Limnodrilus sulphurensis Fend, Liu & Erséus, 2016
|
publication ID |
https://doi.org/ 10.11646/zootaxa.4066.4.6 |
|
publication LSID |
lsid:zoobank.org:pub:87790552-1DFF-41DF-B6C4-5BD43D19E998 |
|
DOI |
https://doi.org/10.5281/zenodo.5624975 |
|
persistent identifier |
https://treatment.plazi.org/id/03B77177-FFEE-A26A-FF25-ACA9FFA3FC6E |
|
treatment provided by |
Plazi |
|
scientific name |
Limnodrilus sulphurensis Fend, Liu & Erséus |
| status |
sp. nov. |
Limnodrilus sulphurensis Fend, Liu & Erséus n. sp.
( Figures 1–3 View FIGURE 1 View FIGURE 2 View FIGURE 3 )
Holotype. DMNS ZE.46275, a whole mounted, sexually mature and DNA barcoded specimen; i.e., a small posterior part of the worm was used for DNA extraction. COI barcode = haplotype 1 (CE10482, GenBank KT692957 View Materials ; see also "Barcodes" below).
Type locality. Colorado, Routt Co., City of Steamboat Springs, Sulphur Cave, high H2S stream in dark zone, W106.841050°, N40.84759°. 11-Apr-2010. Collected by David Steinmann and Fred Luiszer.
Paratypes. All from the type locality. DMNS ZE.46276–46280: 11-Apr-2010, 2 whole-mounts, 3 dissected. SMNH Type 8779, 20-Sep-2008, 1 whole-mounted, mature specimen with COI barcode (CE7491, haplotype 2, GenBank KT692956 View Materials ). USNM 1283518, 18-Aug-2007, 1 whole-mount, mature, with COI barcode (CE7489, haplotype 1); USNM 1283512-1283516, 11-Apr-2010, 2 whole-mounts, 3 dissected; USNM 1283517, 1-Aug- 2009, 1 sagittally sectioned. CASIZ 197459, 1-Aug-2009, 1 whole-mount; CASIZ 197460, 11-Apr-2010, 1 wholemount, 3 dissected. ZMH Ol 15446 -15450, 1-Aug-2009, 2 whole-mounts; 11-Apr-2010, 3 dissected. All collected by David Steinmann and Fred Luiszer.
Additional material. The type locality, 18-Aug-2008, SMNH 150175 (CE7486); 20-Sep-2008, SMNH 150176 (CE7490); both whole-mounts with COI barcodes (haplotype 2). 9-Mar-2009, 10 whole-mounts (6 immature), 1 sagittally sectioned. 1-Aug-2009, 5 whole mounts. 11-Apr-2010, 5 whole mounts, 12 dissected. Colorado, Routt Co., Surface spring near Sulphur Cave, 11-Apr-2010, 4 whole mounts (immature). SMNH 150177, 11-Apr-2010, 1 whole-mount, immature, with COI barcode (CE10479, haplotype 1). Colorado, Routt Co., Black Sulphur Springs, 11-Apr-2010, 1 whole mount, 1 whole mount with COI barcode ( SMNH 150178, CE10477, haplotype 1). All collected by David Steinmann and Fred Luiszer.
Etymology. Named for the type locality.
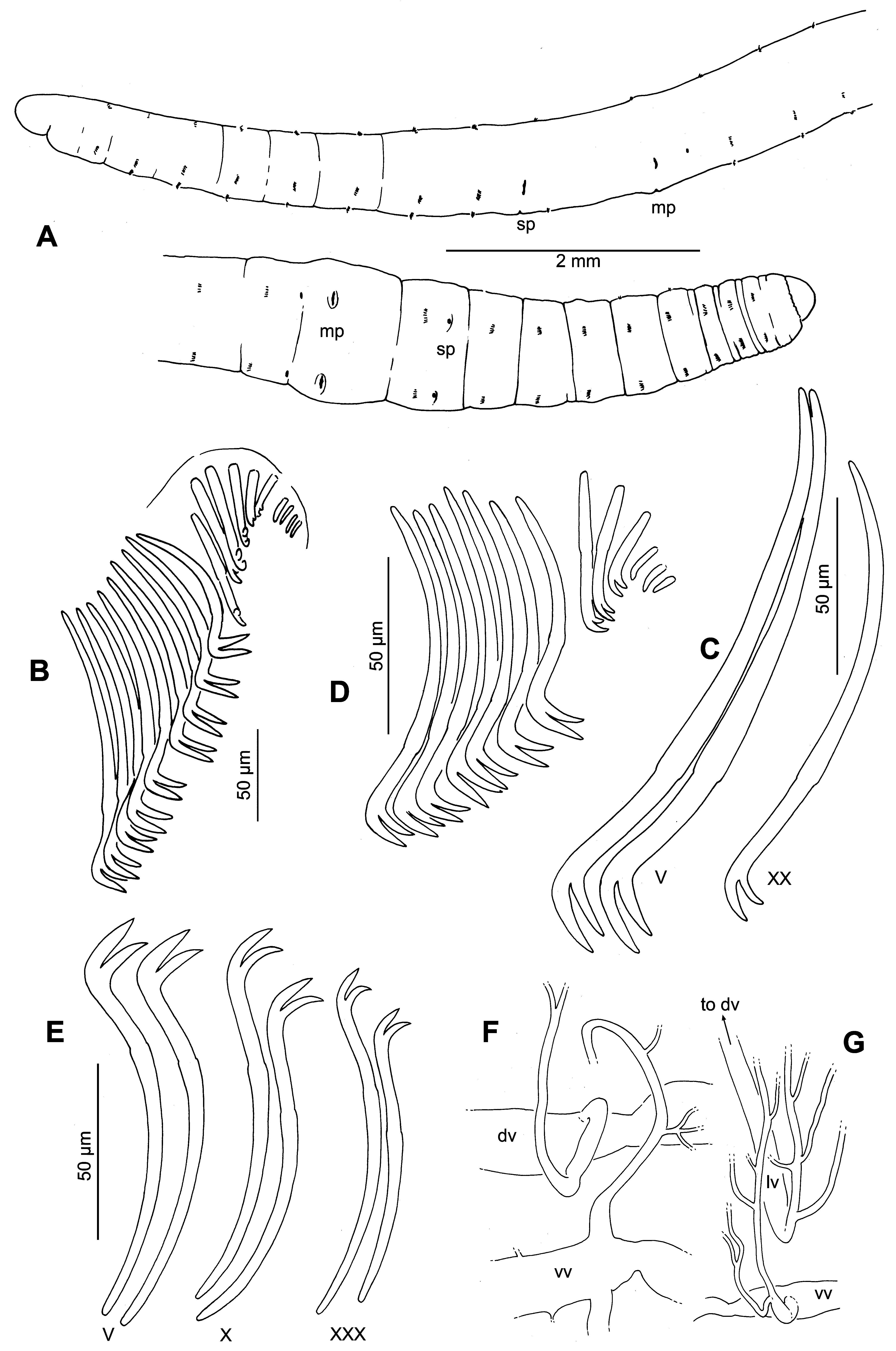
Description. Specimens relaxed in dilute alcohol and fixed in FAA 18–25 mm long; formalin-preserved worms more contracted, 12–19 mm. Width 0.52–0.99 mm in VIII, to 1.05 mm in XI, tapering in posterior segments. 51–78 (median 64) segments. Prostomium rounded, slightly wider than long in FAA-preserved worms; broadly conical in formalin-fixed specimens ( Fig. 1 View FIGURE 1 A). Clitellum weak in all available material, X–XII.
Segments II–VII or VIII with 5–11 fully developed chaetae per bundle (median 7 dorsal, 8 ventral), and a similar but indeterminate number of partially developed replacement chaetae, all in a descending, fan-like series ( Fig. 1 View FIGURE 1 B). Number of chaetae per bundle decreasing posteriorly; by XX 2–3, posterior segments at most 1–2; most specimens with few or no chaetae from about the posterior 1/4 of the worm. All chaetae bifid, sigmoid, with variably developed nodulus at about the distal 1/3 ( Fig. 1 View FIGURE 1 B–E). Chaetae in anterior part of body (to about X) with teeth approximately equal in length and thickness; teeth long (12–20 µm), nearly straight (never strongly hooked), and nearly perpendicular to the distal part of the shaft ( Fig. 3 View FIGURE 3 A–C). Chaetae gradually smaller, and teeth shorter and less strongly angled relative to shaft in posterior segments ( Figs. 1 View FIGURE 1 C–E, 3D). Ventral chaetae absent in XI in mature worms; no modified spermathecal chaetae in X. Chaetal length 84–185 µm in II–X (maximum length in IV–VI); 90–125 in XI–XX; 50–100 µm in posterior segments. In anterior (preclitellar) segments, the ventral chaetae about 30% longer and slightly thicker than the corresponding dorsal ones ( Fig. 1 View FIGURE 1 C vs. 1E).
Pharynx in II–III, weakly differentiated from esophagus; about equally developed dorsally and ventrally. Chloragogen cells dense on gut beginning in VI, with dark inclusions in worms fixed in the field ( Fig. 3 View FIGURE 3 E,F).
Branches of dorsal blood vessel join to form the ventral vessel at about 2/3. A well-developed supra-intestinal vessel from about 5/6, joining the dorsal vessel in IX; the conjoined "dorsal" vessel curving ventrad along the left side of the gut, and remaining in a lateral or even ventrolateral position until the posterior segments. Dorsal and ventral vessels connected by long, convoluted, commissural vessels in II–VII and in IX–XI. In VIII, commissures shorter and modified as "hearts"; these greatly dilated (to 150 µm), but not obviously muscular, and joining the supra-intestinal vessel instead of the dorsal. A network of fine peripheral, capillary blood vessels visible in posterior segments, extending into the epidermal layer ( Fig. 3 View FIGURE 3 F); relatively sparse beginning in about XXV, forming a dense network beginning in about XXXV. Branches of capillary vessels coalesce to a short ventrolateral vessel, which joins the ventral vessel near the posterior septum. A second main branch from the capillary blood vessels joins a pair of dorsolateral vessels extending from the dorsal vessel to both sides of the ventral body wall. These dorsolateral vessels join the dorsal vessel near the posterior septum, the one going to the right side passing over the gut; apparently no direct connection to the ventral vessel ( Fig. 1 View FIGURE 1 F,G).
Nephridia in XIII and many posterior segments; a convoluted duct covered with indistinct, granular cells forming a dense mass, extending ventrally through most of segment, terminating in a short ectal duct, which widens into a small vesicle at the nephridiopore, anterior to the ventral chaetae.
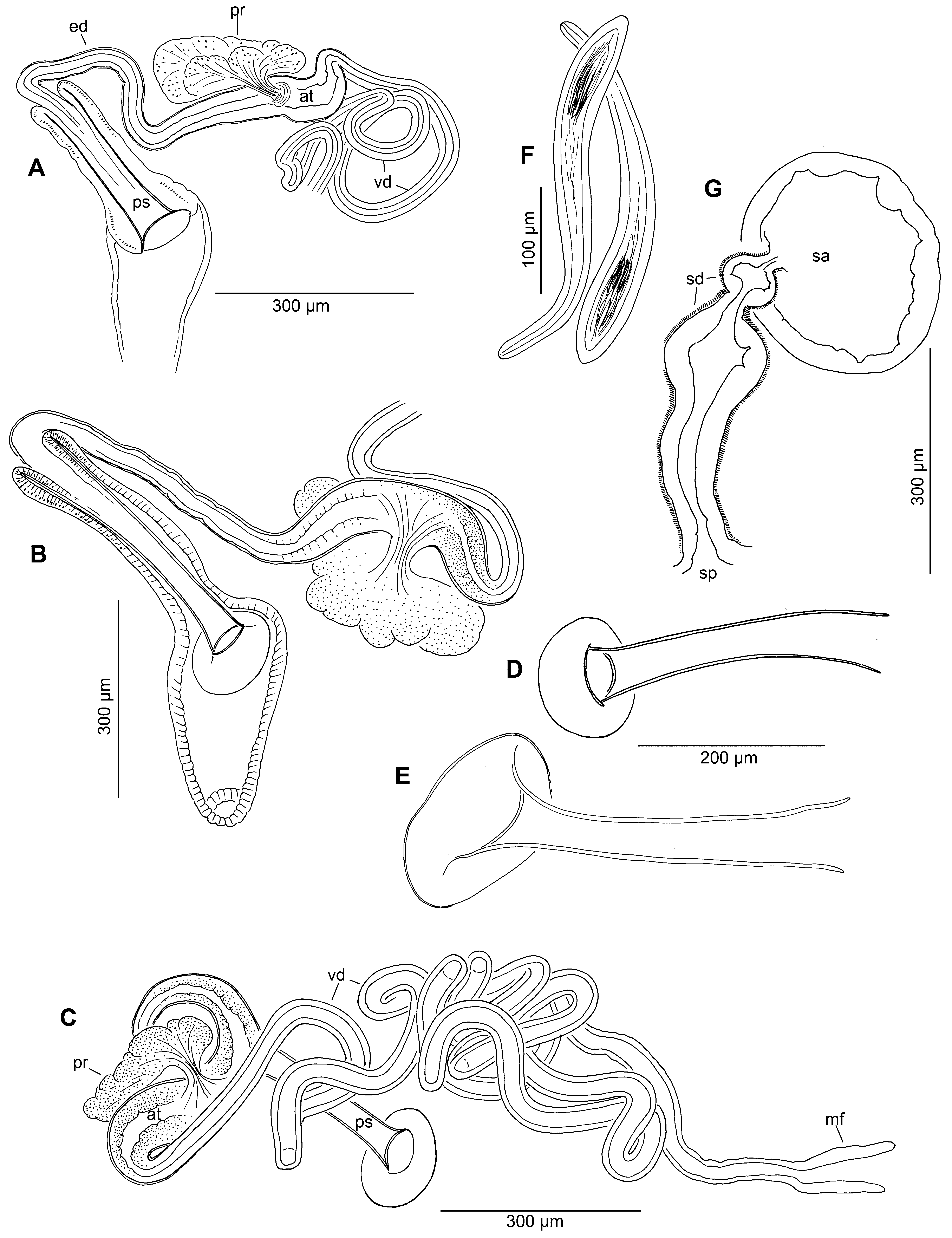
Male ducts and spermathecae paired. Male pores narrow, transverse openings, in line with ventral chaetae, at about the posterior 1/4 of segment XI ( Fig. 1 View FIGURE 1 A). Spermathecal pores prominent and transverse, in line with ventral chaetae, midway between chaetae in X and anterior septum (9/10). Male funnel 100–120 µm long, either narrowly conical with upper and lower lips about the same length, or with lips laterally spread; projecting into X from 10/11. Vas deferens very long, about 4000–6400 µm, diameter 22–48 µm, generally thinnest in formalin-preserved worms; of nearly uniform diameter and histology ( Fig. 2 View FIGURE 2 C). Vas deferens composed of a single layer of ciliated epithelium 7–10 µm thick, without obvious muscle layer. Atrium narrowly fusiform, narrowing gradually to vas deferens and ejaculatory ducts; length 240–490 µm, diameter to 65–94 µm ental to prostate attachment, 42–85 µm in ectal part ( Fig. 2 View FIGURE 2 A–C). Atrial epithelium ciliated, cells granular, irregular, indistinct in ental part; often more regular and less granular in ectal part ( Fig. 3 View FIGURE 3 H). Atrium joined near midpoint by a single, stalked prostate gland ( Figs. 2 View FIGURE 2 B, 3G,H); prostate a fan-like cluster of irregular lobes; entire mass 240–380 µm wide; prostatic cells granular and indistinct. Ectal (ejaculatory) duct of atrium 250–480 µm long by 26–38 µm wide, with a thin (5–7 µm), non-ciliated epithelium and indistinct muscle layer about 2–3 µm thick; appearing slightly wrinkled and less evenly cylindrical than vas deferens. Penis without obvious spiral muscles; penis sheath surrounded by a closefitting sac to 120–240 µm deep, consisting of columnar cells and a thin, indistinct muscle layer. Shaft of cuticular penis sheath nearly tubular, expanded slightly at ends; straight or somewhat curved in slide-mounted specimens ( Figs. 2 View FIGURE 2 D, 3I,J); wall thickness up to 3–4 µm. Sheath length 240–410 (median 333) µm; diameter in middle 34–49 µm, 43–82 µm at ental end, 40–60 µm at ectal end (below head); ratio of length to basal width 4.1–5.7 (median 4.9). Ectal end ("head") broad and plate-like, appearing flat or recurved ( Fig. 3 View FIGURE 3 I,K,L); plate circular and symmetrical, diameter 120–170 µm in fully mature worms. Plate may be indistinct, with very thin cuticle, particularly in unmated specimens ( Fig. 2 View FIGURE 2 A).
Ectal part of spermathecal duct about 200–300 µm long, thick and pyriform; widest entally, to 121–145 µm thick; ectally narrowing to 80–95 µm ( Fig. 2 View FIGURE 2 G). Duct composed of an outer, transverse-circular muscle layer, and an irregular epithelium ( Fig. 3 View FIGURE 3 M,O); lumen narrow ectally, wider entally. Duct surrounded by an irregular layer of cells. Remaining (ental) part of duct narrower ( Fig. 3 View FIGURE 3 O), but often medially inflated ( Fig. 2 View FIGURE 2 G); about 60–100 µm long. Spermathecal ampulla nearly spherical to irregular, sacciform. Spermatozeugmata 200–310 µm long, narrow, slightly widened at one end, to 37–55 µm ( Figs. 2 View FIGURE 2 F, 3N).
Barcodes. Data on the COI barcodes of seven specimens are summarized in Table 2 View TABLE 2 . Six of them were successfully sequenced to the standard length of 658 base pairs (bp), all barcodes covered positions 2–658 in the alignment. Two different haplotypes (Ht 1 and 2 in Table 2 View TABLE 2 ) within this interval were recognized, with variation (uncorrected p-distance 0.15%) only in one position (181). When blasting the haplotypes against the BOLD and NCBI/Genbank databases,>80% resemblance was found with several other North American clitellates, including a barcoded worm (about 82 % similar) identified as Limnodrilus hoffmeisteri (Genbank EF089358 View Materials ; http:// www.ncbi.nlm.nih.gov/nucleotide/). The closest match (90.21%) was between haplotype 1 and an unidentified " Tubificidae " collected in Manitoba, Canada, and in Early-Release status in BOLD (http://www.boldsystems.org/).
Remarks. The new species clearly belongs to Limnodrilus as defined by Brinkhurst (1971): vas deferens long and ciliated, atrium short with a single large prostate, ejaculatory duct long, leading to a thick, cylindrical penis sheath; genital chaetae absent, somatic chaetae all bifids; spermatozeugmata present in spermathecae; prominent coelomocytes absent.
Limnodrilus sulphurensis is most easily differentiated from congeners by the very long, sharply angled teeth of both ventral and dorsal chaetae in anterior segments. The distinctive chaetae are not restricted to mature worms, and are consistently seen in very small, immature specimens from Sulphur Cave ( Fig. 1 View FIGURE 1 D), as well as in worms from nearby surface springs. The large number and continuous progression of developing, replacement chaetae in anterior segments of L. sulphurensis are also unusual. Interestingly, Fig. 1 View FIGURE 1 A in Snimshchikova (1998) shows several replacement chaetae in anterior ventral bundles of Limnodrilus dybowskii ( Grube, 1873) .
Based on proportions and morphology of the penis sheath, the characters most often used to distinguish Limnodrilus species, L. sulphurensis appears closest to the widespread L. profundicola , and will key to that species using Brinkhurst (1971: 463). It also somewhat resembles the widespread Limnodrilus udekemianus Claparède, 1862 , the Jamaican Limnodrilus variesetosus Brinkhurst, 1979 , and a group of endemic Lake Baikal species associated with L. dybowskii .
Taxonomy of L. profundicola is problematic, due to a very limited original description and lack of useable type material; in the following we attempt to clarify some of these issues with observations on penis sheaths and chaetae in museum specimens and in new collections (see below). Based on new and previously published measurements, the penis sheath is similar in general shape and proportions to that of L. sulphurensis , but is smaller ( Table 1 View TABLE 1 , Fig. 4 View FIGURE 4 ), and the terminal expansion ("head") is smaller, but usually more distinct. The profundicola atrium is also smaller ( Table 1 View TABLE 1 ). Limnodrilus profundicola has rather ordinary chaetae for the genus, with short, nearly equal teeth. Chaetal bundles have a similar number of well-developed chaetae in the two species ( Table 1 View TABLE 1 ), although profundicola does not have the large number of developing chaetae seen in L. sulphurensis (see below; Fig. 1 View FIGURE 1 B vs. 4G). Chaetae of Limnodrilus alpestris Eisen, 1879 , and Limnodrilus monticola Eisen, 1879 —both taxa that have since been synonymized with profundicola ( Brinkhurst 1965) —are not well described.
There seems to be good agreement in the literature on the status and diagnostic characters of L. udekemianus . Penis sheaths of this species may have similar proportions to those of L. sulphurensis (length/width ratio 2–5 [ Kennedy 1969], or up to 4 [ Timm 2009]), but despite high variability, they are usually illustrated as shorter and broader, gradually expanded, and without a plate-like head ( Claparède 1862, Pl. I, Fig. 4 View FIGURE 4 ; Brinkhurst 1971, Fig. 8.4B; Kennedy 1969, Fig. 9). Nevertheless, the distal end may be variably expanded and reflexed ( Dzwillo 1984, Fig. 3 View FIGURE 3 ; Piguet 1913, Fig. 10a). The anterior chaetae of L. udekemianus are considered diagnostic: distal teeth are enlarged, thickened, and sometimes strongly curved, but the proximal teeth are of normal size, differing from those of L. sulphurensis (e.g., Kennedy 1969, Fig. 1 View FIGURE 1 ; Brinkhurst 1965, Fig. 4 View FIGURE 4 ). Timm (2009) also considers the long, thin posterior end of the body to be distinctive. Penis sheaths of L. variesetosus appear quite similar to those of L. udekemianus ; the anterior ventral chaetae also have a very long distal tooth, but posterior chaetae have a short, thin distal tooth ( Brinkhurst 1979, Figs. 1 View FIGURE 1 A,E; Rodriguez 2002, Fig. 2 View FIGURE 2 B,C).
The male duct of L. dybowskii , as illustrated in Fig. 2 View FIGURE 2 of Snimshchikova (1998), appears quite similar to that of L. sulphurensis ; measurements of dybowskii atria and penis sheaths (400–520 and 350 µm, respectively) are also similar. Two other Lake Baikal endemics, Limnodrilus nitens ( Semernoy, 1982) and Limnodrilus tendens ( Semernoy, 1982) appear to have similar penial morphology to dybowskii ( Semernoy 2004, Figs. 117,118); the latter was synonymized with dybowskii by Snimshchikova (1998). It should be noted that Semernoy (1982) compared the penis sheath of L. tendens to that of L. helveticus , and gave similar measurements for L. nitens . All three of the Baikal species are distinguished from congeners by enlarged and highly modified chaetae in some anterior ventral (but not dorsal) segments. The enlarged chaetae are fewer in number and simple-pointed (or with small distal tooth), and thus bear little resemblance to those of L. sulphurensis n. sp. ( Snimshchikova 1998, Figs. 1 View FIGURE 1 , 3 View FIGURE 3 ; Semernoy 2004, Figs. 116, 117, 118).
The material. The morphological description is largely based on material collected on three dates in March 2009 (fixed in FAA), August 2009, and April 2010 (fixed in 10% formalin). The March collection included only two mated specimens (only one with developed eggs), a few "nearly mature", and many immature specimens. Penis sheaths in particular were not well developed, and the ectal ends were indistinct. Most measurements used in the description were based on the large series of mature, formalin-fixed worms collected in April 2010. The formalin-fixed material from August contained several mated worms and many immatures.
Some histological differences among collection dates appear related to the fixation method, as formalin-fixed specimens were highly contracted, and tissues appeared shrunken and separated (e.g., Fig. 3 View FIGURE 3 K [formalin] vs. 3L [ FAA]). Chaetae of specimens mounted in CMC-10 are similar to those in Canada balsam, but teeth appear slightly thicker ( Fig. 3 View FIGURE 3 B vs. 3A). Penis sheaths have similar length and proportions, although the thinner cuticle of the head may be indistinct in CMC-10.
* Holotype (sexually mature); ** Paratypes (mature)
Earlier (September 2007 and August 2008) collections included several mature worms, but they had been preserved in strong alcohol, and thus were brittle and contracted. Chaetae were mostly broken, male ducts invisible against the dark chloragogen, and penis sheaths broken and twisted.
Distribution. Other than the cave habitat, L. sulphurensis has only been collected at the adjacent surface spring and at Black Sulphur Spring. The distinctive chaetal morphology allows the attribution of immature specimens, confirmed by reproductive characters in the two mature worms ( Fig. 2 View FIGURE 2 E, Table 1 View TABLE 1 ). DNA, as well as proximity of the three sites, suggests that all are from the same population.
TABLE 2. COI barcodes of 7 specimens of Limnodrilus sulphurensis n. sp., from Sulphur Cave dark zone (SC), Sulphur Cave surface spring (SS) and Black Sulphur Spring (BS). Two haplotypes (Ht # 1, # 2) are recognized in positions 2 - 658, i. e. the interval covered by all sequences; 658 base pairs (bp) are the targeted length of this barcode, but first nucleotide is unknown for 1 specimen.
| GenBank# | Individual# | Length | Ht# | Site | Museum voucher |
|---|---|---|---|---|---|
| - | CE7486 | 658 bp | 2 | SC, 18 Aug 2008 | SMNH 150175 |
| - | CE7489 | 658 bp | 1 | SC, 20 Sep 2008 | USNM 1283518 ** |
| - | CE7490 | 658 bp | 2 | SC, 20 Sep 2008 | SMNH 150176 |
| KT692956 View Materials | CE7491 | 658 bp | 2 | SC, 20 Sep 2008 | SMNH Type 8779** |
| - | CE10477 | 657 bp | 1 | BS, 11 Apr 2010 | SMNH 150178 |
| - | CE10479 | 658 bp | 1 | SS, 11 Apr 2010 | SMNH 150177 |
| KT692957 View Materials | CE10482 | 658 bp | 1 | SC, 11 Apr 2010 | DMNS ZE.46275* |
TABLE 1. Selected measurements of L. sulphurensis n. sp. and L. profundicola from new material, compared with literature values for profundicola. Values are mean and (range), where available.
| L. sulphurensis n. sp. | Number of chaetae Maximum chaeta in V, ventral bundle length in V | Penis sheath length Penis sheath Penis sheath head Atrium length length/ basal width width |
|---|---|---|
| Sulphur Cave (n=26–33) | 8.1 (4–11) 165 (146–185) | 333 (240–410) 4.9 (4.1–5.7) 148 (120–170) 366 (240–492) |
| Black Sulphur Spring (n=2) | (8–9) (150–175) | (395–415) (4.1–5.6) (168–192) (385–550) |
| L profundicola new material | ||
| Estonia and European Russia (n=12–16) | 6.4 (4–8) 96 (79–110) | 191 (156–221) 4.6 (3.8–5.7) 65 (48–77) 171 (150–190) |
| Kamchatka (Lake Kuril'skoe) (n=3) | 5.8 (5–7) 114 (103–122) | 241 (226–270) 4.9 (4.3–5.3) 66 (58–72) 236 (220–252) |
| Japan, Lake Biwa (n=4) | 6.3 (6–7) 94 (89–98) | 194 (180–221) 4.8 (4.4–5.1) 59 (53–65) 171 (145–190) |
| Lake Michigan (n=15–22) | 6.1 (4–9) 113 (89–139) | 222 (178–270) 4.7 (3.8–5.7) 63 (53–79) 186 (145–252) |
| L. profundicola literature values | ||
| Sokol’skaya 1972, Kamchatka (as L. helveticus ) | (252–340) (4–6) | |
| Piguet 1913, Switzerland ( L. helveticus ) | maximum8 | 206 (148–264) 5 69 |
| Brinkhurst 1965 and Kennedy 1969, Europe and North America (incl. helveticus , monticola , profundicola ) | 6.4 (5–9) | 240, 340, 270 4.5 (2–7) |
| Ohtaka 1992, Japan | 3.2 | |
| Cui et al. 2015, Tibet, China | (6–11) | 290 5 238 |
| Semernoy 2004, Lake Baikal | (198–234) (4–5) |
| DMNS |
Denver Museum of Nature and Science |
| DNA |
Department of Natural Resources, Environment, The Arts and Sport |
| COI |
University of Coimbra Botany Department |
| SMNH |
Saskatchewan Museum of Natural History |
| USNM |
Smithsonian Institution, National Museum of Natural History |
| ZMH |
Zoologisches Museum Hamburg |
| FAA |
Universidad Nacional del Centro de la Provincia de Buenos Aires |
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
SubFamily |
Tubificinae |
|
Genus |
Limnodrilus sulphurensis Fend, Liu & Erséus
| Fend, Steven V., Liu, Yingkui, Steinmann, David, Giere, Olav, Barton, Hazel A., Luiszer, Fred & Erséus, Christer 2016 |
Limnodrilus variesetosus
| Brinkhurst 1979 |
Limnodrilus dybowskii (
| Grube 1873 |
Limnodrilus udekemianus Claparède, 1862
| Claparede 1862 |