Siren sphagnicola, Fedler & Enge & Moler, 2023
|
publication ID |
https://doi.org/10.11646/zootaxa.5258.4.1 |
|
publication LSID |
lsid:zoobank.org:pub:73F90E50-168F-4441-8B68-8DDFFF8E17D4 |
|
DOI |
https://doi.org/10.5281/zenodo.7786180 |
|
persistent identifier |
https://treatment.plazi.org/id/03BB87B1-FF80-0F37-43D5-FB19FD2DFE5E |
|
treatment provided by |
Plazi (2023-03-30 08:21:49, last updated 2024-11-28 04:35:20) |
|
scientific name |
Siren sphagnicola |
| status |
sp. nov. |
Siren sphagnicola sp. nov.
( Figs. 1‒6 View FIGURE 1 View FIGURE 2 View FIGURE 3 View FIGURE 4 View FIGURE 5 View FIGURE 6 , 11–14 View FIGURE 11 View FIGURE 12 View FIGURE 13 View FIGURE 14 )
Common name. Seepage Siren
Holotype. UF Herp 185209 ( Fig. 11A View FIGURE 11 ), adult female from Junior Walton Pond in Okaloosa Co., Florida, USA (30.69270°N, 86.47250°W, datum WGS84, elev. 30 m) ( Fig. 12 A & 12B View FIGURE 12 ). Collected on 18 January 2019 by Matthew Fedler, Paul Moler, and Pierson Hill. GoogleMaps
Paratypes. UF Herp 161516, 162498, 162568, 163271, 164240, 164241, 164242, 164243, 184285, 185195, 185200, 185201, 185208, 185209, 185214, 185215, 188766, 188767, 190036, 190037, 185205, 185216, 185197, 185198, 185210. Locality information for the various paratype localities is available via FLMNH’s UF Herpetology database (http://specifyportal.flmnh.ufl.edu/herps/).
Description of holotype. The holotype has 32 costal grooves and faint black dorsal spots extending from the head to the forelimbs. It lacks approximately 3 mm of its tail tip. In life, it was mouse gray on its venter and sides with a grayish brown dorsum. Sensory pits on the head are well defined and beige in color. Measurements are 97 mm SVL, 49 mm TaL, 3.9 mm interorbital distance, 7.1 mm head width, 11.1 mm head length, 3.6 mm eye-snout distance, and 4.4 mm chest width.
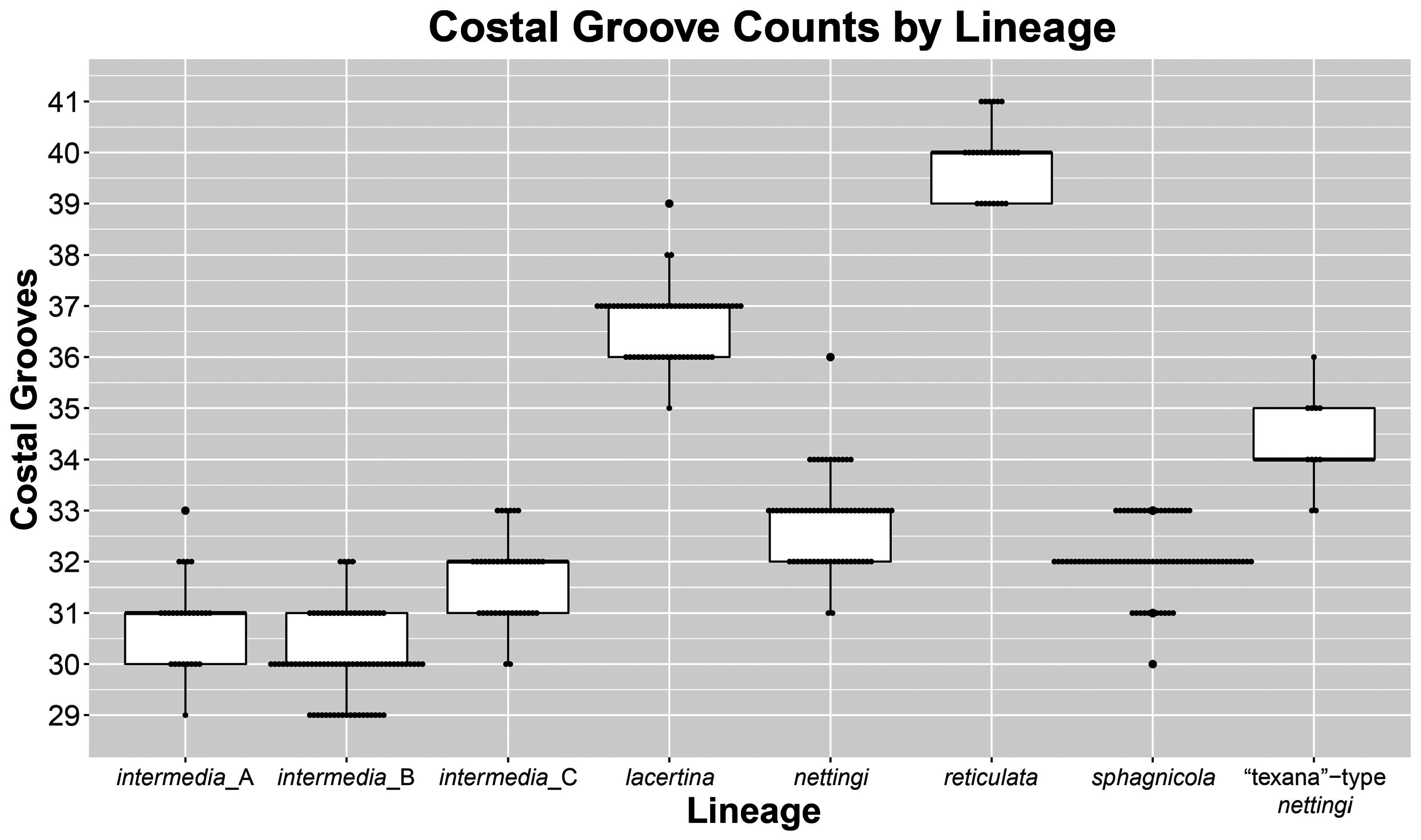
Diagnosis. Siren sphagnicola has typical Siren characteristics: external gills with three fimbriate gill stalks, three associated gill slits, four toes on the forelimbs, lack of pelvic girdle and hindlimbs, and a thin, pigment-bearing mucus layer that overlies the keratinized skin. A combination of traits distinguishes it from other members of the genus. It has 30‒33 costal grooves ( Fig. 3 View FIGURE 3 ) and a mouse gray base color with occasionally a light, grayish brown sheen on the dorsum ( Fig. 11 View FIGURE 11 ). Small juveniles in the post-macrocephalic larval stage, which is>2 months of age according to diagrams of growth/transition rates of S. nettingi provided by Noble & Marshall (1932), have the same gray coloration as adults ( Fig. 11 View FIGURE 11 ) and lack the orange, red, or yellow highlights present on other Siren juveniles of similar age ( Fig. 13 View FIGURE 13 ). A few adult specimens examined have small, well-defined black spotting on the head and occasionally on the dorsum ( Fig. 11 View FIGURE 11 ). Sensory pits on the head are more visible than those on heads of other Siren species and are typically ivory to beige colored, which may denote an absence of gray pigment rather than the presence of chromatophores ( Fig. 11 View FIGURE 11 ). This species lacks the yellow labial stripe present in young S. lacertina ( Figs. 13B & 13F View FIGURE 13 ), S. intermedia ( Figs. 13A, 13C, 13G, & 13I View FIGURE 13 ), and S. nettingi examined from the Mobile Bay drainage. Some juvenile S. intermedia in eastern populations also lack the light labial stripe. A few small juvenile S. sphagnicola have yellow spots or a short, broken stripe where a labial stripe is present in other species ( Figs. 13E & 13H View FIGURE 13 ). Siren sphagnicola also lacks the post-cranial yellow or gold flecking found in many S. lacertina ( Fig. 8 View FIGURE 8 ), S. intermedia ( Fig. 10 View FIGURE 10 ), and S. nettingi . Gill stalk coloration is typically rosy pink to red in recently captured specimens but fades to grayish pink in captivity, likely due to changes in acidity or oxygenation of water. Intact tail tips are rounded, whereas partially regenerated tails (frequently observed) often taper to an abrupt point after the tail fin blade ( Fig. 11 View FIGURE 11 ). Regenerated portions of the tail seem to lack the density of gray pigment found in non-regenerated portions; thus, the regenerated portion is easily distinguished by its pinkish gray hue. Regenerated portions of the tail of other Siren species examined match the normal body coloration or have a brownish hue.
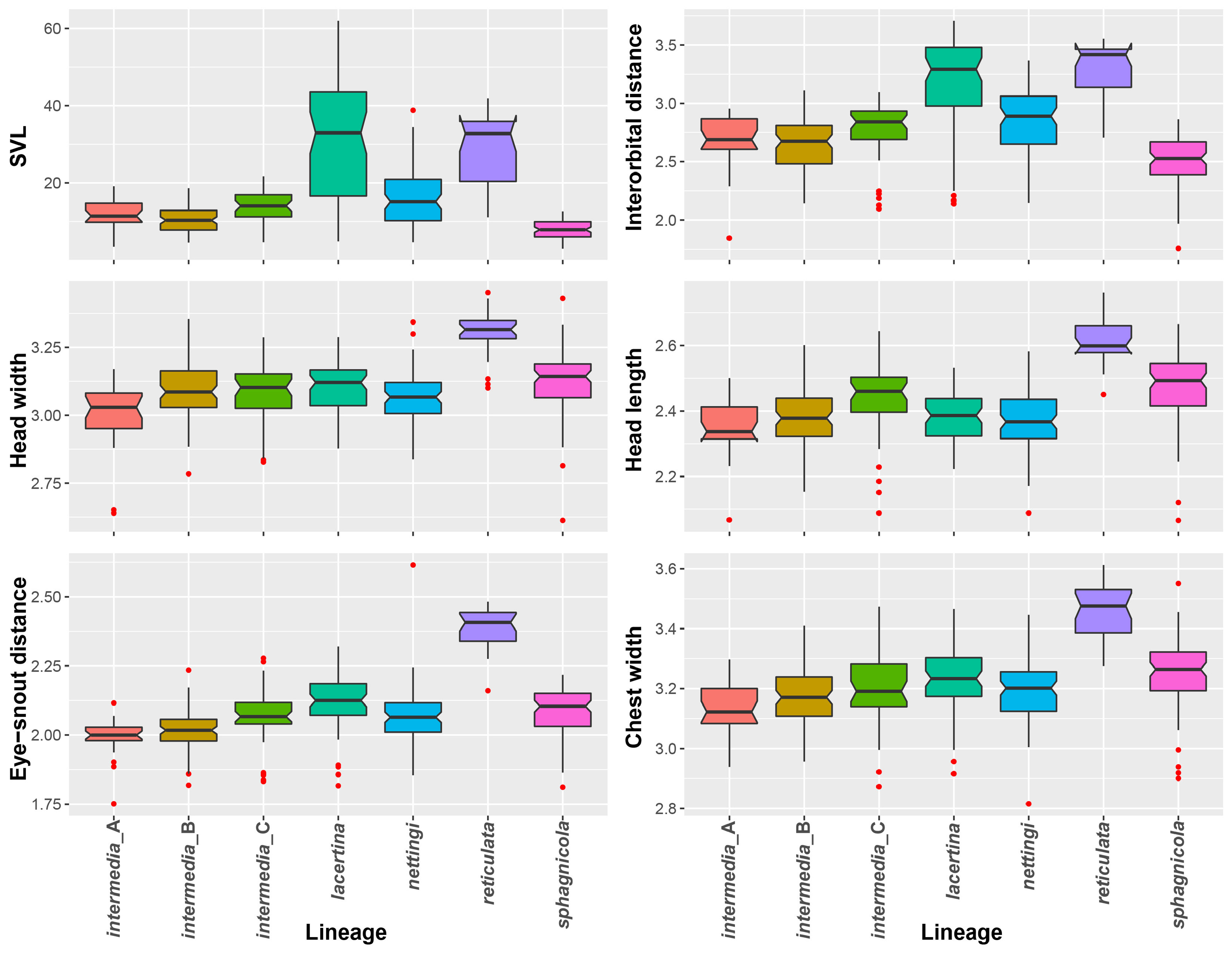
Size. Siren sphagnicola is the smallest known species in the genus Siren . Additionally, all specimens examined are shorter than the maximum length given for both species of Pseudobranchus ( Moler 2019b, c), making S. sphagnicola the smallest member of Sirenidae based on our current understanding. The largest specimen examined (AUM 27973) had an SVL of 126 mm, but it lacked a complete tail. The largest specimen with a complete tail (AUM 8960) had an SVL of 120 mm and a TaL of 76 mm (196 mm TL). We attributed these AUM specimens to S. sphagnicola based upon costal groove count, lack of labial striping and gold flecking found on sympatric S. nettingi and S. intermedia , and presence of beige-colored facial pores. Reproductive females have been found as small as 71 mm SVL (111 mm TL). When comparing measurement distributions of Siren lineages, S. sphagnicola was not distinct from any other single lineage in, at most, two of seven measurements (Table 4).
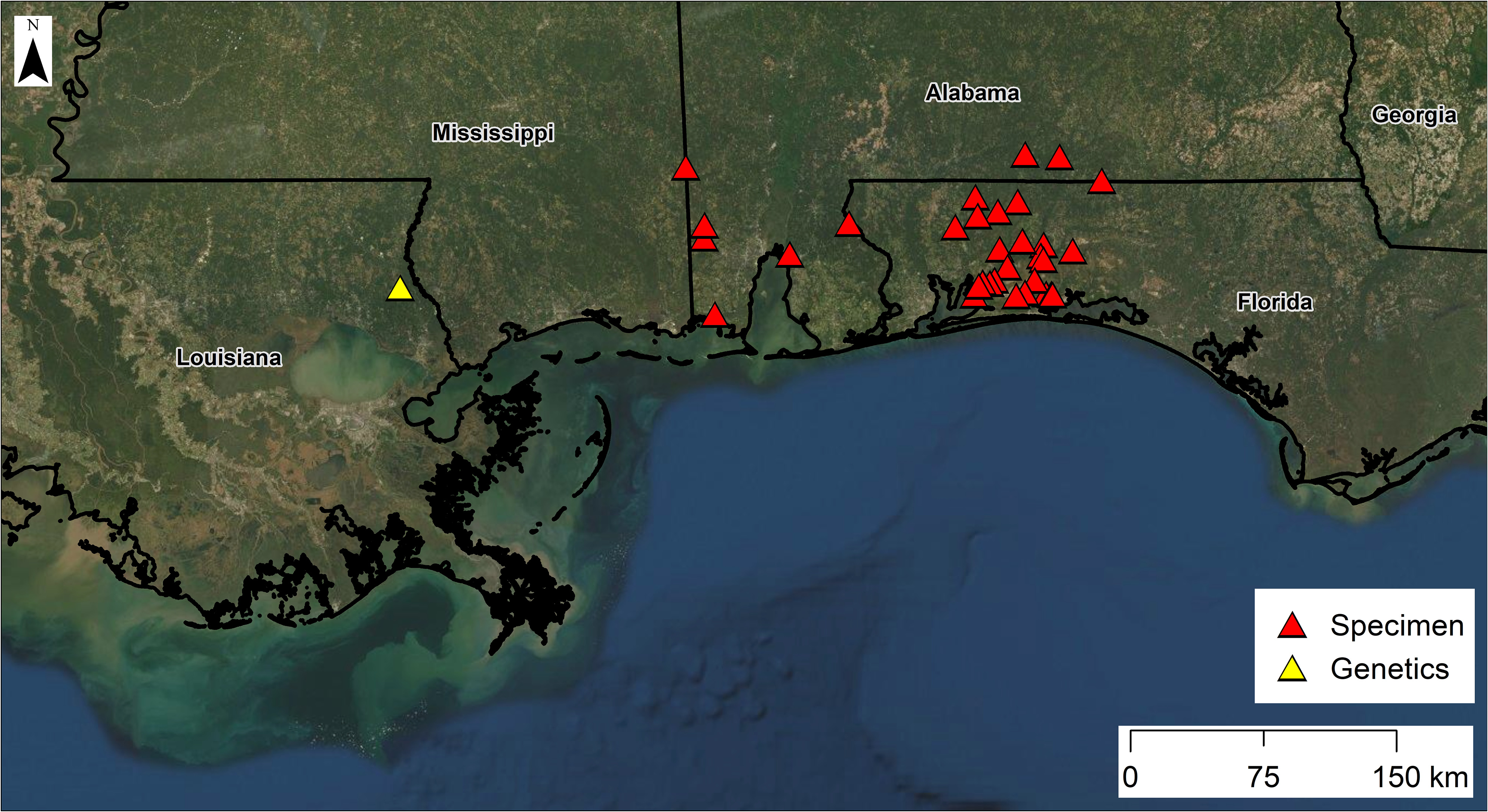
Natural history and distribution. Based on our surveys in Florida, populations appear to be robust and widely distributed in suitable microhabitats in the Blackwater and Yellow river drainages and the western two-thirds of Eglin Air Force Base, including several streams that flow into the western side of Choctawhatchee Bay ( Fig. 14 View FIGURE 14 ). This suspected microhabitat specialist has been found in headwater seepage areas of steephead streams, mucky seeps farther downstream, muddy and/or densely vegetated seepage bogs, shallow-water depressions lined with dense sphagnum moss or filled with leaves along seepage-fed streamside terraces, and other types of shallow streams with mucky, detrital, or sandy bottoms ( Enge 2005) ( Fig. 12 View FIGURE 12 ). In contrast, S. intermedia collected at localities near (<200 m) S. sphagnicola were found in leaf packs not associated with seeps and adjacent to deeper water. Incised (gully-eroded) first- and second-order streams ( Strahler 1964) lack the microhabitats used by S. sphagnicola (and many other salamander species), because accumulations of leaf litter and other detritus are constantly flushed from streams and scoured from surface pools by heavy rainfall events. Common, syntopic amphibian species are the Southern Cricket Frog ( Acris gryllus [Le Conte]), Bronze Frog ( Lithobates clamitans Latreille ), Southern Two-lined Salamander ( Eurycea cirrigera [Green]), and Southern Red Salamander ( Pseudotriton ruber vioscai Bishop ). Onetoed Amphiuma ( Amphiuma pholeter Neill ) and Two-toed Amphiuma ( A. means Garden ) may be present but are less abundant than the aforementioned species ( Enge 2005).
Deep, steephead ravine systems ( Means 1981, 2000) and more shallow-gradient, seepage bogs in upland habitats near the Gulf of Mexico may have served as “evolutionary engines” during periods of elevated sea levels, producing the Florida Bog Frog ( Lithobates okaloosae Moler, 1985 ), Bog Dwarf Salamander ( Eurycea sphagnicola Wray, Means, & Steppan, 2017 ), and S. sphagnicola . A sea level rise of only 2‒5 m would have led to saltwater inundation of the mouths of these deep steephead valleys, thus isolating ancestral populations of freshwater species ( Means 2000). We suspect the range of S. sphagnicola is similar to that of E. sphagnicola , which also inhabits the sphagnum-lined margins of streams and associated seepage habitats ( Wray et al. 2017).
Siren sphagnicola has a smaller geographic distribution than other Siren species. Most specimens have been found in the Blackwater,Yellow, and Escambia/Conecuh river drainages of Florida and Alabama ( Fig. 14 View FIGURE 14 ). Elsewhere, its range is poorly known, but we believe it is restricted to the environs of sandy, seepage-fed creeks in the lower Gulf Coastal Plain as far west as the Florida Parishes of Louisiana ( Fig. 14 View FIGURE 14 ). Locality information from outside Florida is entirely based on preserved AUM specimens and sequence data from a GenBank specimen that match both mtDNA and scnDNA markers. Few Siren museum vouchers with genetic material exist from Mississippi (42 total specimens via Vertnet search and only one with available tissue, which we sequenced) and the Florida Parishes of Louisiana (63 total via Vertnet search; one of two tissues requested yielded DNA), and we did not examine most museum specimens from this area that lacked tissue samples.
Etymology and common name. The specific epithet is derived from Sphagnum, the generic name for sphagnum moss, and the Latin suffix -cola, meaning inhabitant or dweller. The species epithet is used as noun in apposition. This siren is frequently found in and under mats of Sphagnum in and along streams and the margins of other bodies of water. Because of its affinity for seepage-fed streams and wetlands, we suggest Seepage Siren as the common name.
Specimens examined. See Supplemental Table 1 View TABLE 1 .
Enge, K. M. (2005) Herpetofaunal drift-fence surveys of steephead ravines in the Florida panhandle. Southeastern Naturalist, 4 (4), 657 - 678. https: // doi. org / 10.1656 / 1528 - 7092 (2005) 004 [0657: HDSOSR] 2.0. CO; 2
Means, D. B. (1981) Steepheads: Florida's little-known canyon lands. ENFO (Florida Conservation Foundation), 81 (6), 1 - 4.
Means, D. B. (2000) Southeastern U. S. Coastal Plain habitats of the Plethodontidae: the importance of relief, ravines, and seepage. In: Bruce, R. C., Jaeger, R. J. & Houck, L. D. (Eds.), The Biology of Plethodontid Salamanders. Plenum, New York, New York, pp. 287 - 302. https: // doi. org / 10.1007 / 978 - 1 - 4615 - 4255 - 1 _ 14
Moler, P. E. (1985) A new species of frog (Ranidae: Rana) from northwestern Florida. Copeia, 1985 (2), 379 - 383. https: // doi. org / 10.2307 / 1444847
Moler, P. E. (2019 b) Pseudobranchus axanthus Nettingi and Goin 1942, Southern Dwarf Siren. In: Krysko, K. L., Enge, K. M. & Moler, P. E. (Eds.), Amphibians and Reptiles of Florida. University of Florida Press, Gainesville, Florida, pp. 124 - 126.
Noble, G. K. & Marshall, B. C. (1932) The validity of Siren intermedia Le Conte, with observations on its life history. American Museum Novitates, 532, 1 - 17.
Strahler, A. N. (1964) Section 4 - II. Geology. Part II. Quantitative geomorphology of drainage basins and channel networks. In: Te Chow, V. (Ed.), Handbook of Applied Hydrology. McGraw-Hill, New York, New York, pp. 39 - 76.
Wray, K. P., Means, D. B. & Steppan, S. J. (2017) Revision of the Eurycea quadridigitata (Holbrook 1842) complex of dwarf salamanders (Caudata: Plethodontidae: Hemidactyliinae) with a description of two new species. Herpetological Monographs, 31 (1), 18 - 46. https: // doi. org / 10.1655 / HERPMONOGRAPHS-D- 16 - 00011
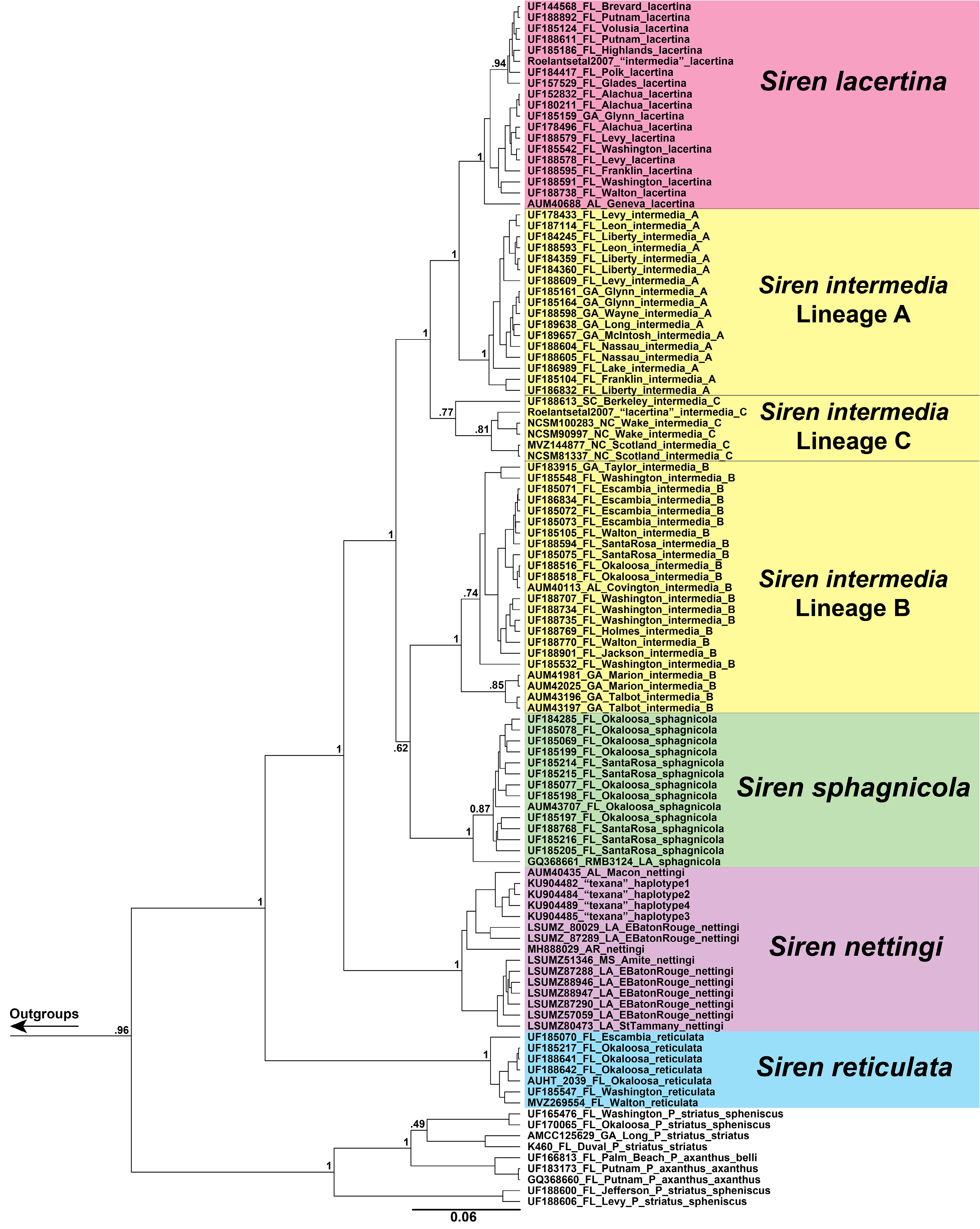
FIGURE 1. Bayesian probability tree of concatenated 16S and ND4 regions inferred using BEAST2. Individual specimen labels include voucher ID or GenBank number if no voucher, state, county, and lineage.
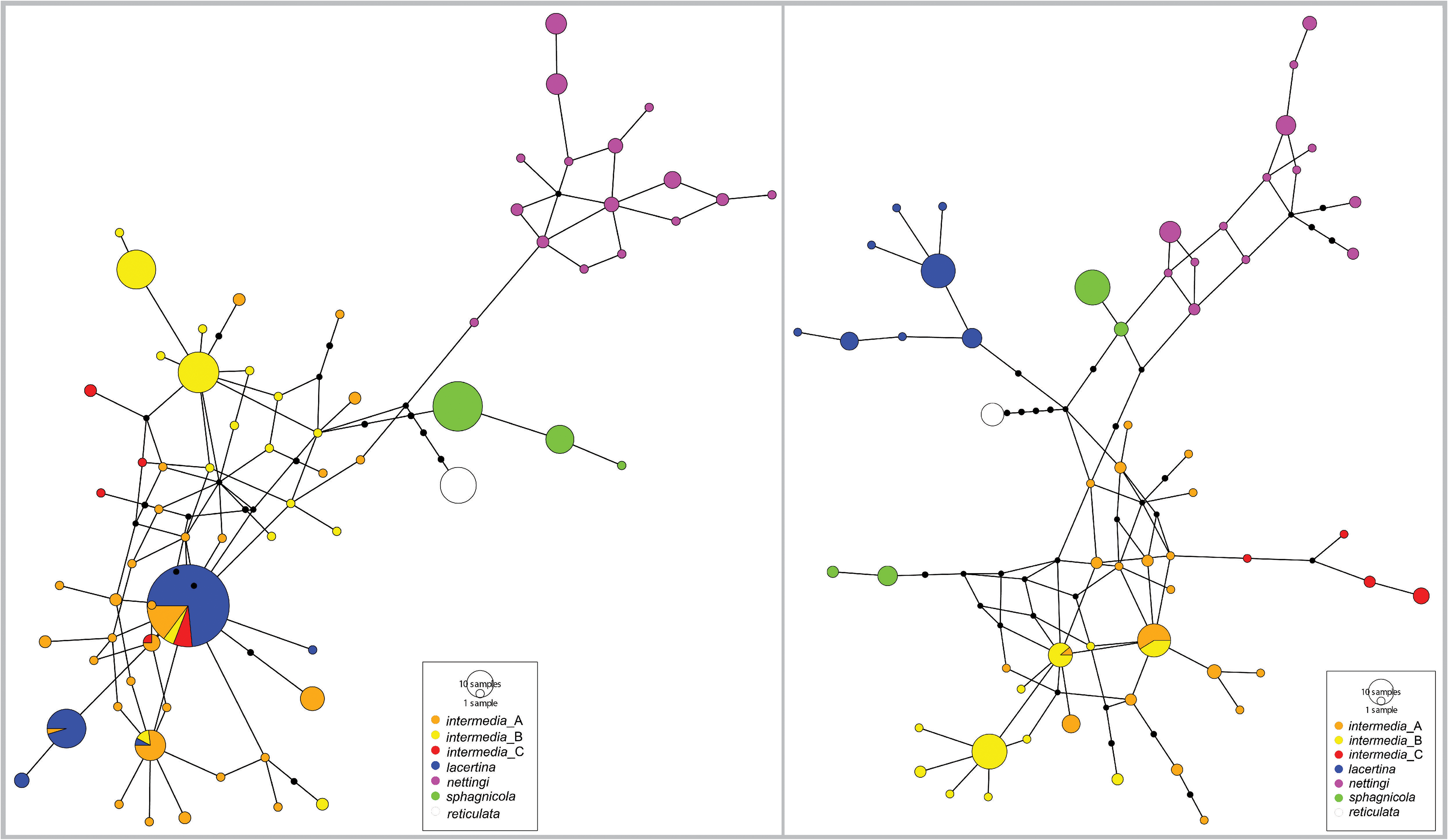
FIGURE 2. Median joining networks of Siren haplotypes for RAG1 (left) and NCX1 (right) regions made using PopART.
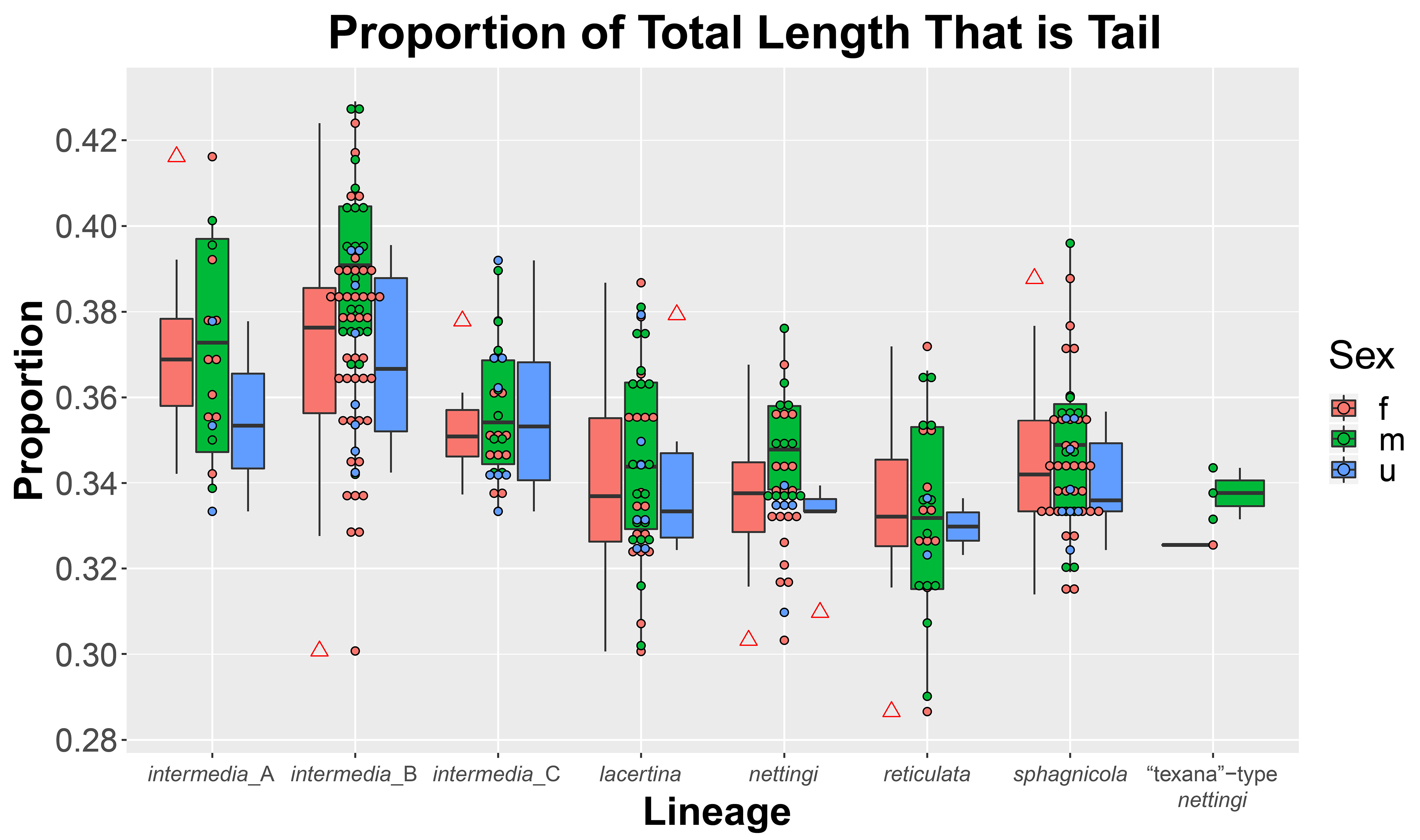
FIGURE 3. Box plots with points colored by sex showing distribution of tail proportions of Siren lineages. Outliers are noted with red triangles on the associated box plot.
FIGURE 4. Box plots with points representing specimens per showing distribution of costal groove counts of Siren lineages.
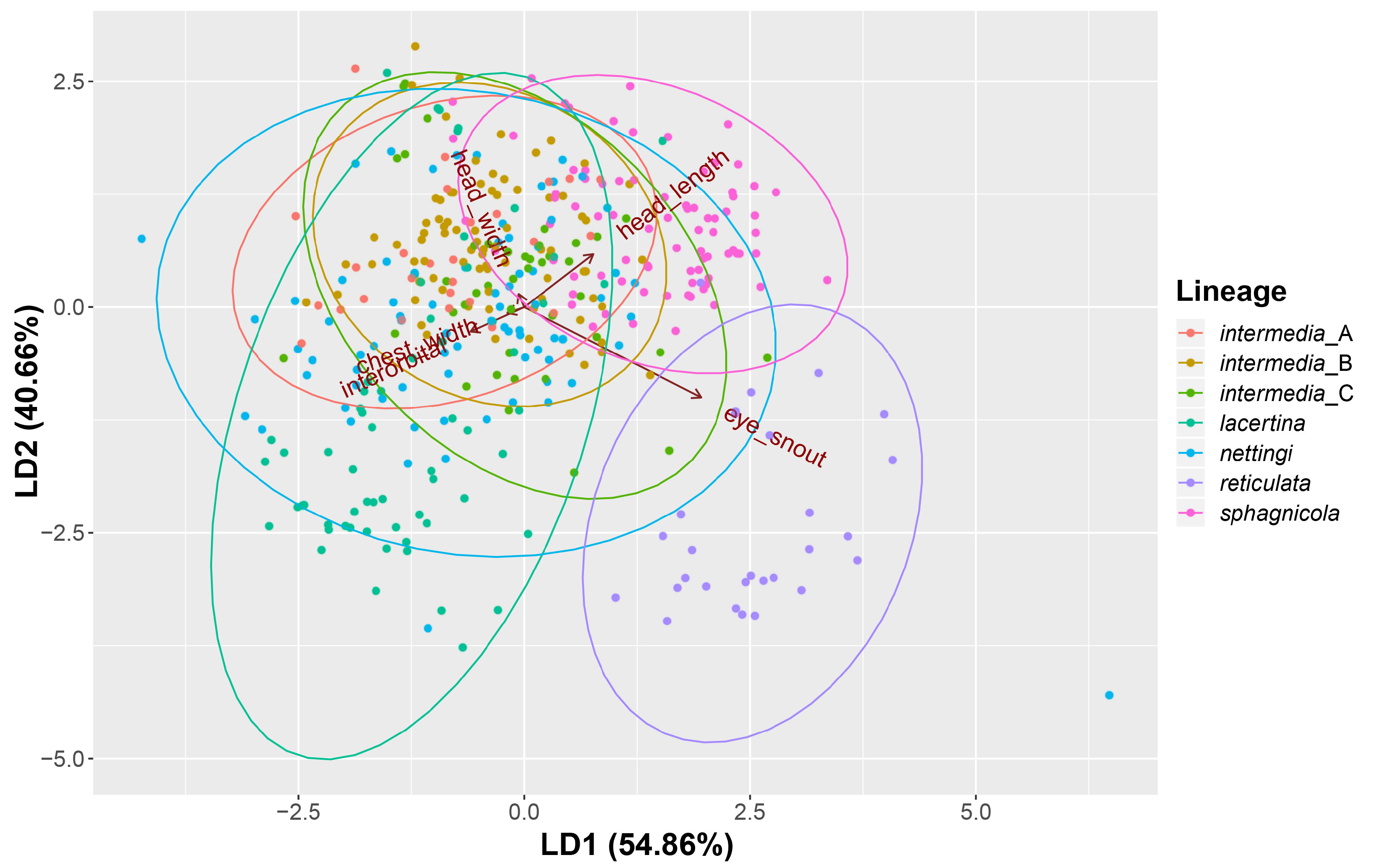
FIGURE 5. Box plots of the distributions of snout-vent length and the five size-corrected linear measurements compared using linear discriminant analysis.
FIGURE 6. Results from our linear discriminant function analysis based on the five size-corrected linear measurements taken from 396 Siren specimens. Except for S. reticulata, each lineage failed to create meaningful separation.
FIGURE 8. A Subadult Siren lacertina from McIntosh Co., Georgia (UF Herp 190368). B Subadult S. lacertina from Alachua Co., Florida. C Large juvenile S. lacertina from Alachua Co., Florida. D Adult S. lacertina from Levy Co., Florida.
FIGURE 10. Live Siren intermedia specimens belonging to mtDNA lineages B and C. A Lineage C from Berkeley Co., South Carolina (UF Herp 188612). B Lineage C from Berkeley Co., South Carolina (UF Herp 188613). C Lineage B from Holmes Co., Florida (UF Herp 188769). D Lineage B from Jackson Co., Florida (UF Herp 188901). E Head of lineage B specimen from Holmes Co., Florida (UF Herp 188769). F Head of lineage C specimen from Berkeley Co., South Carolina (UF Herp 188612) (lineage C). G Head of lineage C specimen from Berkeley Co., South Carolina (UF Herp 188613). The latter two specimens are from the same locality but differ dramatically in pattern and coloration.
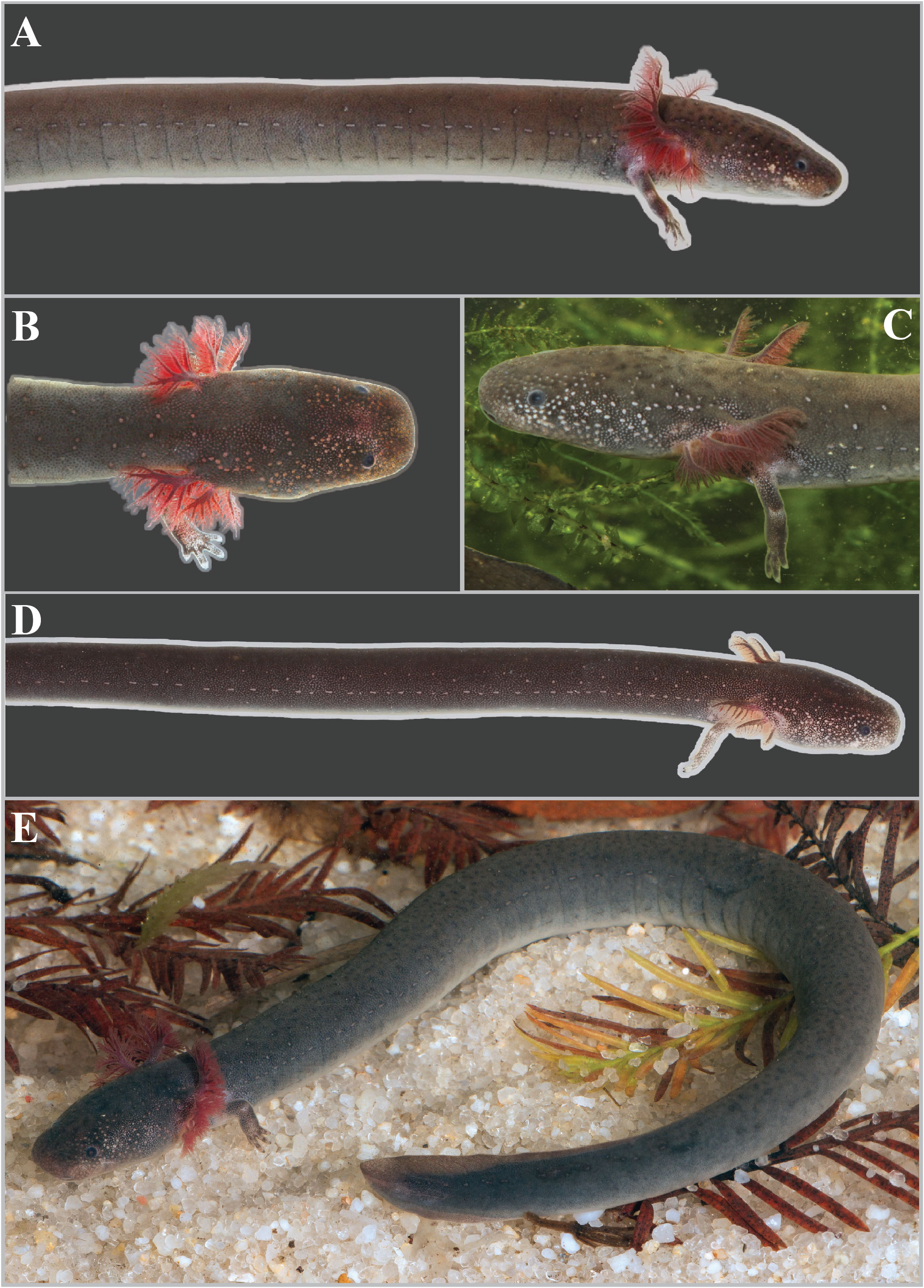
FIGURE 11. Live Siren sphagnicola specimens.A Holotype specimen from Okaloosa Co., Florida (UF Herp 185209). B Dorsal view of an adult found in shallow water where specimens were buried in the sandy bottom. C Dorsolateral view of an adult found in a deeper creek with deep muck deposits where specimens were found by digging through sphagnum root masses and fluid muck. D Juvenile from a shallow, sand-bottomed creek. E Adult hypertrophic male with a partially regenerated tail.
FIGURE 12. Siren sphagnicola habitats. A Type locality at Junior Walton Pond, Okaloosa Co., Florida, showing the feeding stream channel lined with thick muck and vegetation. B Type locality at Junior Walton Pond, Okaloosa Co., Florida, showing sphagnum mats and assorted vegetation covering a thick layer of muck and root masses. C Surface of a sphagnum bog on Bull Pen Branch, Okaloosa Co., Florida, where dense vegetation and 60 cm of muck provide ideal habitat for S. sphagnicola. D Seep at the head of Krul Lake, Santa Rosa Co., Florida, where shallow surface water flows over 15‒90 cm of fluid muck covered by grassy vegetation and sphagnum mats. E & F Hillside seepage pools along Carr Spring Branch, Okaloosa Co., Florida, where intermittent pools are deceptively deep and filled with fluid muck, like many other localities inhabited by S. sphagnicola.
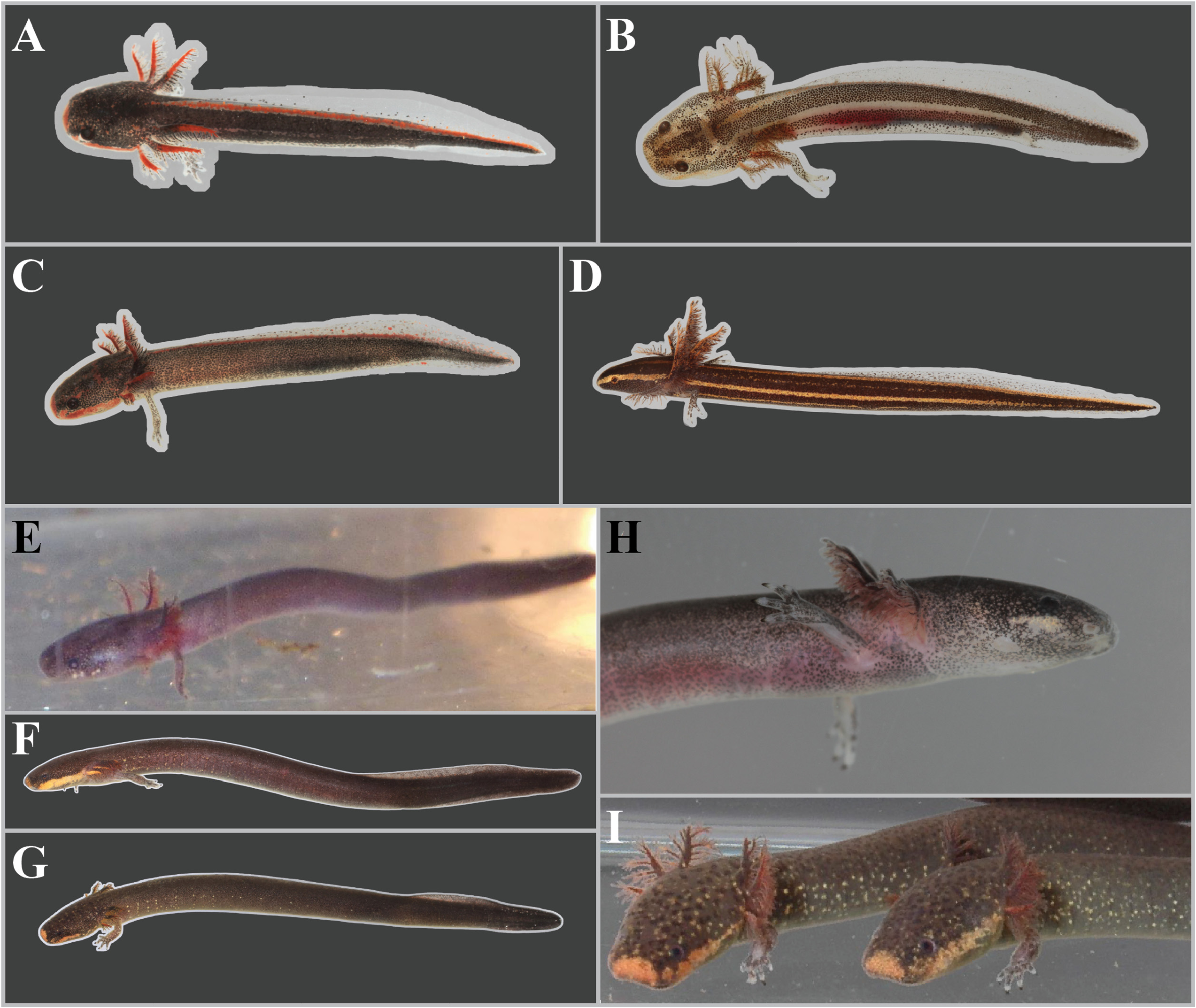
FIGURE 13. A comparison of larval and juvenile sirens. A Larval lineage B Siren intermedia from Escambia Co., Florida. B Larval S. lacertina from Jefferson Co., Florida (UF Herp 188601). C Larval lineage A S. intermedia from Liberty Co., Florida, that is slightly farther along in development than UF Herp 188601. D Larval Pseudobranchus striatus spheniscus from Jefferson Co., Florida (UF Herp 188600). E Juvenile S. sphagnicola (~3 cm TL) from Okaloosa Co., Florida, no voucher. F Juvenile S. lacertina from Walton Co., Florida (UF Herp 188739). G Juvenile lineage B S. intermedia from Washington Co., Florida (UF Herp 188735). H Head of a juvenile S. sphagnicola from Okaloosa Co., Florida (UF Herp 185068. I Juvenile lineage B S. intermedia from Escambia Co., Florida (UF Herp 185073 & UF Herp 185074).
FIGURE 14. Distribution map of specimens assigned to Siren sphagnicola. Red triangles represent localities with museum voucher specimens. Species of these specimens were determined using sequence data if tissue samples were available or by comparing costal groove counts and patterns to data from sequenced specimens. The yellow triangle represents a locality with genetic data only.
| UF |
Florida Museum of Natural History- Zoology, Paleontology and Paleobotany |
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.