Tripyla bioblitz, Zhao, Zeng Qi, 2009
|
publication ID |
https://doi.org/ 10.5281/zenodo.191403 |
|
DOI |
https://doi.org/10.5281/zenodo.5678171 |
|
persistent identifier |
https://treatment.plazi.org/id/03B8842B-FF9F-FFCE-FF11-F8B2CC35FCB7 |
|
treatment provided by |
Plazi |
|
scientific name |
Tripyla bioblitz |
| status |
sp. nov. |
Tripyla bioblitz sp. nov.
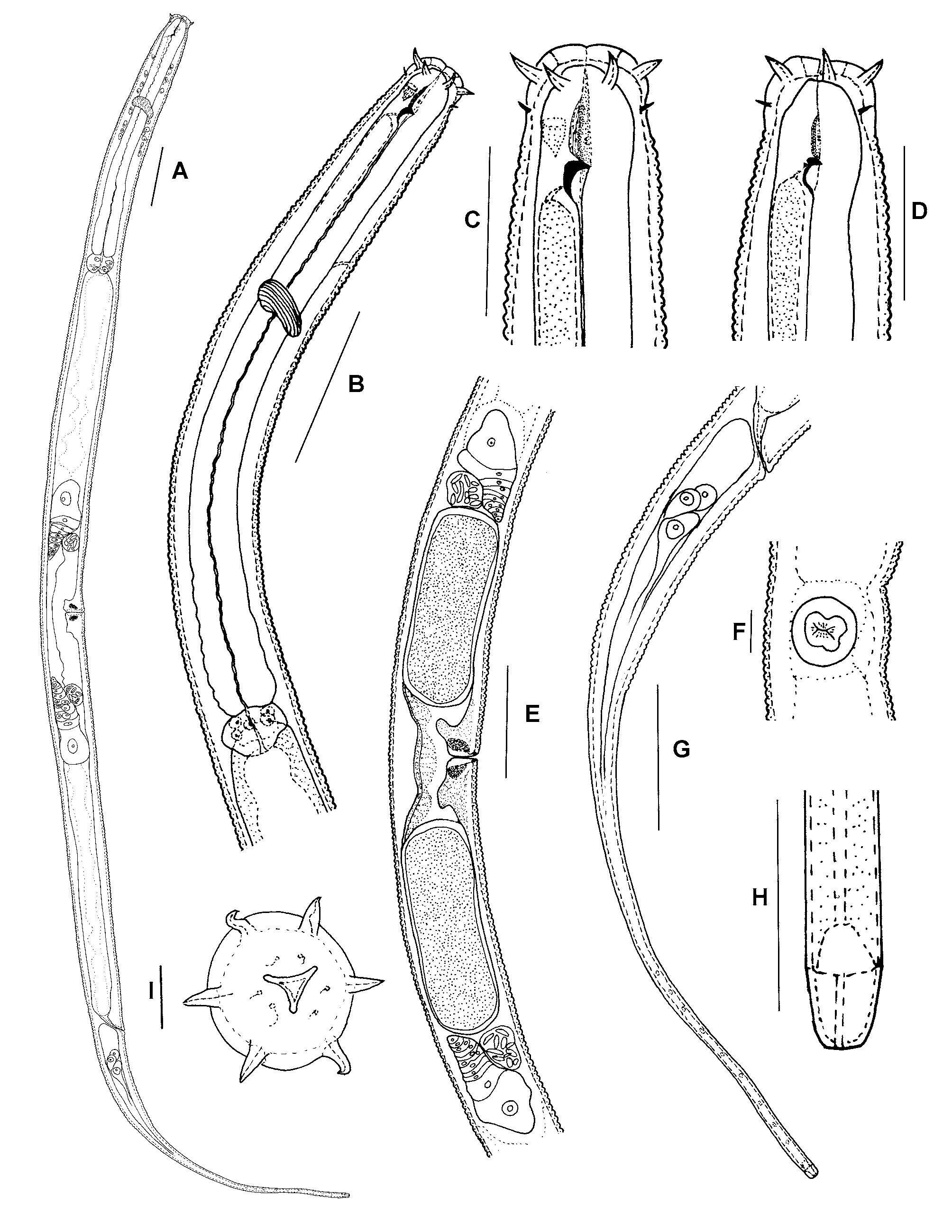
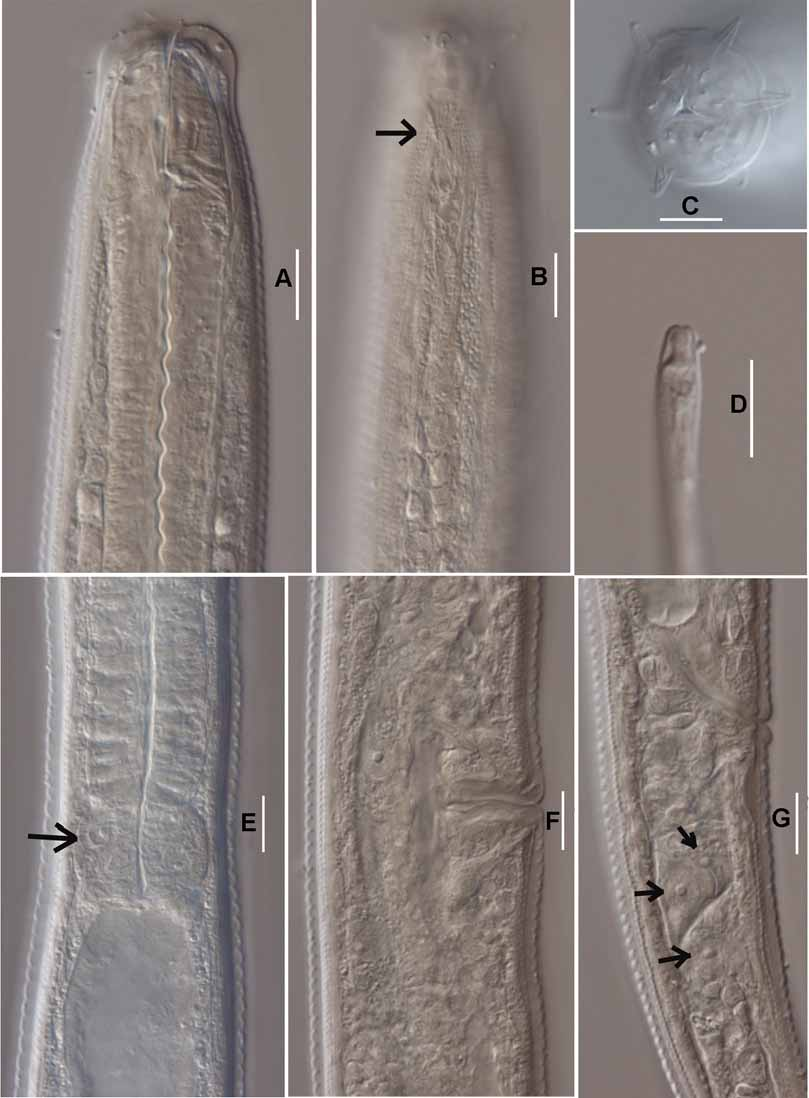
( Figs 1 View FIGURE 1 , 2 View FIGURE 2 )
Measurements. Table 1 View TABLE 1 .
Holotype Paratype females Paratype females
female (Smith’s Bush) (Botanic Garden)
Mean ± S.D. (range) Mean ± S.D. (range) Material examined. Holotype: National Nematode Collection of New Zealand (NNCNZ), slide No. 264. Paratype: Seven females and seven juveniles. NNCNZ, slide Nos 2559–2572.
Description. Female. Body ventrally arcuate when fixed ( Fig. 1 View FIGURE 1 A), posterior more curved than anterior. Cuticle distinct, about 2–3 μm thick at mid-body part, cuticular annules 2–3 μm wide. Maximum body diameter generally at level of vulva, occasionally at the level of base of pharynx. Body pores not seen.
Head rounded, smooth, continuous with body contour, narrower than adjacent body, cuticle 3–4 μm thick ( Figs 1 View FIGURE 1 C, D; 2A). Labial papillae short and conical. Six long and four short cephalic setae arranged in two separate circles; six longer cephalic setae 6–7 μm long, or 28–35% of head diameter, more or less arcuate and directed anteriorly; four short setae 3–4 μm long and thinner compared with the six longer cephalic setae, more or less arcuate near tip, situated nearly one length of a longer seta from the anterior circle. Stoma walls thickened distinctively, dorsal tooth large, hook shaped, triangular; two tiny subventral teeth in stomatal chamber 4–5 μm anterior to dorsal tooth ( Fig. 1 View FIGURE 1 C). Amphids cup-like with transverse oval opening, 8–19 μm from anterior end ( Figs 1 View FIGURE 1 C; 2B).
Excretory pore 70–73 μm, or 27–30% of pharyngeal length, from anterior end ( Fig. 1 View FIGURE 1 B). Nerve ring 87– 98 μm, or 36–40% of pharyngeal length, from anterior end. Pharynx cylindrical, muscular, 233–254 μm long. Three prominent cells located at the pharyngeal-intestinal junction ( Figs 1 View FIGURE 1 B; 2E). Coelomocytes not seen. Female genital system amphidelphic, gonad lying ventro-lateral to intestine, 233–337 μm long, or 19–25% of body length between points of flexure ( Fig. 1 View FIGURE 1 E). Ovaries reflexed 1/3–1/2 of the way back to vulva. Eggs present in female reproductive systems ( Fig. 1 View FIGURE 1 E). Vulva simple, without protuberant lips, vagina occupying one-fourth to one-third of corresponding body diameter, pore-shaped in lateral view ( Figs 1 View FIGURE 1 E; 2F), in ventral view vulva appears to have an X-shaped opening ( Fig. 1 View FIGURE 1 F), sclerotised pieces seen in the vaginal area.
Intestine showing undulating walls and a broad lumen. No distinct prerectum. Rectum less than anal body diameter (23 vs 26 μm). Tail tapering in anterior part reaching nearly to middle point, then becoming cylindrical ( Fig. 1 View FIGURE 1 G), c = 4.0–4.4 ( Table 1 View TABLE 1 ). Numerous tiny dots evenly distributed on posterior part of middle field of tails ( Fig. 1 View FIGURE 1 G). Three tandem caudal glands ( Fig. 1 View FIGURE 1 G); spinneret terminal, 4–6 μm long ( Figs 1 View FIGURE 1 H; 2D).
Male. Not known.
Locality and habitat. Holotype and six paratypes (NNCNZ slide nos 2559–2561 females; 2562–2564 juveniles) from soil and litter mixture, 0–10 cm depth under a native tree, Dacrycarpus dacrydioides (A. Rich.) de Laub. (common names: Kahikatea, white pine), surrounded by several trees, Geniostoma ligustrifolium A. Cunn. , Smith’s Bush, North Shore City, Auckland, New Zealand (36º 48.782 S, 174º 45.026 E), coll. Zeng Qi Zhao, 4. iv. 2008; eight paratypes (NNCNZ slide nos 2565–2568 females; 2569–2572 juveniles) from soil and litter mixture, 0–10 cm depth under D. dacrydioides , Auckland Botanical Garden, South Auckland, New Zealand (37˚ 0.657 S, 174˚ 54.491 E), coll. Zeng Qi Zhao, 23. iv. 2008.
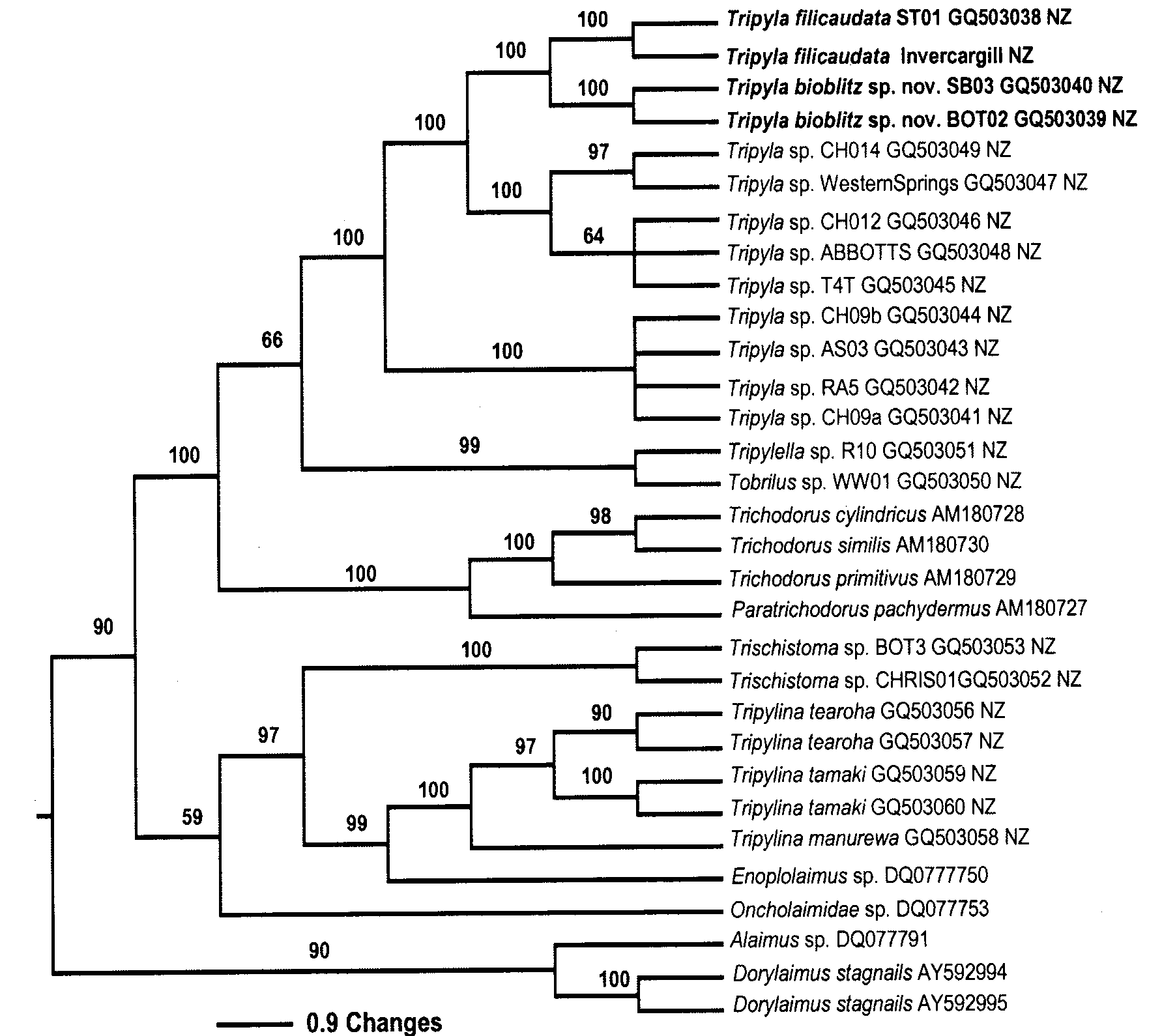
Diagnosis and relationships. The genus Tripyla is divided into two groups—some with “long-tails” (c <5) and others with “short-tails” (c>5.9) ( Tsalolikhin 2003). T. bioblitz sp. nov. belongs to the former group, bringing its members to eight (Table 2).
TABLE 2. Comparative morphometrics of eight long-tailed Tripyla species.
L of female Spi- Guber-
Species a b c c’ V/T% References
(µm) cule naculum
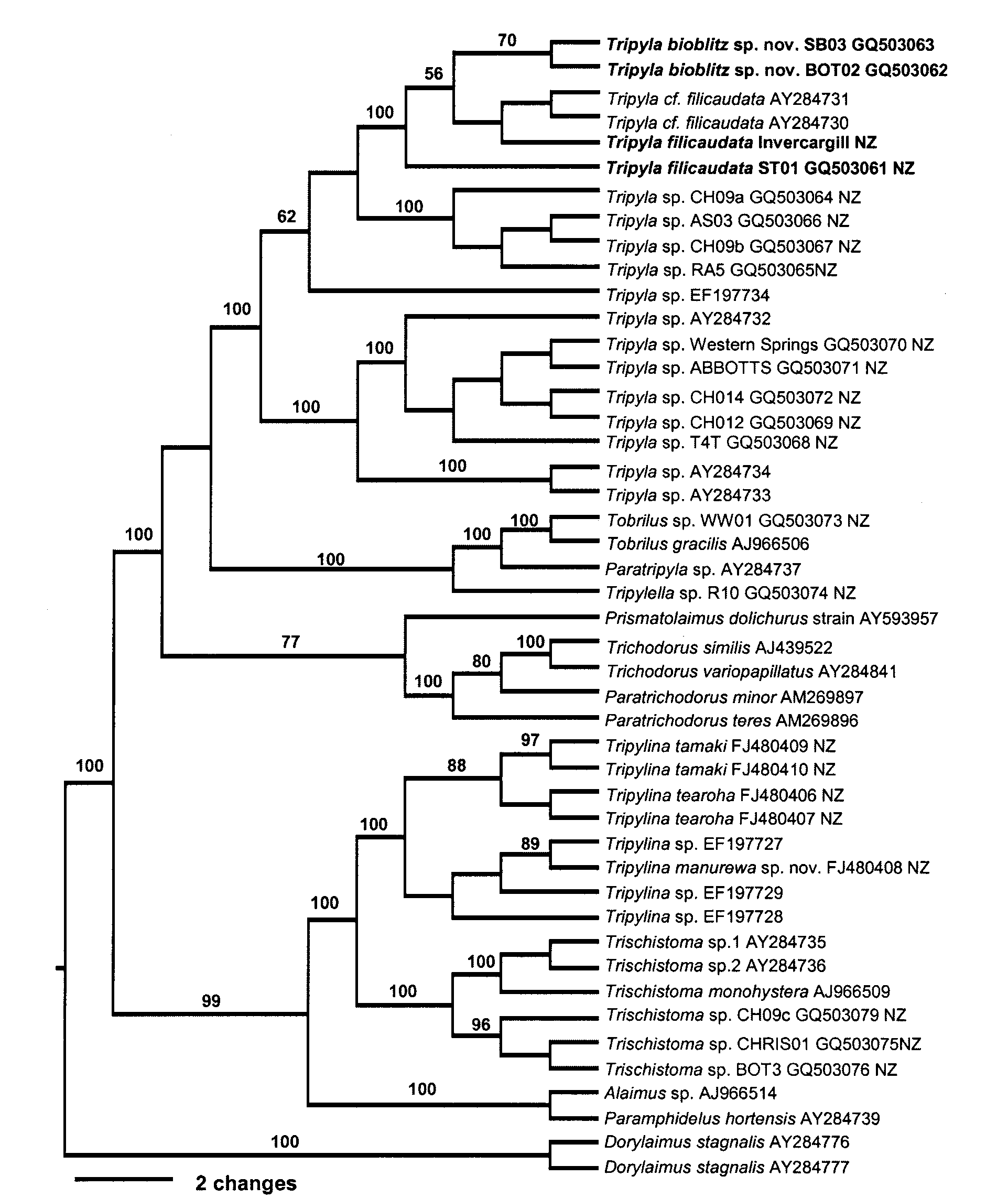
Females 2402 55.2 6.2 4.3 48 Tsalolikhin italica 1952 –2686 40–64.8 5.4–6.8 3.6–4.8 45–51 2003 Tripyla bioblitz sp. nov. is characterized by its short body length (L = 1150–1410 μm) and anterior vulva (V = 44–45%) position. Its status as a distinct species is confirmed by molecular data from sequencing of SSU and LSU ( Figs 5 View FIGURE 5 , 6 View FIGURE 6 ).
Six species in the long-tails group of Tripyla have body lengths greater than 1490 μm, so that T. bioblitz sp. nov. (1150–1410 μm) is easily differentiated from them (Table 2). T. bioblitz sp. nov. overlaps the body length of T. filicaudata but differs from it in vulva position (44–45 vs 46–53%) (see Table 2).
Etymology. The name BioBlitz is applied to a series of public scientific events in New Zealand and other countries. It is used here as a noun in apposition.
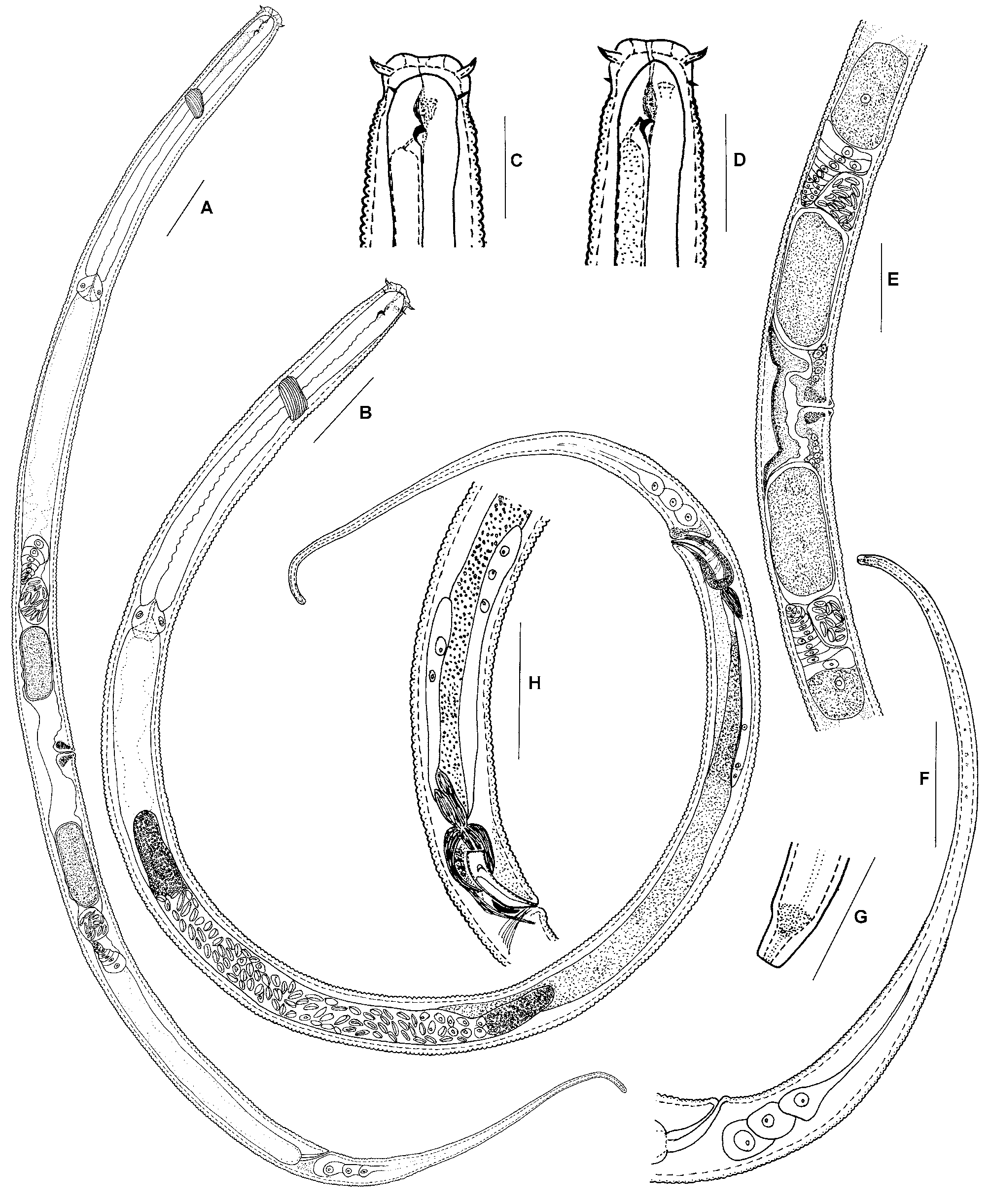
Remarks. Some T. bioblitz sp. nov. were collected that looked like normal adult females except: 1) the vulval opening was covered by cuticle; 2) body length and diameter were smaller than in normal adult females; 3) the shape of the dorsal tooth differed from that of adults, i.e., a small tooth can be clearly observed at the dorsal part of dorsal tooth which is not found in normal adult female; and 4) the stoma wall was thinner than in normal adult females ( Fig. 1 View FIGURE 1 D). The reproductive system of these nematodes appeared to be fully developed, but no eggs were observed. It is probable that these nematodes were mature fourth stage juveniles. Here, they are referred to as ‘sub-females’ ( Fig. 1 View FIGURE 1 D), and were also seen in T. filicaudata ( Fig. 3 View FIGURE 3 D).
Species of Tripyla are thought to be predacious ( Goodey 1963; Yeates et al. 1993). The biology of T. bioblitz sp. nov. is unknown. However, some prey debris (possibly nematode or rotifer) was observed in the intestine of some specimens examined here, confirming that it is a predator.
TABLE 1. Morphometric data for Tripyla bioblitz sp. nov. (measurements in μm ± S. D.)
| n | 1 | 3 | 4 |
|---|---|---|---|
| a b | 30.3 5.4 | 30.8 ± 1.5 (29.4–32.4) 5.2 ± 0.3 (4.9–5.5) | 31.1 ± 0.7 (30.4–31.7) 5.3 ± 0.2 (5.0–5.5) |
| c | 4.3 | 4.2 ± 0.2 (4.0–4.4) | 4.3 ± 0.1 (4.2–4.4) |
| c’ | 11.0 | 11.1 ± 0.5 (10.5–11.4) | 11.5 ± 0.9 (10.1–12.3) |
| V | 44.9 | 44.6 ± 0.9 (43.7–45.4) | 44.7 ± 0.5 (44.1–45.2) |
| Body length Head diameter | 1301 19 | 1266.2 ± 103.3 (1150–1348) 20.2 ± 0.3 (19–20) | 1269.8 ± 99.9 (1174–1410) 22.1 ± 1.5 (21–24) |
| Body diameter Dorsal tooth from anterior | 43 21 | 41.2 ± 5.0 (35–44) 22.1 ± 1.3 (21–23) | 40.9 ± 4.0 (37–46) 20.7 ± 0.5 (20–21) |
| Excretory pore from anterior | 74 | 73.0 ± 2.2 (70–76) | 70.6 ± 0.9 (70–71) |
| Vulva from anterior | 585 | 564.6 ± 36.6 (523–589) | 569.9 ± 47.5 (522–635) |
| Pharynx length Amphid from anterior | 239 12 | 242.7 ± 7.7 (234–248) 13.0 ± 5.0 (8–19) | 240.6 ± 9.6 (234–255) 10.9 ± 1.2 (9–12) |
| Nerve ring from anterior | 88 | 90.0 ± 3.3 (87–93) | 94.2 ± 3.2 (91–98) |
| Tail length | 303 | 299.9 ± 15.2 (286–316) | 296.9 ± 29.9 (271–340) |
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |