Calapnita Simon, 1892
|
publication ID |
https://doi.org/ 10.5281/zenodo.273086 |
|
publication LSID |
lsid:zoobank.org:pub:0FA0F51A-3868-4F13-A93D-E34CA5A689F8 |
|
DOI |
https://doi.org/10.5281/zenodo.6040194 |
|
persistent identifier |
https://treatment.plazi.org/id/03B66F68-8537-073B-FF6A-FBBB2FD3FE0E |
|
treatment provided by |
Plazi |
|
scientific name |
Calapnita Simon, 1892 |
| status |
|
Calapnita Simon, 1892 View in CoL View at ENA
Calapnita Simon, 1892: 42 View in CoL (type species: C. vermiformis Simon, 1892 View in CoL ). Deeleman-Reinhold 1986b: 205, 212 (removed from synonymy with Micromerys View in CoL ). Deeleman-Reinhold 1986a: fig. 3 (distribution). Huber 2011: 41, 44.
Micromerys— Simon 1893: 473–474 (synonymy rejected in Deeleman-Reinhold 1986b).
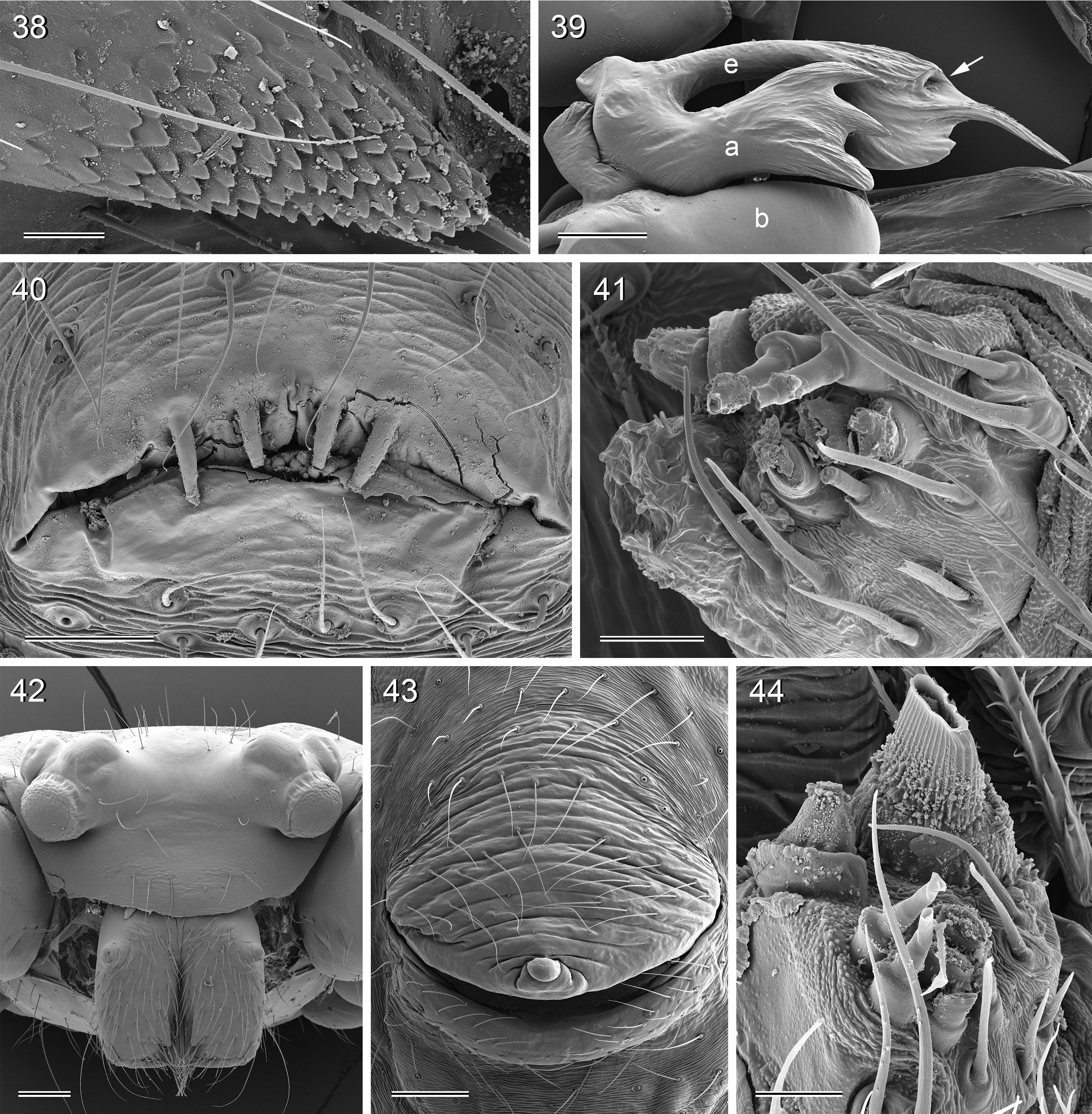
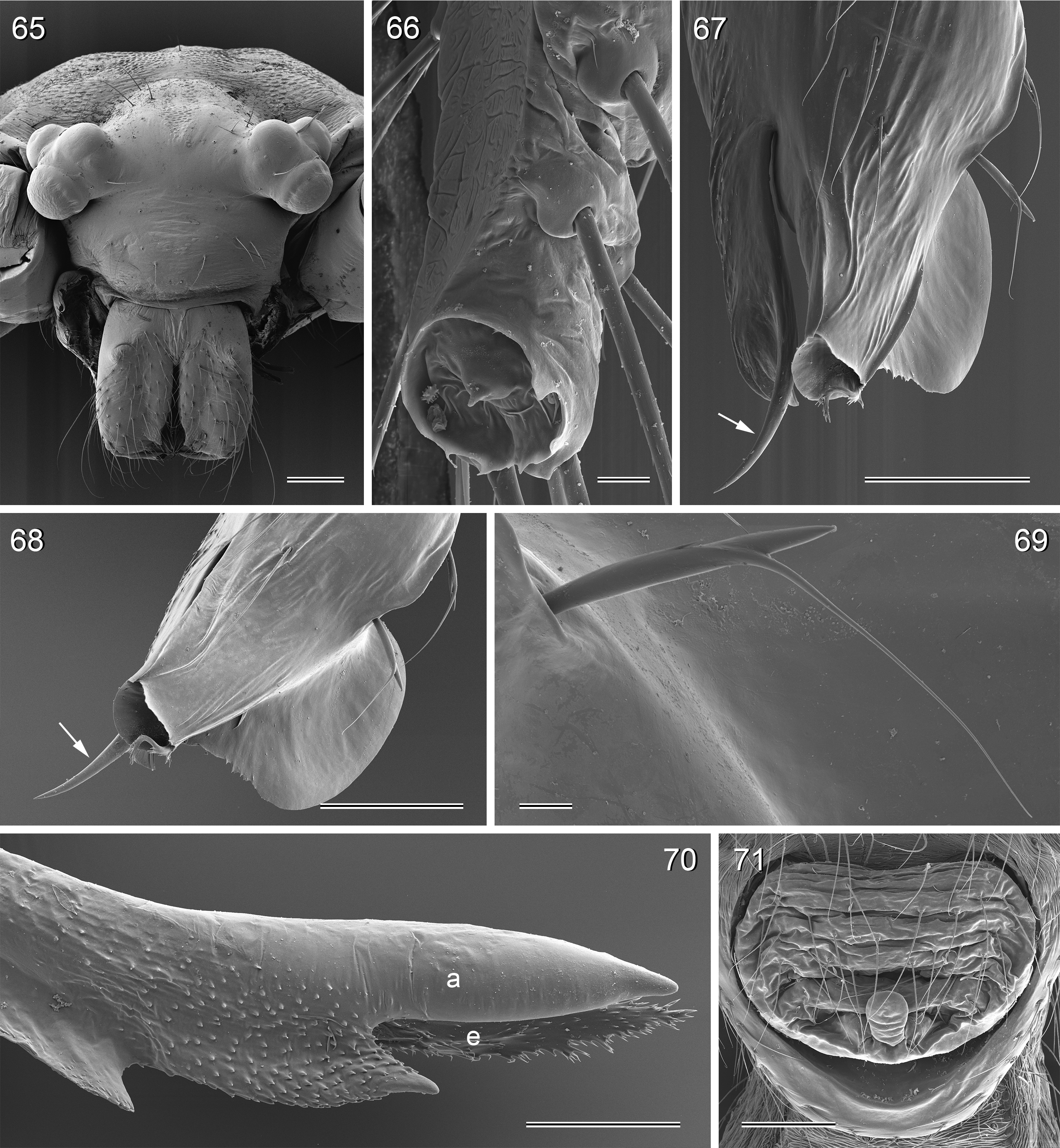
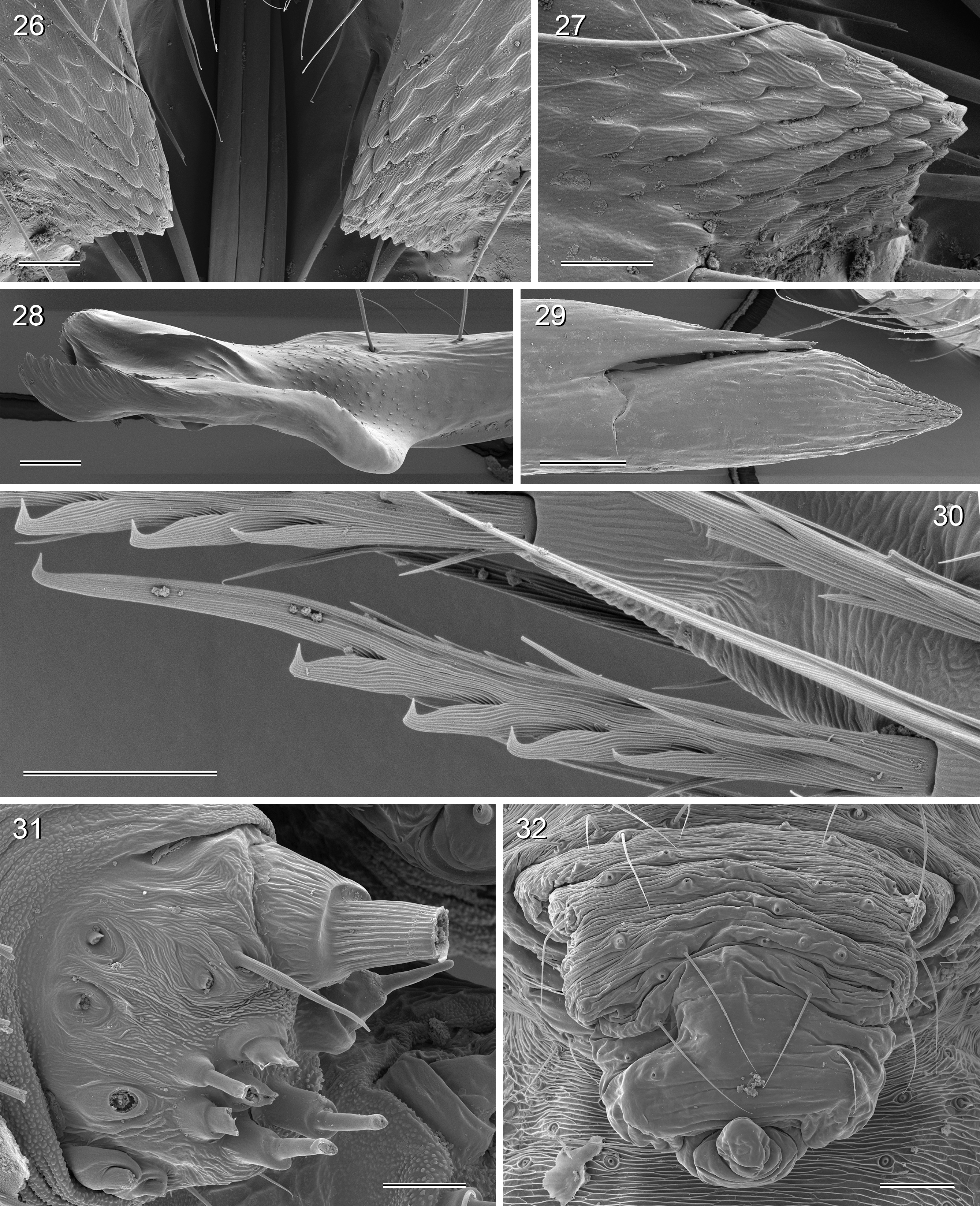
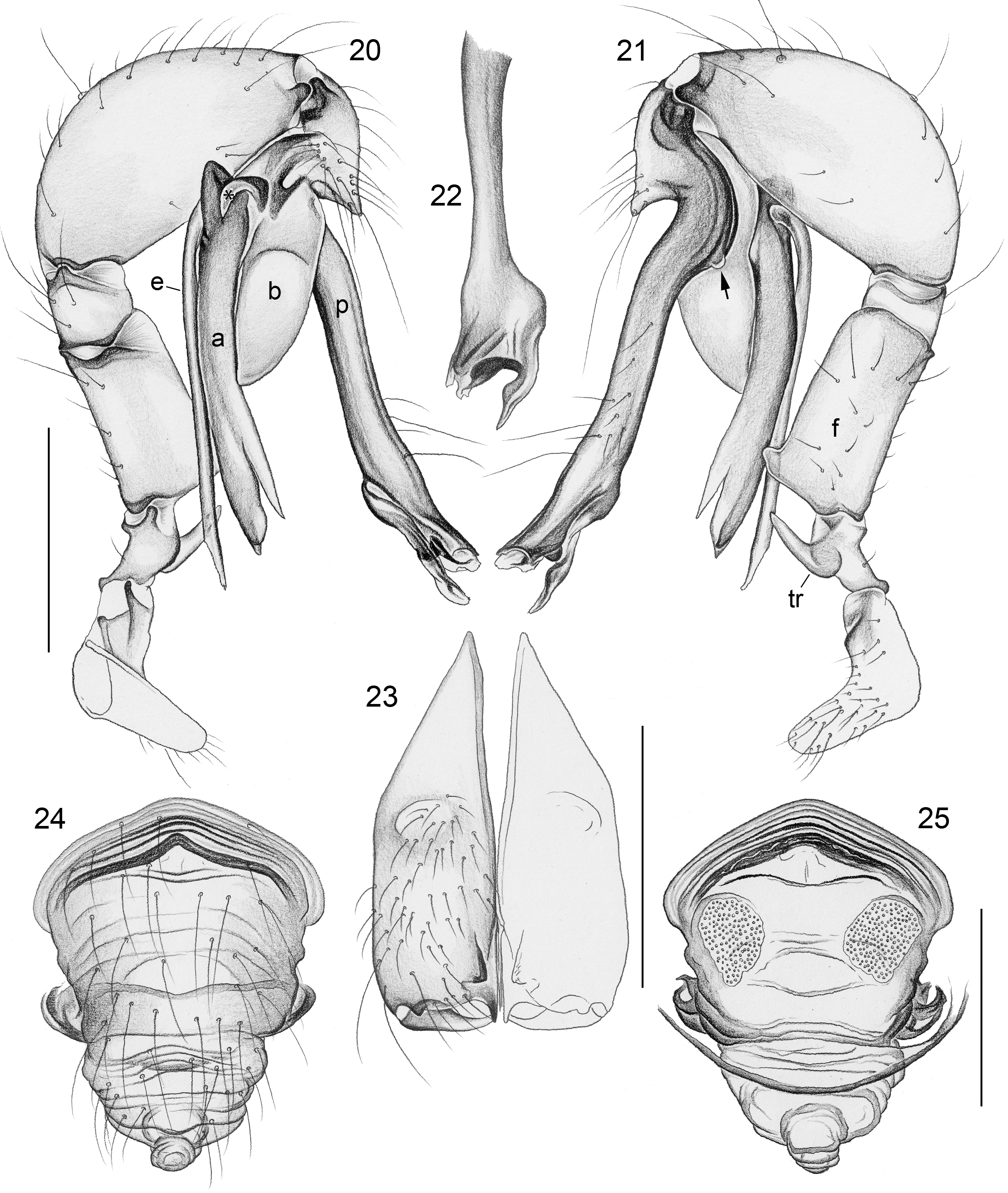
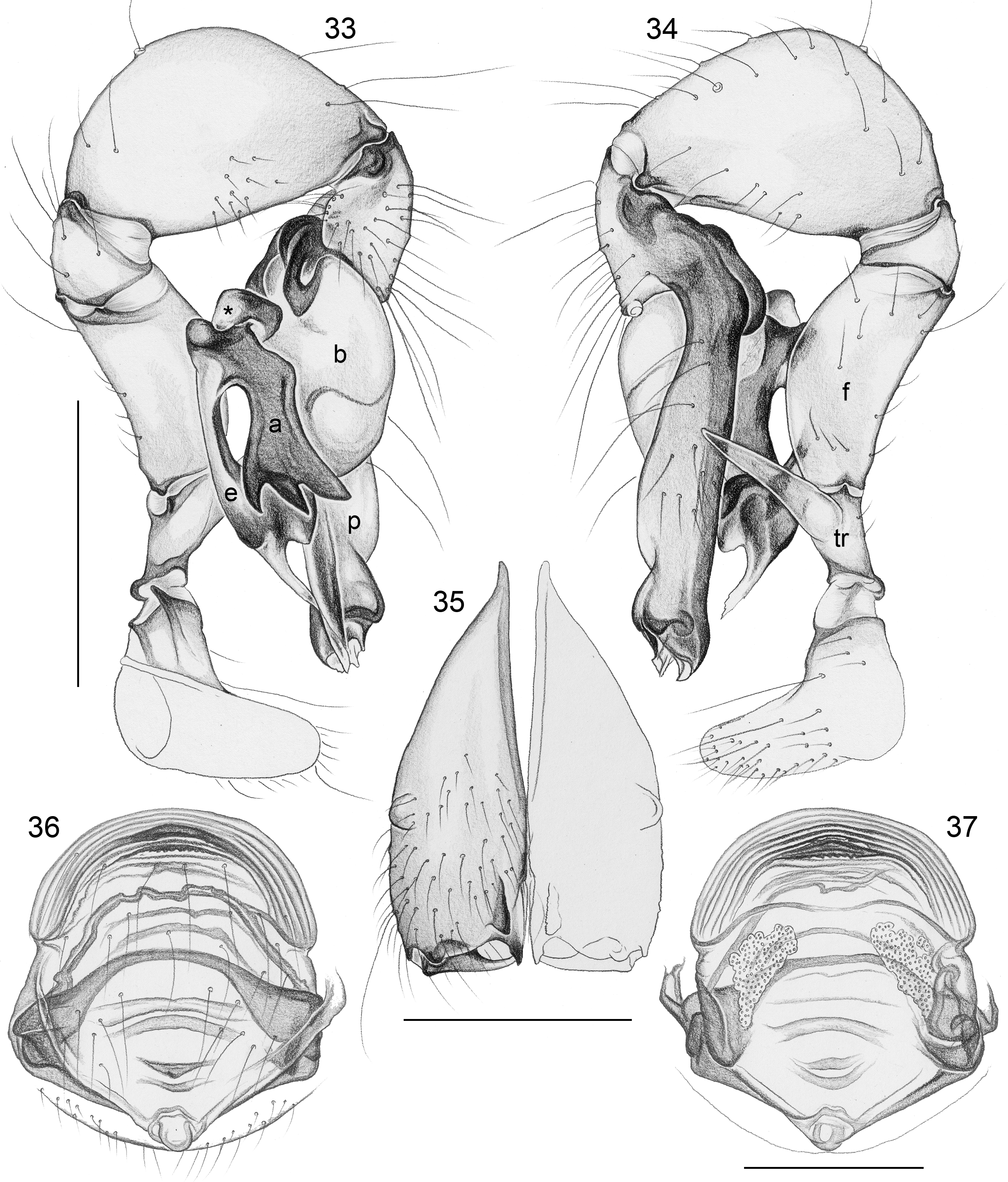
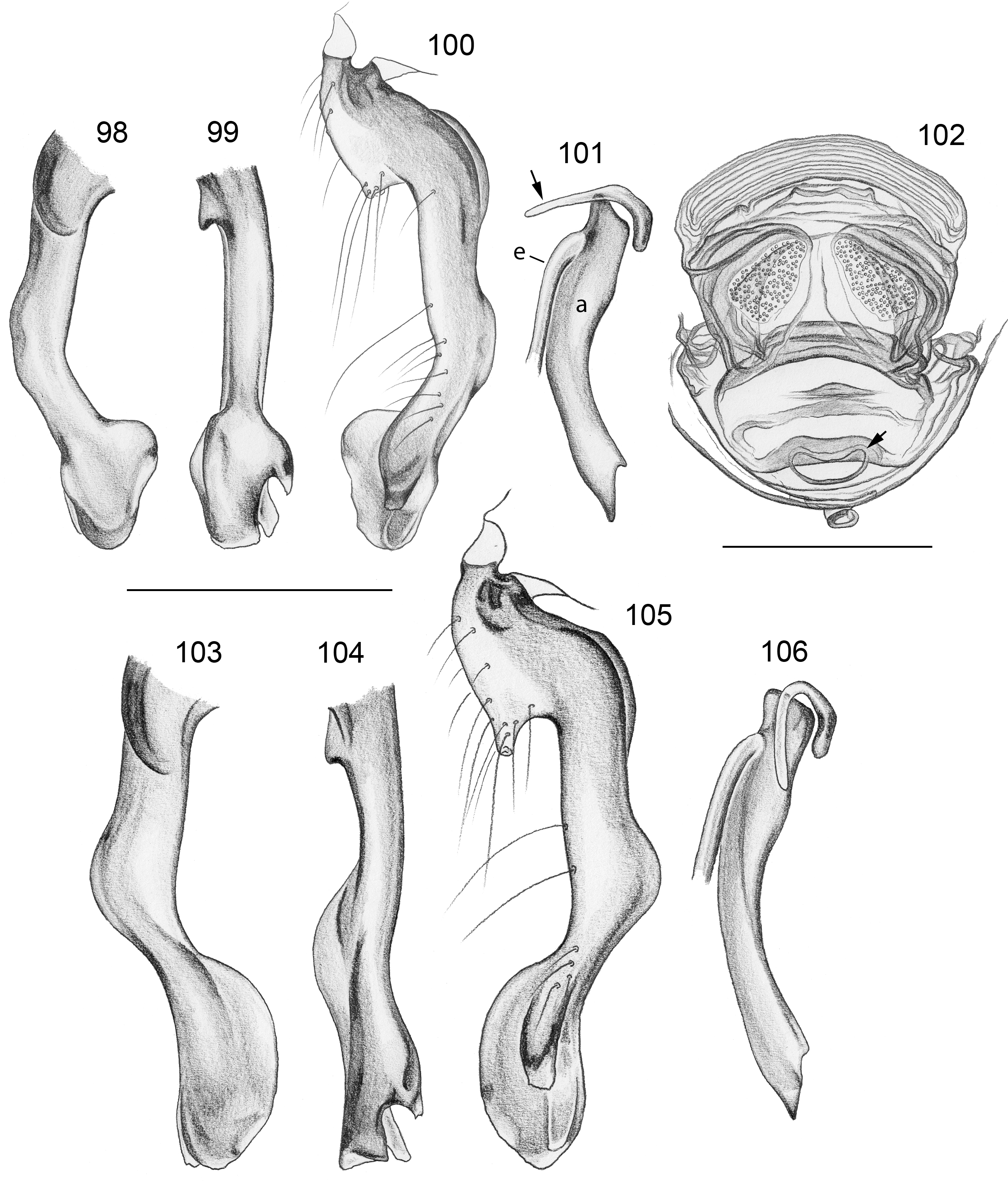
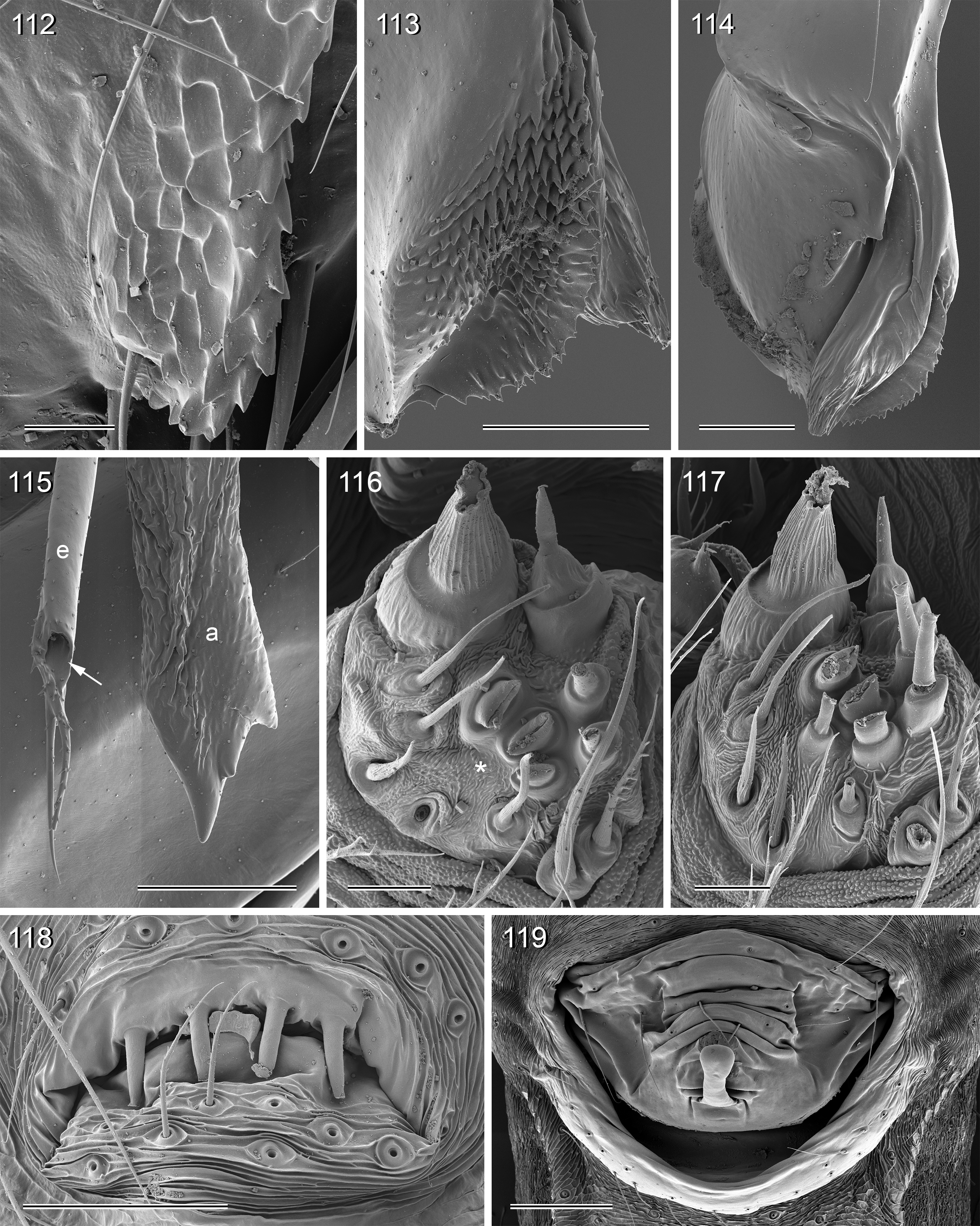
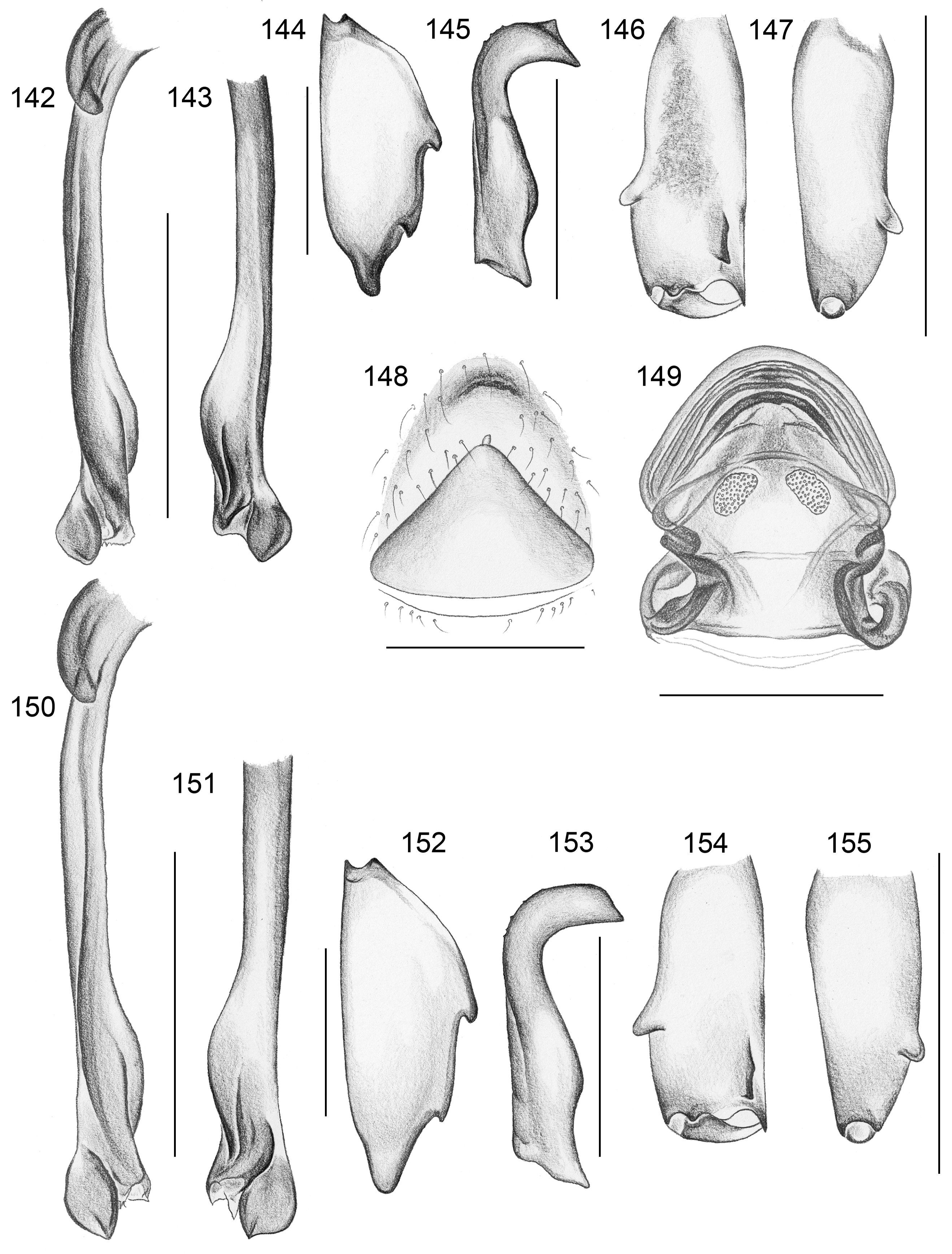
Diagnosis. Leaf-dwelling, pale whitish, long-legged pholcids with six eyes and long cylindrical abdomen ( Figs 3– 19 View FIGURES 3 – 12 View FIGURES 13 – 19 , 120–133 View FIGURES 120 – 127 View FIGURES 128 – 133 ); distinguished from similar species in other genera ( Leptopholcus , Micromerys , Pholcus , Panjange ) by combination of: (1) eye triads barely elevated above carapace ( Figs 42 View FIGURES 38 – 44 , 65 View FIGURES 65 – 71 , 79 View FIGURES 72 – 80 ); (2) abdomen posteriorly not drawn out into dorsal cone ( Figs 9, 10 View FIGURES 3 – 12 , 121 View FIGURES 120 – 127 ); (3) male chelicerae frontal apophyses present, with scaly cuticle but without modified hairs ( Figs 26, 27 View FIGURES 26 – 32 , 38 View FIGURES 38 – 44 , 273 View FIGURES 272 – 280 ); (4) tip of male palpal trochanter apophysis not serrated (e.g., Figs 21 View FIGURES 20 – 25 , 34 View FIGURES 33 – 37 , 138 View FIGURES 134 – 141 ); (5) procursus ‘knee’ without long transparent process (e.g. Figs 98 View FIGURES 98 – 106 , 134 View FIGURES 134 – 141 ); (6) embolus parallel to bulbal appendix (e.g., Figs 20 View FIGURES 20 – 25 , 274 View FIGURES 272 – 280 ); (7) epigynum with ‘knob’ (e.g. Figs 43 View FIGURES 38 – 44 , 71 View FIGURES 65 – 71 , 280 View FIGURES 272 – 280 ).
Description. Total body length ~3.5–7.0; carapace width 0.8–1.1; leg 1 length ~26–43; tibia 1 length ~5.5– 11.0; tibia 2/tibia 4 length <0.95 in C. vermiformis group,> 1.05 in C. phyllicola group; tibia 1 L/d ~95–125. Color in life ( Figs 3–19 View FIGURES 3 – 12 View FIGURES 13 – 19 , 120–133 View FIGURES 120 – 127 View FIGURES 128 – 133 ) mostly pale ochre-yellow to whitish, usually without any darker dorsal marks, sternum mostly whitish but in males of one species group brown, legs with brown patellae and tibia-metatarsus joints. Carapace without median furrow; ocular area not raised, eye triads barely elevated above carapace ( Figs 42 View FIGURES 38 – 44 , 65 View FIGURES 65 – 71 , 79 View FIGURES 72 – 80 ). AME absent. Clypeus moderately high, unmodified. Abdomen very long cylindrical, never angular (or drawn out into cone) above spinnerets ( Figs 9, 10 View FIGURES 3 – 12 , 121 View FIGURES 120 – 127 ). Male gonopore with four epiandrous spigots ( Figs 40 View FIGURES 38 – 44 , 96 View FIGURES 89 – 97 , 118 View FIGURES 112 – 119 ). Each ALS either with only two spigots ( C. vermiformis group: large widened spigot and pointed spigot; Figs 278, 279 View FIGURES 272 – 280 ) or with eight spigots ( C. phyllicola group: large widened spigot and pointed spigot, and six (rarely five) cylindrically shaped spigots of varying sizes; Figs 31 View FIGURES 26 – 32 , 41, 44 View FIGURES 38 – 44 , 78 View FIGURES 72 – 80 , 94, 95 View FIGURES 89 – 97 , 116, 117 View FIGURES 112 – 119 ); PMS with two spigots each ( Fig. 95 View FIGURES 89 – 97 ). Male chelicerae distal apophyses with scaly cuticle, without modified hairs ( Figs 26, 27 View FIGURES 26 – 32 , 38 View FIGURES 38 – 44 , 273 View FIGURES 272 – 280 ), proximal lateral processes either in usual proximal position ( C. phyllicola group; e.g., Figs 42 View FIGURES 38 – 44 , 72 View FIGURES 72 – 80 ) or in unusually distal position ( C. vermiformis group; e.g., Figs 136 View FIGURES 134 – 141 , 146 View FIGURES 142 – 155 ); chelicerae without stridulatory ridges. Male palpal coxa unmodified; trochanter with retrolatero-ventral apophysis either simple ( C. phyllicola group; Figs 21 View FIGURES 20 – 25 , 34 View FIGURES 33 – 37 ) or distinctively hooked and sclerotized ( C. vermiformis group; e.g., Figs 138 View FIGURES 134 – 141 , 145, 153 View FIGURES 142 – 155 ); femur either barely modified ( C. phyllicola group; with ventral process in C. lehi ; Figs 21 View FIGURES 20 – 25 , 34 View FIGURES 33 – 37 ) or with distinctive series of three ventral sclerotized processes ( C. vermiformis group; e.g., Figs 139 View FIGURES 134 – 141 , 144, 152 View FIGURES 142 – 155 ); patella usually short ( Figs 21 View FIGURES 20 – 25 , 34 View FIGURES 33 – 37 ), unusually long in C. semengoh (figs 189–190 in Huber 2011); tibia with two trichobothria; palpal tarsus with short coneshaped or turret-shaped whitish dorsal process carrying tarsal organ ( Figs 21 View FIGURES 20 – 25 , 34 View FIGURES 33 – 37 , 66 View FIGURES 65 – 71 , 73 View FIGURES 72 – 80 ); palpal tarsal organ exposed or capsulate ( Figs 66 View FIGURES 65 – 71 , 73 View FIGURES 72 – 80 ; figs 150, 180 in Huber 2011); procursus without parallel ridges, proximal ventral ‘knee’ never with long transparent process (short process in C. lehi , Fig. 21 View FIGURES 20 – 25 ), without hinged parts (except possibly C. lehi , Figs 20–22 View FIGURES 20 – 25 ); bulb with strong proximal sclerite connecting to tarsus ( Figs 20 View FIGURES 20 – 25 , 33 View FIGURES 33 – 37 ), with weakly sclerotized embolus (with unique sclerotized widening in C. kubah only, Figs 33 View FIGURES 33 – 37 , 39 View FIGURES 38 – 44 ; in some species with serrated edge, Fig. 75 View FIGURES 72 – 80 ) and variable appendix ( C. vermiformis group: weakly sclerotized pointed process, Fig. 274 View FIGURES 272 – 280 ; C. phyllicola group: sclerotized process, e.g., Figs 20 View FIGURES 20 – 25 , 33 View FIGURES 33 – 37 , 47 View FIGURES 45 – 54 ); uncus absent, possibly present in C. phyllicola group in form of small membranous (sometimes worm-shaped) process ( Figs 33 View FIGURES 33 – 37 , 47 View FIGURES 45 – 54 , 74 View FIGURES 72 – 80 , 93 View FIGURES 89 – 97 ). Legs without spines, without curved hairs, few vertical hairs; retrolateral trichobothrium very proximal (tibia 1: at 1.5–3.5% of tibia length), prolateral trichobothrium absent on tibia 1, present on other tibiae; tarsal pseudosegments usually not or barely visible in dissecting microscope; tarsus 4 with single row of ventral comb-hairs of Pholcus - type (cf. Huber & Fleckenstein 2008) ( Figs 30 View FIGURES 26 – 32 , 277 View FIGURES 272 – 280 ). Females in general similar to males, eye triads usually slightly closer together, legs slightly shorter. Epigynum weakly sclerotized, either a roughly triangular plate with anterior scape or ‘knob’ ( C. vermiformis group; e.g., Figs 141 View FIGURES 134 – 141 , 148 View FIGURES 142 – 155 ) or roughly rectangular or trapezoidal area with folded (extensible) cuticle and posterior ‘knob’ ( C. phyllicola group; Figs 43 View FIGURES 38 – 44 , 71 View FIGURES 65 – 71 , 80 View FIGURES 72 – 80 , 97 View FIGURES 89 – 97 , 119 View FIGURES 112 – 119 ); internal genitalia with pair of pore plates of variable shape, sometimes with paired or unpaired membranous ‘sacs’ (e.g., Figs 49, 54 View FIGURES 45 – 54 , 102 View FIGURES 98 – 106 ).
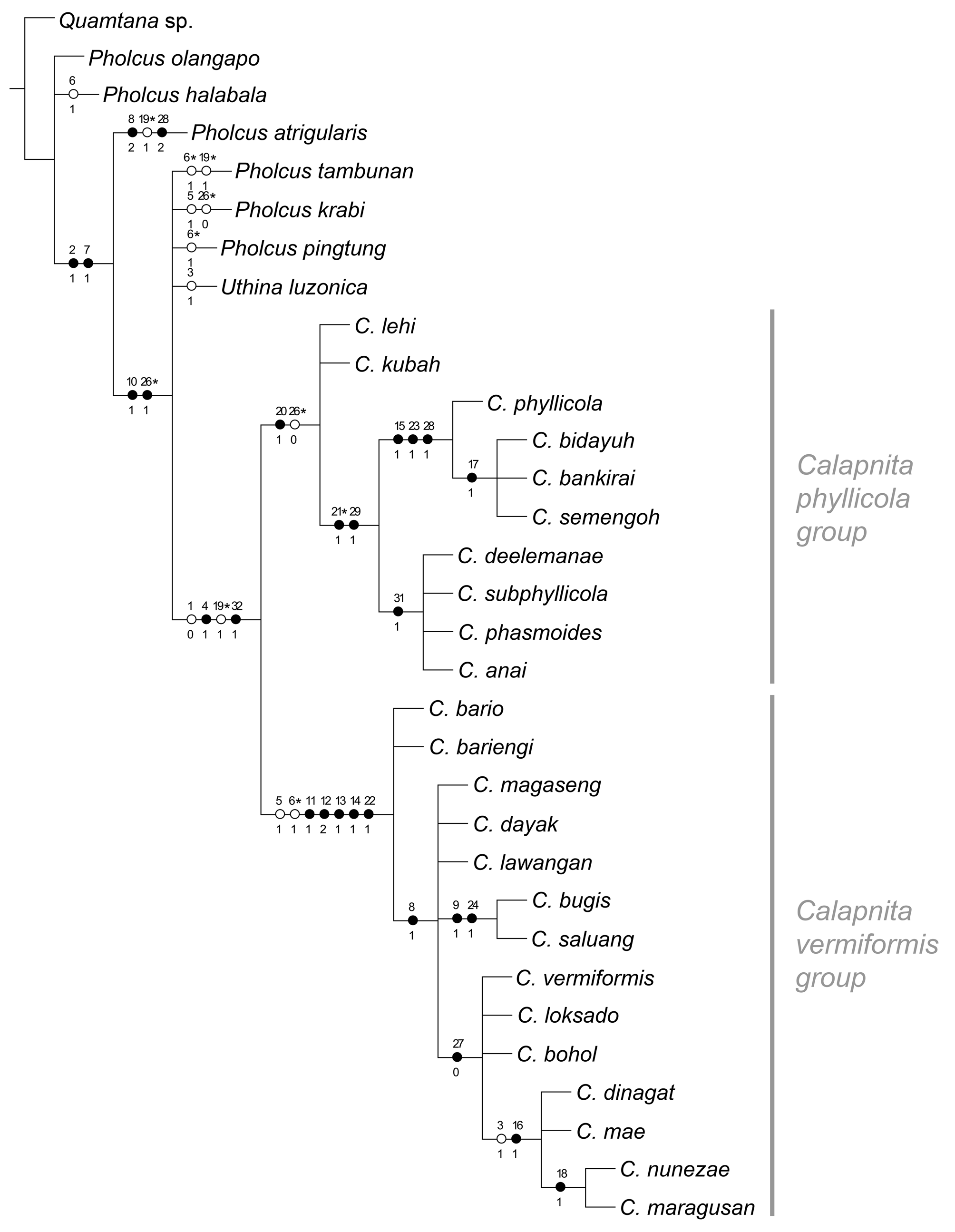
Relationships. Using NONA with “hold/100, mult*200” (or “hold/10; mult*10.000”), “amb-”, and equal character weights for the matrix in Appendix 1 resulted in two most parsimonious cladograms with a length of 43 (Ci = 81; Ri = 95). The same two cladograms were obtained when using TNT with implicit enumeration, Pee-Wee with implied character weighting (with the constant of concavity K = 1–5), and NONA with successive character weighting. The cladogram shown in Fig. 1 View FIGURE 1 is the strict consensus of these two cladograms. Variation occurred mostly among outgroup taxa (not further discussed here), and in the character support for (but not the relationships among) Calapnita and its two major subgroups, the C. phyllicola group and the C. vermiformis group.
As expected from the low number of characters, Jackknifing (in TNT, 5000 replicates) resulted in low support values (<70) for most clades. Exceptions were the C. vermiformis group (99), C. phyllicola + closest relatives (94), and the clade including C. dinagat to C. maragusan in Fig. 1 View FIGURE 1 (86). The C. phyllicola group had a support of 66, the genus Calapnita only 46 (new technology search) and 44 (traditional search) respectively.
Calapnita is resolved as monophyletic, supported by the flat ocular area, the worm-shaped abdomen, the reduction of the web to a layer of silk closely attached to the leaf surface, and (in the consensus cladogram only) the absence of an uncus. In a previous analysis ( Huber 2011), the bulbal processes were coded differently, which resulted in different support for Calapnita . The only bulbal process in the C. vermiformis group and the membranous process in the C. phyllicola group were both considered to be an uncus; the former is here seen as an appendix, while the latter is seen as a newly evolved structure. In the absence of intermediate forms or ontogenetic data, the question of homology in these cases is difficult to solve convincingly. An alternative plausible coding (uncus present in C. phyllicola group, represented by membranous bulbal process) resulted in longer most parsimonious cladograms (44 steps) and was thus discarded.
Both species groups within Calapnita are recovered, but as in the previous analysis ( Huber 2011), only the C. vermiformis group is strongly supported by several characters, while the C. phyllicola group remains weakly supported. Six characters unambiguously support the C. vermiformis group: reduction of ALS spigots to two (per spinneret); tibia 2 significantly shorter (<0.96 x) than tibia 4; femur 1 significantly longer (>1.05 x) than tibia 1; male palpal trochanter apophysis strongly curved and sclerotized; male palpal femur ventrally serrated; and appendix reduced to slender membranous process. The C. phyllicola group is unambiguously supported by only one character, the membranous bulbal process that may or may not be derived from an uncus.
Within the C. phyllicola group, most species share a membranous process on the bulb that is worm-shaped and a paired or unpaired membranous ‘sac’ in the female internal genitalia. Four of the species included ( C. deelemanae to C. anai in Fig. 1 View FIGURE 1 ) share an egg-sac where all eggs are lined up in a single row. The other four species ( C. phyllicola to C. semengoh in Fig. 1 View FIGURE 1 ) are united by several characters: male palpal tarsal organ situated on a turret-like process of the tarsus; serrated edge of the embolus; and drop-shaped pore plates. Within this clade, all species but C. phyllicola share the distinctive split hairs dorsally on the procursus.
Within the C. vermiformis group, most species are united by a bipartite male cheliceral apophysis. In two species ( C. saluang , C. bugis ), the proximal part of this bipartite apophysis is very thin; these two species also share unusually strong and regular fringes distally on the embolus. Several species (mostly from the Philippines, and C. loksado from Borneo) share a continuous rather than distinct transition between epigynal plate and ‘knob’; within this clade, four species from Mindanao ( C. dinagat to C. maragusan in Fig. 1 View FIGURE 1 ) share a dark sternum and a distinct prolateral process on the procursus. In two of these species ( C. nunezae , C. maragusan ) the tip of the procursus is characteristically widened or ‘inflated’.
Natural history. The spiders require suitable leaves but are not strictly restricted to well preserved forests. By contrast, they were sometimes easier to find in disturbed habitats at forest edges and along trails near buildings (even on cultivated plants such as banana and taro) than deeper in the forest. In forests, the distribution was sometimes very patchy, with several specimens on one or a few plants close together and no further specimens for several hours of searching.
Usually, two or three species were found per locality, especially in Borneo, Sumatra, and southern Malay Peninsula . One locality in Central Kalimantan (Tumbang Tahai) is known to have four species ( C. dayak , C. lawangan , C. phasmoides , C. phyllicola ). Calapnita spiders were often found together with other leaf dwelling pholcids (sharing the habitat, sometimes the plant, very rarely the individual leaf) of the genera Belisana , Pholcus (minang group, domingo group, tambunan group, andulau group, krabi group, halabala group, kerinci group), Panjange , and Leptopholcus .
Some species accept a variety of large leaves (monocot and dicot) while others seem to be more restricted to certain taxa, such as palm ( Arecaceae ) or aroid ( Araceae ) leaves. During the day, the spiders press their bodies and legs against the leaf in a very cryptic resting position, with the prolateral sides of the legs close to the leaf surface ( Figs 17 View FIGURES 13 – 19 , 127 View FIGURES 120 – 127 ). Unforced activity was only seen at night ( Fig. 133 View FIGURES 128 – 133 ).
The webs consist of a very fine but usually relatively dense sheet directly attached to the leaf surface, never extending beyond the leaf. Usually the threads are barely visible unless viewed in a specific way (e.g. against light when leaf is viewed from side). In a few cases, webs seemed to consist of very few threads or were entirely invisible or non-existent (e.g., C. semengoh males).
Egg-sacs contain only about 6–16 (usually 8–12) eggs, and are always elongated. Usually there are at least proximally three (rarely two) eggs per diameter (e.g., Figs 4, 8 View FIGURES 3 – 12 , 121, 123 View FIGURES 120 – 127 ), but in one clade ( C. deelemanae to C. anai in Fig. 1 View FIGURE 1 ) all eggs are usually perfectly lined up in a single row ( Figs 9, 10, 12 View FIGURES 3 – 12 ).
Egg-parasitism seems to be relatively common and has been observed in several species: C. phyllicola on Gunung Liang ( Fig. 15 View FIGURES 13 – 19 ); C. nunezae on Mount Matutum; and C. subphyllicola on Mount Mupo and Camp Abubakar.
When disturbed, Calapnita spiders do never vibrate but press their bodies against the leaf. If the disturbance continues, they eventually run away or reluctantly drop on a silk line.
Composition. The genus now includes 25 named species; of these, 15 are in the C. vermiformis group, ten in the C. phyllicola group. Judging from distribution patterns, further species of the C. phyllicola group are expected to occur mainly on Borneo; further species of the C. vermiformis group are expected to occur mainly on Borneo and the Philippines.
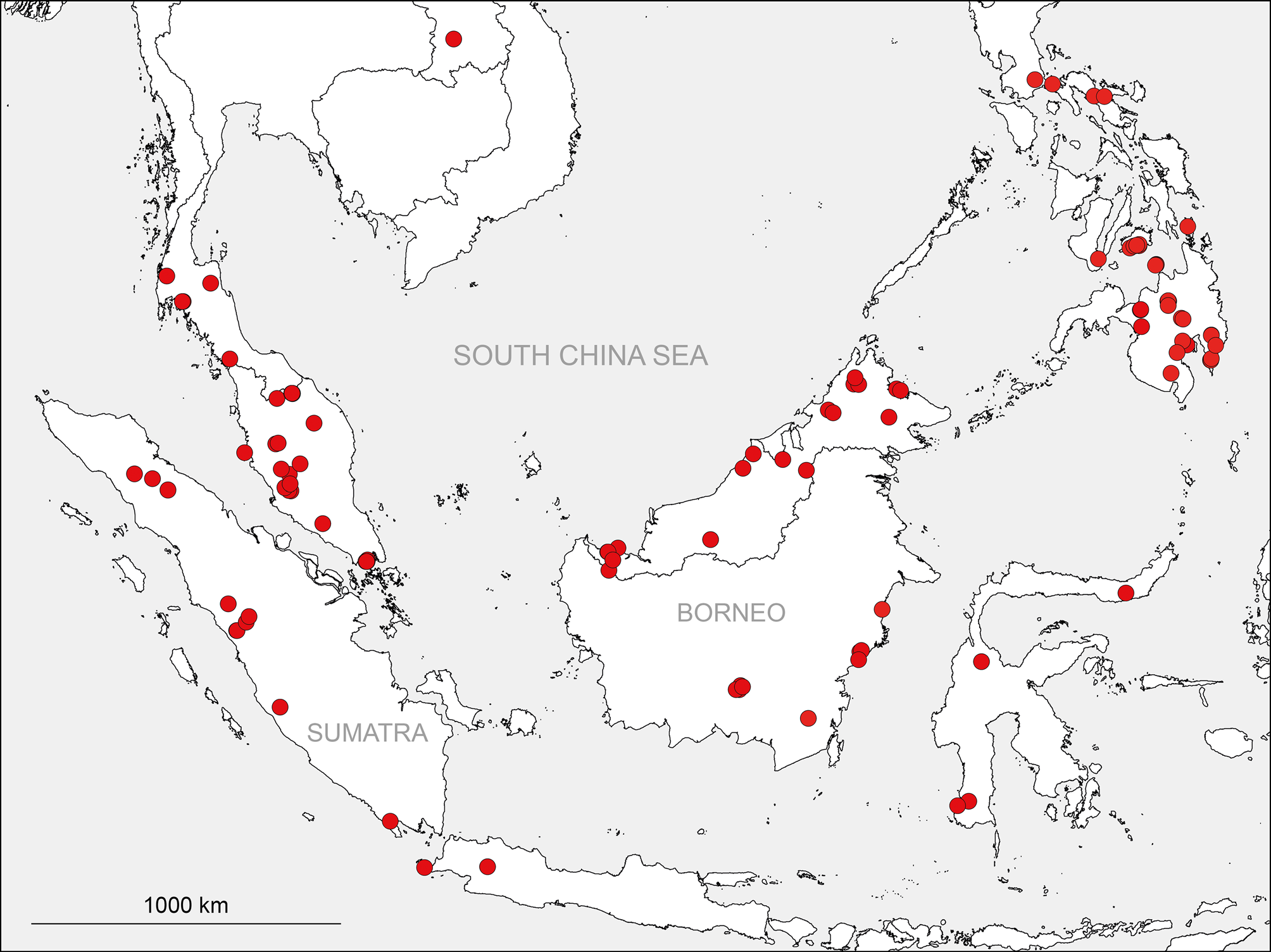
Distribution. Southeast Asia, from southern Laos, the Malay Peninsula, and Sumatra in the west to the Philippines and Sulawesi in the east ( Fig. 2 View FIGURE 2 ); apparently absent from the Moluccas and the Lesser Sunda Islands. Whether the single unidentified female in Muséum d’histoire naturelle, Genève from Thailand, Chiang Mai Prov., Doi Suthep, assigned to Calapnita in Huber 2011 , is actually a Calapnita or not is uncertain.
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
Calapnita Simon, 1892
| Bernhard A. Huber 2017 |
Calapnita
| Huber 2011: 41 |
| Deeleman-Reinhold 1986: 205 |
| Simon 1892: 42 |