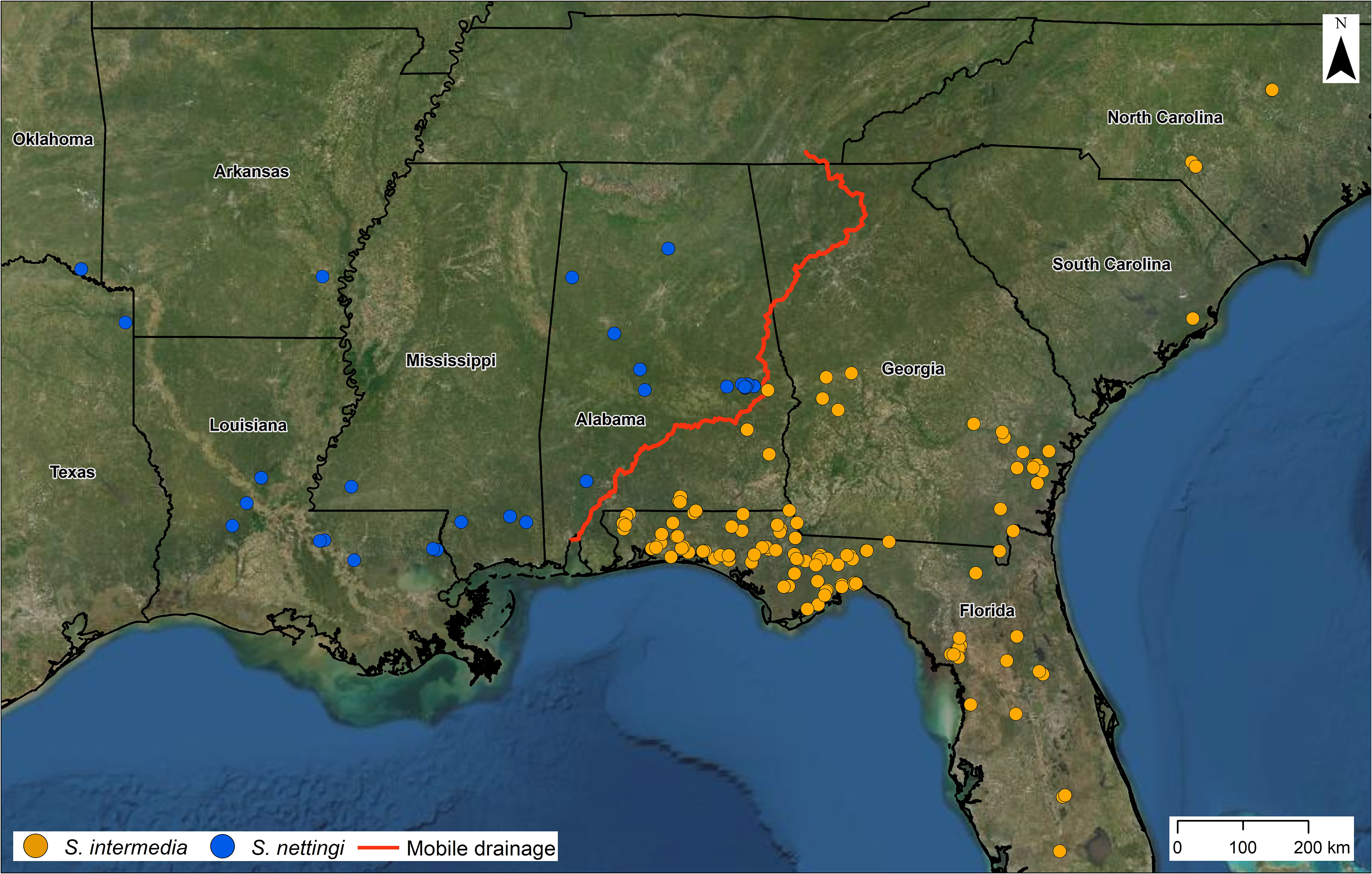
Distribution. Based on our examination of museum specimens and genetic analyses, S. nettingi occurs from the Mobile Bay drainage westward, and native populations do not occur in any Florida drainage ( Fig. 9 View FIGURE 9 ). A single specimen that sequenced as S. nettingi was collected from Tates’s Hell State Forest, Franklin Co., Florida, but we suspect this specimen was translocated as live fish bait. Based on phenotypes and costal groove counts, Goin (1942) suggested that Florida Parishes of Louisiana represented the break between the then-recognized subspecies intermedia and nettingi . This boundary was later considered to be a hybridization zone that stretched into Mississippi ( Boyd & Vickers 1963) and then to the Mobile Bay drainage ( Caldwell & Howell 1966). We suspect part of this confusion resulted from examining S. sphagnicola and attributing them to S. i. intermedia because of their resemblance to grayish S. intermedia that can be found farther east. Furthermore, S. nettingi is currently thought to be the only Siren species that occurs from the Mississippi River west and south to Veracruz, Mexico.
Common Name. We suggest the common name Western Siren . Except for the Mobile Bay drainage system, where S. reticulata is present, S. nettingi represents the largest or only siren (from the Mississippi River westward) present throughout its range; thus, the “Lesser” moniker is misleading.
Specimens examined. See Supplemental Table 1 View TABLE 1 .
Boyd, C. & Vickers, D. H. (1963) Distribution of some Mississippi amphibians and reptiles. Herpetologica, 19 (3), 202 - 205.
Caldwell, R. D. & Howell, W. M. (1966) Siren intermedia nettingi from Alabama. Herpetologica 22 (4), 310 - 311.
Flores-Villela, O. & Brandon, R. A. (1992) Siren lacertina (Amphibia: Caudata) in northeastern Mexico and southern Texas. Annals of the Carnegie Museum, 61, 289 - 291. https: // doi. org / 10.5962 / p. 226656
Goin, C. J. (1942) Description of a new race of Siren intermedia Le Conte. Annals of the Carnegie Museum, 29, 211 - 217. https: // doi. org / 10.5962 / p. 215158
Goin, C. J. (1957) Description of a new salamander of the genus Siren from the Rio Grande. Herpetologica, 13 (1), 37 - 42. https: // doi. org / 10.2307 / 1439392
Highton, R., Bonett, R. M. & Jockusch, E. L. (2017) Caudata salamanders. In: Crother, B. I. (Ed.), Scientific and Standard English Names of Amphibians and Reptiles of North America North of Mexico, with Comments Regarding Confidence in our Understanding. Society for the Study of Amphibians and Reptiles Herpetological Circular No. 43. 8 th Edition. Society for the Study of Amphibians and Reptiles, s. n., pp. 18 - 30.
LaFortune, T. C. (2015) Species Identification and Habitat Assessment of the South Texas Siren. M. S. Thesis, University of Texas at Brownsville, Brownsville, Texas, 91 pp.
Martof, B. S. (1973 a) Siren intermedia Le Conte. Lesser Siren. Catalogue of American Amphibians and Reptiles, 127, 1 - 3.
Martof, B. S. (1973 b) Siren lacertina Linnaeus. Greater Siren. Catalogue of American Amphibians and Reptiles, 128, 1 - 2.
Reinhard, S., Voitel, S. and Kupfer, A. (2013) External fertilisation and paternal care in the paedomorphic salamander Siren intermedia Barnes, 1826 (Urodela: Sirenidae). Zoologischer Anzeiger-A Journal of Comparative Zoology, 253 (1), 1 - 5. https: // doi. org / 10.1016 / j. jcz. 2013.06.002
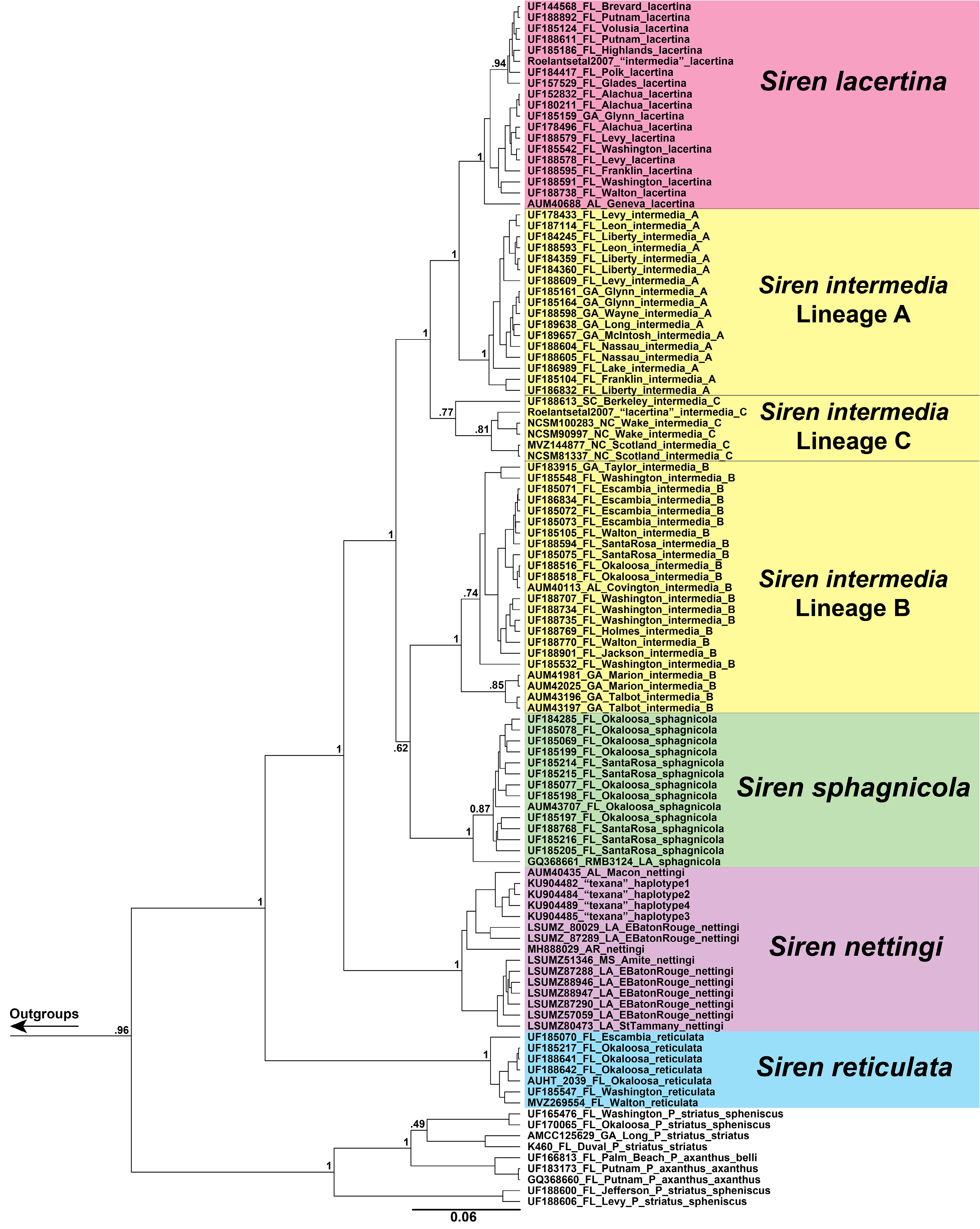
FIGURE 1. Bayesian probability tree of concatenated 16S and ND4 regions inferred using BEAST2. Individual specimen labels include voucher ID or GenBank number if no voucher, state, county, and lineage.
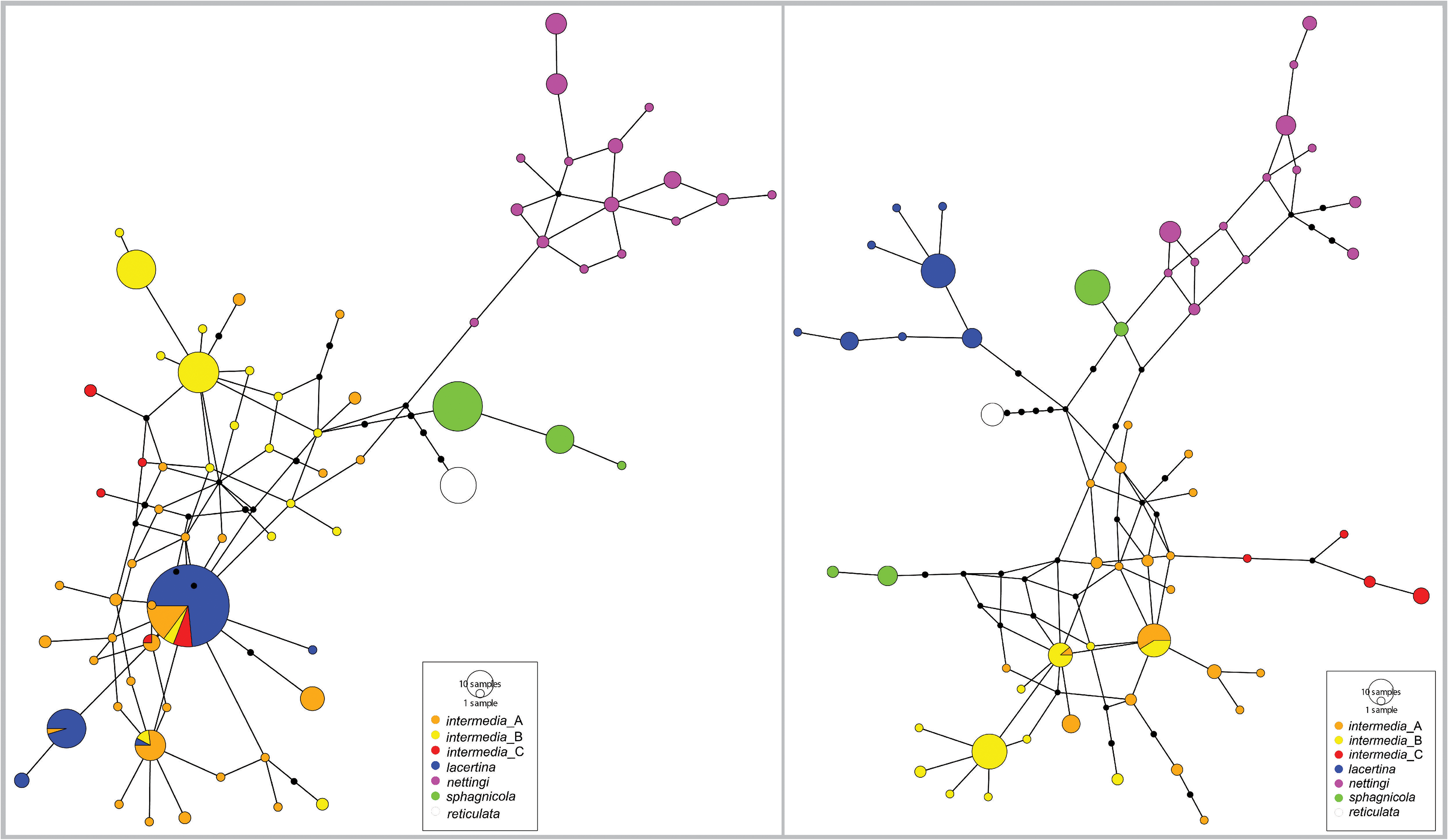
FIGURE 2. Median joining networks of Siren haplotypes for RAG1 (left) and NCX1 (right) regions made using PopART.
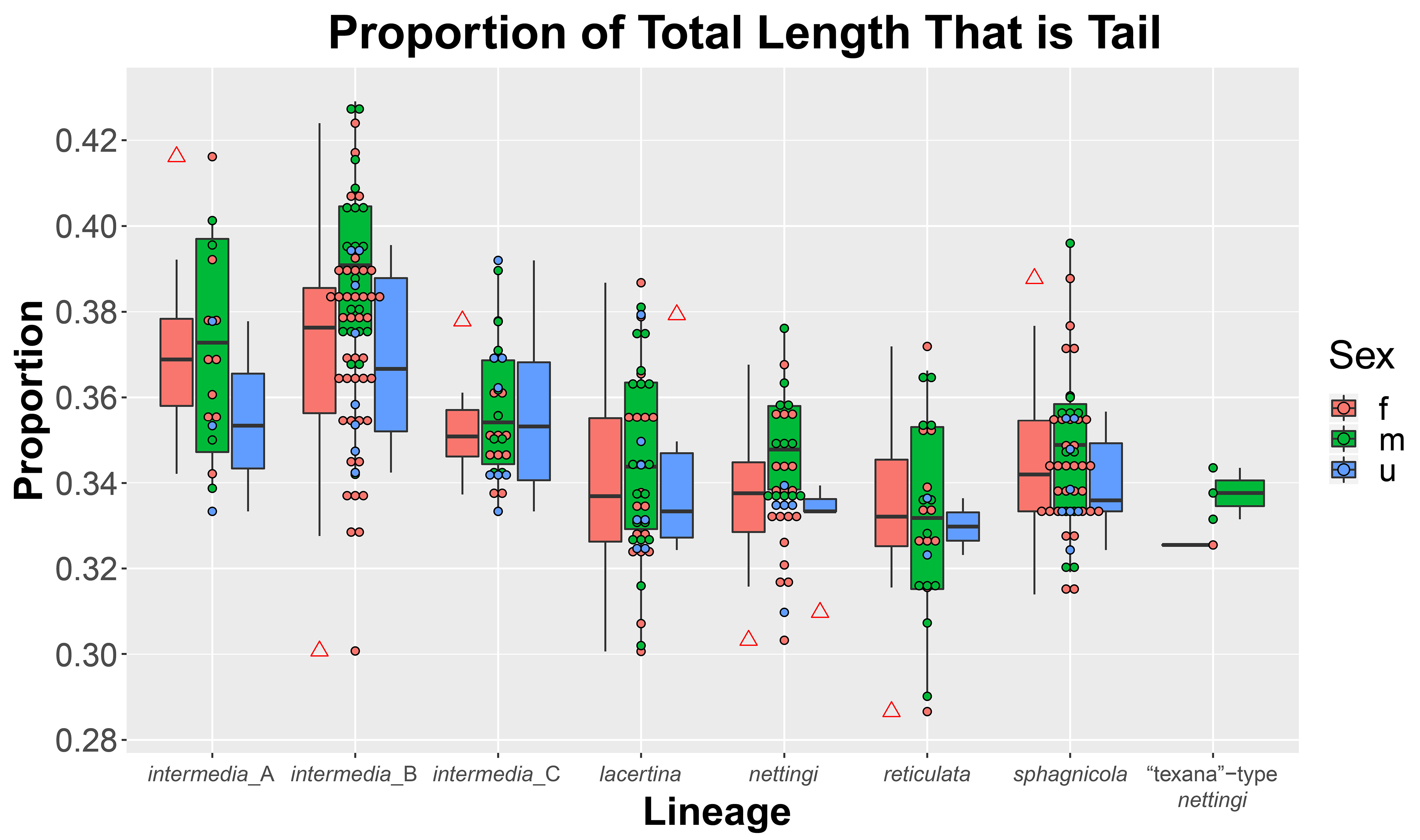
FIGURE 3. Box plots with points colored by sex showing distribution of tail proportions of Siren lineages. Outliers are noted with red triangles on the associated box plot.
FIGURE 4. Box plots with points representing specimens per showing distribution of costal groove counts of Siren lineages.
FIGURE 5. Box plots of the distributions of snout-vent length and the five size-corrected linear measurements compared using linear discriminant analysis.
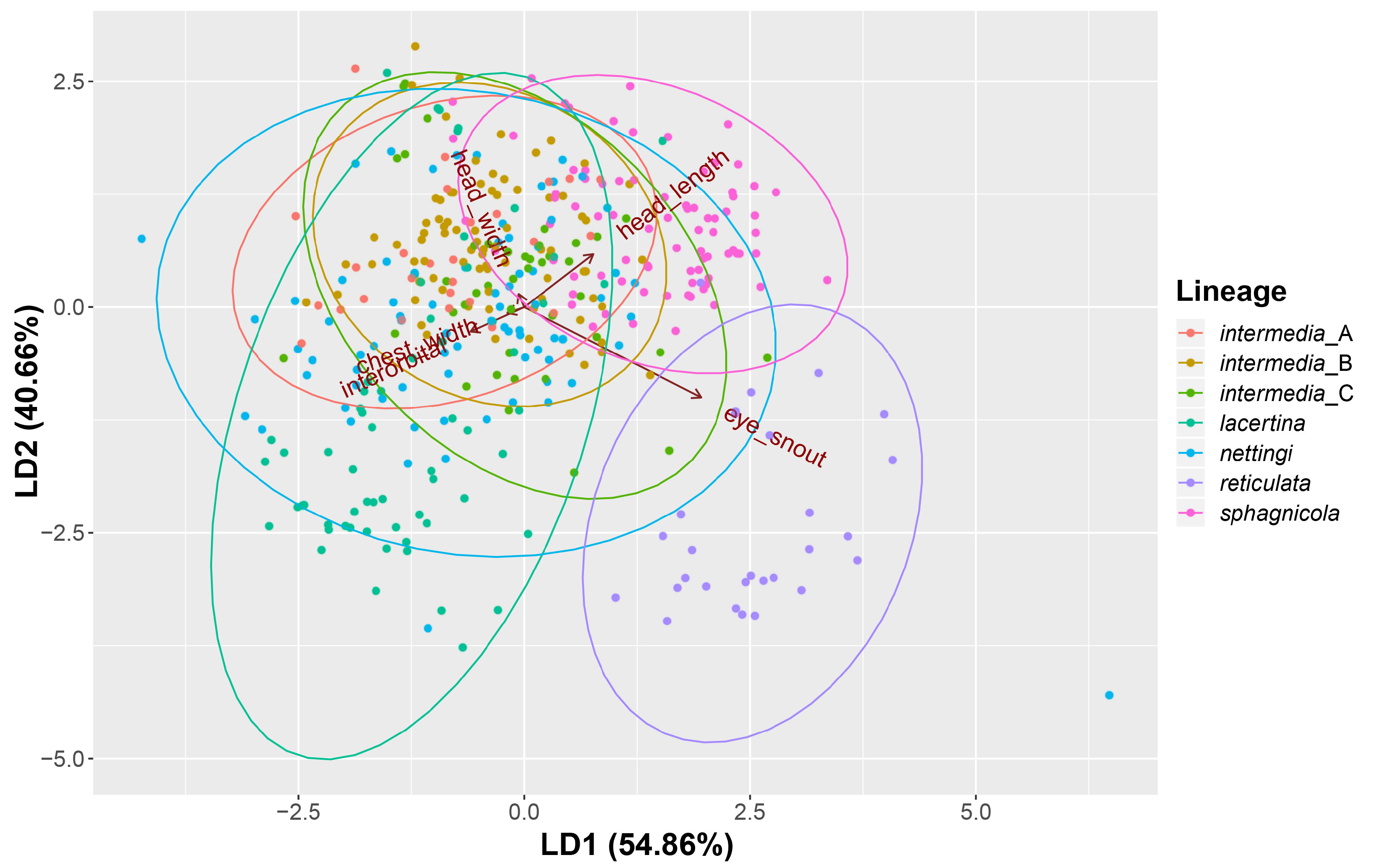
FIGURE 6. Results from our linear discriminant function analysis based on the five size-corrected linear measurements taken from 396 Siren specimens. Except for S. reticulata, each lineage failed to create meaningful separation.
FIGURE 9. Distribution map of specimens assigned to Siren intermedia (orange circles) and S. nettingi (blue circles). The red line is the eastern boundary of the Mobile drainage from EDNA Derived Watersheds for Major Named Rivers (https://edna. usgs.gov/watersheds/kml_index.htm). The Mobile drainage lacks S. intermedia records and is the easternmost drainage where S. nettingi occurs. Our westernmost records of S. intermedia are from the Escambia drainage.
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.