Caliroa Costa, 1859
|
publication ID |
https://doi.org/10.11646/zootaxa.4768.3.1 |
|
publication LSID |
lsid:zoobank.org:pub:C8036F69-F881-4727-96E7-C78AA6C7F920 |
|
DOI |
https://doi.org/10.5281/zenodo.3794889 |
|
persistent identifier |
https://treatment.plazi.org/id/03B387A9-FFD2-FF85-1DC6-24C032B5FE8D |
|
treatment provided by |
Plazi (2020-05-04 07:11:17, last updated 2024-11-26 08:51:24) |
|
scientific name |
Caliroa Costa, 1859 |
| status |
|
Caliroa Costa, 1859 View in CoL View at ENA
Caliroa Costa, 1859: 59 View in CoL ; Takeuchi, 1952: 56; Lorenz & Kraus, 1957: 111; Smith, 1971: 12; Zhelochovtsev & Zinovjev, 1988: 57, 191; Wei, 1997: 52; Vasu, 1998: 286; Wei, 1998: 26; Lacourt, 1999: 54; Taeger et al., 2010: 364; Koch & Smith, 2011: 445. Type-species: Caliroa sebetia Costa, 1859 [= Caliroa cothurnata (Serville, 1823) ], by monotypy.
Eriocampoides Konow, 1890: 233 . Type species: Tenthredo limacina Retzius, 1783 [= Caliroa cerasi ( Linné, 1758) ], by subsequent designation of MacGillivray (1909).
Periclistoptera Ashmead, 1898: 255 . Type species: Monostegia alba (Norton, 1867) [= Caliroa obsoleta (Norton, 1867) ], by original designation.
For more synonymy, see Smith (1971) and Lacourt (1999).
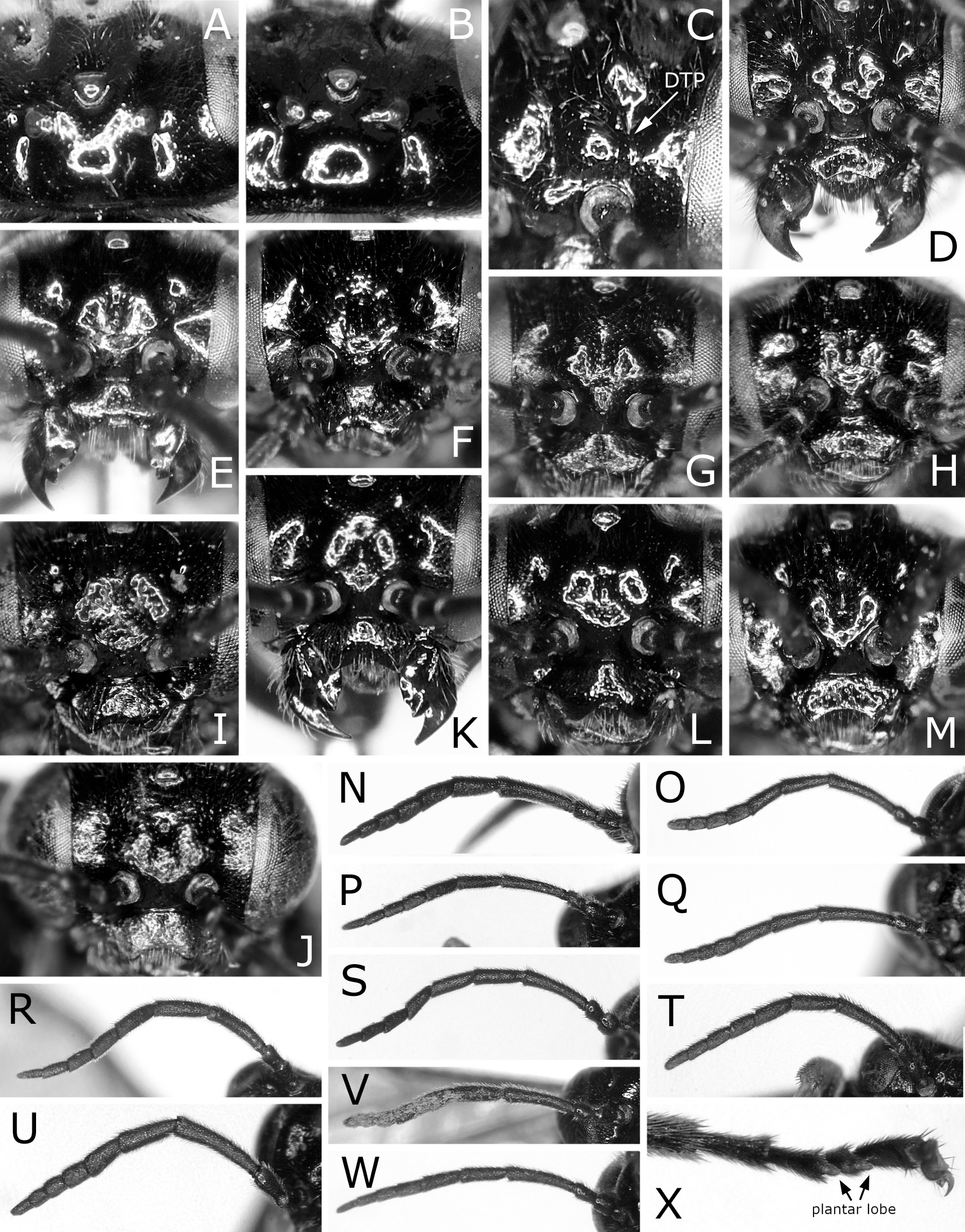
Diagnosis. Length 3.5–6.5 mm. Anterior margin of postocellar area not grooved ( Fig. 3A View FIGURE 3 ), sometimes laterally grooved ( Fig. 3B View FIGURE 3 ). Dorsal tentorial pit (DTP in Fig. 3C View FIGURE 3 ) widely separated from torulus; distance between dorsal tentorial pit and torulus as long as or slightly longer than height of torulus. Distance between toruli 1.1–1.6 × distance between torulus and eye ( Fig. 3 View FIGURE 3 D–M). Outer margin of eye without large punctures. Genal carina absent or only distinct in very short ventral part. Malar space less than 0.2 × width of median ocellus [except for C. feri Vasu, 1998 from India with malar space 0.5 × diameter of median ocellus ( Vasu, 1998)]. Antenna with pedicel longer than wide, shorter than scape ( Fig. 3 View FIGURE 3 N–W); first flagellomere longer than second; apical four flagellomeres combined 0.9–2.1 × as long as first flagellomere. Epicnemium narrow; epicnemial groove rarely inconspicuous. Mesepisternum continuously setose from dorsal to ventral ends, glabrous along anterior margin. Metapleuron with metapleural groove nearly straight. Basal two tarsomeres without plantar lobe ( Fig. 3X View FIGURE 3 ). Tarsal claw with one outer tooth and large acute basal lobe. Forewing with joint of vein M and crossvein 2r-m located basal to joint of vein M and crossvein 2m-cu ( Figs 1 View FIGURE 1 , 2 View FIGURE 2 ); vein 2A+3A complete, without spur like appendix [in some Afrotropical and Nearctic species, vein 2A+3A incomplete, with its wide middle part obliterated (figs 6, 8 in Koch & Smith, 2011)]; crossvein a present, oblique ( Figs 1 View FIGURE 1 , 2 View FIGURE 2 ). Hind wing of male with or without marginal vein. Cercus short, long oval (fig. 2J in Hara & Shinohara, 2013) [in some Oriental species, cercus slender (figs 3, 4 in Vasu, 1998)]. Lancet sinuate ( Figs 6–10 View FIGURE 6 View FIGURE 7 View FIGURE 8 View FIGURE 9 View FIGURE 10 ). Penis valve with small lateral spine at apical fourth ( Fig. 11 View FIGURE 11 ).
Remarks. Smith (1971), Wei (1997, 1998) and Koch & Smith (2011) adopted the reduction of apical four antennomeres as a generic character of Caliroa . Smith (1971) and Wei (1997) wrote that “apical four segments reduced, together subequal in length to or only slightly longer than third segment”. Wei (1998) wrote that “Apical 4 segments of antenna distinctly reduced, 3rd segment of antenna longer than apical 3 segments together”. However, apical four antennomeres are not very reduced in C. annulipes (fig. 2J in Hara, 2011), C. ouensis ( Fig. 3S View FIGURE 3 ) and C. vaccini ( Fig. 3R View FIGURE 3 ). These three species have the apical four antennomeres together 1.5–2.1 × as long as a third antennomere, and a third antennomere is 0.8–0.9 × as long as the apical three antennomeres together.
Smith (1971) and Vasu (1998) wrote that an epicnemium (or prepectus) is absent in Caliroa , while Wei (1997, 1998) wrote that it is distinct. In our material, an epicnemium is usually well defined by a distinct epicnemial groove.
Wei (1998) stated in his key to genera of the Caliroini that “Hind basitarsus as long as or longer than following 4 joints combined” and “Postocellar furrow absent” for Caliroa and Sinocaliroa Wei, 1998, and “2nd cubital cell distinctly shorter than 3rd cubital cell”, “Penis valve with a large apical hook” and “Lance without serrula-like dent” for Caliroa . These characters are variable within the genus in our material. A hind first tarsomere is 0.7–1.1 × as long as the following four tarsomeres combined. A postocellar furrow is sometimes distinct laterally ( Fig. 3B View FIGURE 3 ). The forewing has the cell 2Cu often as long as the cell 3Cu ( Figs 1A View FIGURE 1 , 2A, E, O, Q View FIGURE 2 ). The apex of a penis valve is not hooked but rounded in C. staphyleae ( Fig. 11R View FIGURE 11 ). A lance is often slightly but distinctly serrate (e.g. Figs 7A View FIGURE 7 , 10G View FIGURE 10 ).
Caliroa is most similar to Arla Malaise, 1957 in Japanese tenthredinid genera. They are distinguished as follows: the anterior margin of a postocellar area is not grooved or shortly grooved laterally in Caliroa ( Fig. 3A, B View FIGURE 3 ), while it is entirely grooved in Arla ; the distance between a dorsal tentorial pit and a torulus is as long as or slightly longer than the height of a torulus in Caliroa ( Fig. 3C View FIGURE 3 ), while it is shorter than that height in Arla ; an epicnemium is usually distinct in Caliroa , while it is absent in Arla ; the mesepisternum is setose from the dorsal to ventral parts uninterruptedly in Caliroa , while it is setose on the dorsal and ventral parts and widely glabrous on the middle part in Arla .
Ashmead, W. H. (1898) Classification of the horntails and sawflies, or the sub-order Phytophaga (Paper No. 5). The Canadian Entomologist, 30 (10), 249 - 257. https: // doi. org / 10.4039 / Ent 30249 - 10
Costa, A. (1859) Fauna del Regno di Napoli. Imenotteri. Parte III. Trivellanti Sessiliventri. [Tentredinidei]. Antonio Cons, Napoli, 116 + 5 pp. [1859 - 1860]
Hara, H. (2011) A new slug sawfly, Caliroa nara sp. nov. (Hymenoptera, Tenthredinidae, infesting oak trees, with taxonomic notes on C. angustata and C. annulipes. Japanese Journal of Systematic Entomology, 17, 369 - 383.
Hara, H. & Shinohara, A. (2013) A slug sawfly, Caliroa matsumotonis (Hymenoptera: Tenthredinidae), injurious to peach and pear trees in Japan and Korea. Applied Entomology and Zoology, 48, 379 - 386. https: // doi. org / 10.1007 / s 13355 - 013 - 0198 - y
Koch, F. & Smith, D. R. (2011) A new species of Caliroa (Hymenoptera: Tenthredinidae) from South Africa. Proceedings of the entomological Society of Washington, 113, 442 - 450. https: // doi. org / 10.4289 / 0013 - 8797.113.4.442
Konow, F. W. (1890) Tenthredinidae Europae. Deutsche Entomologische Zeitschrift, 1890 (2), 225 - 240.
Lacourt, J. (1999) Repertoire des Tenthredinidae ouest-palearctiques (Hymenoptera, Symphyta). Memoires de la SEF, 3, 1 - 432.
Linne, C. (1758) Systema Naturae, per regna tria naturae secundum classes, ordines, genera, species cum characteribus, dif- ferentiis, synonymis, locis. Editio Decima, Reformata. Vol. 1. Laurentius Salvius, Holmiae, 824 pp. https: // doi. org / 10.5962 / bhl. title. 542
Lorenz, H., & Kraus, M. (1957) Die Larvalsystematik der Blattwespen (Tenthredinoidea und Megalodontoidea). Abhandlungen zur Larvalsystematik der Insekten, (1), 1 - 339.
MacGillivray, A. D. (1909) A new genus and some new species of Tenthredinidae. The Canadian Entomologist, 41 (10), 345 - 362. https: // doi. org / 10.4039 / Ent 41345 - 10
Retzius, A. J. (1783) Caroli De Geer (....) Genera et species insectorum e generosissimi auctoris scriptis extraxit, digessit, latine quoad partem reddidit, et terminologiam insectorum Linneanam addidit. Siegfried Lebrecht Crusium, Lipsiae, pp. i-vi + 7 - 220 + 1 - 32.
Smith, D. R. (1971) Nearctic sawflies, III. Heterarthrinae: Adults and larvae (Hymenoptera: Tenthredinidae). U. S. Department of Agriculture, Technical Bulletin, 1420, 1 - 84, pls. 1 - 18.
Taeger, A., Blank, S. M. & Liston, A. D. (2010) World catalog of Symphyta (Hymenoptera). Zootaxa, 2580 (1), 1 - 1064. https: // doi. org / 10.11646 / zootaxa. 2580.1.1
Takeuchi, K. (1952) A Generic Classification of the Japanese Tenthredinidae (Hymenoptera: Symphyta). Kyoto, 90 pp.
Vasu, V. (1998) First records and new species of Caliroa Costa (Hymenoptera: Tenthredinidae: Heterarthrinae) from India. Entomotaxonomia, 20, 285 - 290.
Wei, M. (1998) A review of Caliroini with descriptions of new taxa from China (Hymenoptera: Heterarthridae). Journal of Central South Forestry University, 18 (4), 25 - 34.
Zhelochovtsev, A. N. & Zinovjev, A. G. (1988) [Suborder Symphyta (Chalastogastra), Sawflies and Horntails]. In: Zhelokhovcev, A. N., Tobias, V. I. & Kozlov, M. A. (Eds.), Keys to the Insects of the European Part of the USSR. Vol. 3. Hymenoptera. Part 6. Leningrad, Nauka, pp. 7 - 237. [in Russian, English translation, 1994, Keys to the Insects of the European Part of the USSR. Vol. 3. Hymenoptera. Part 6. Symphyta. E. J. Brill, Leiden, New York, Koln.]
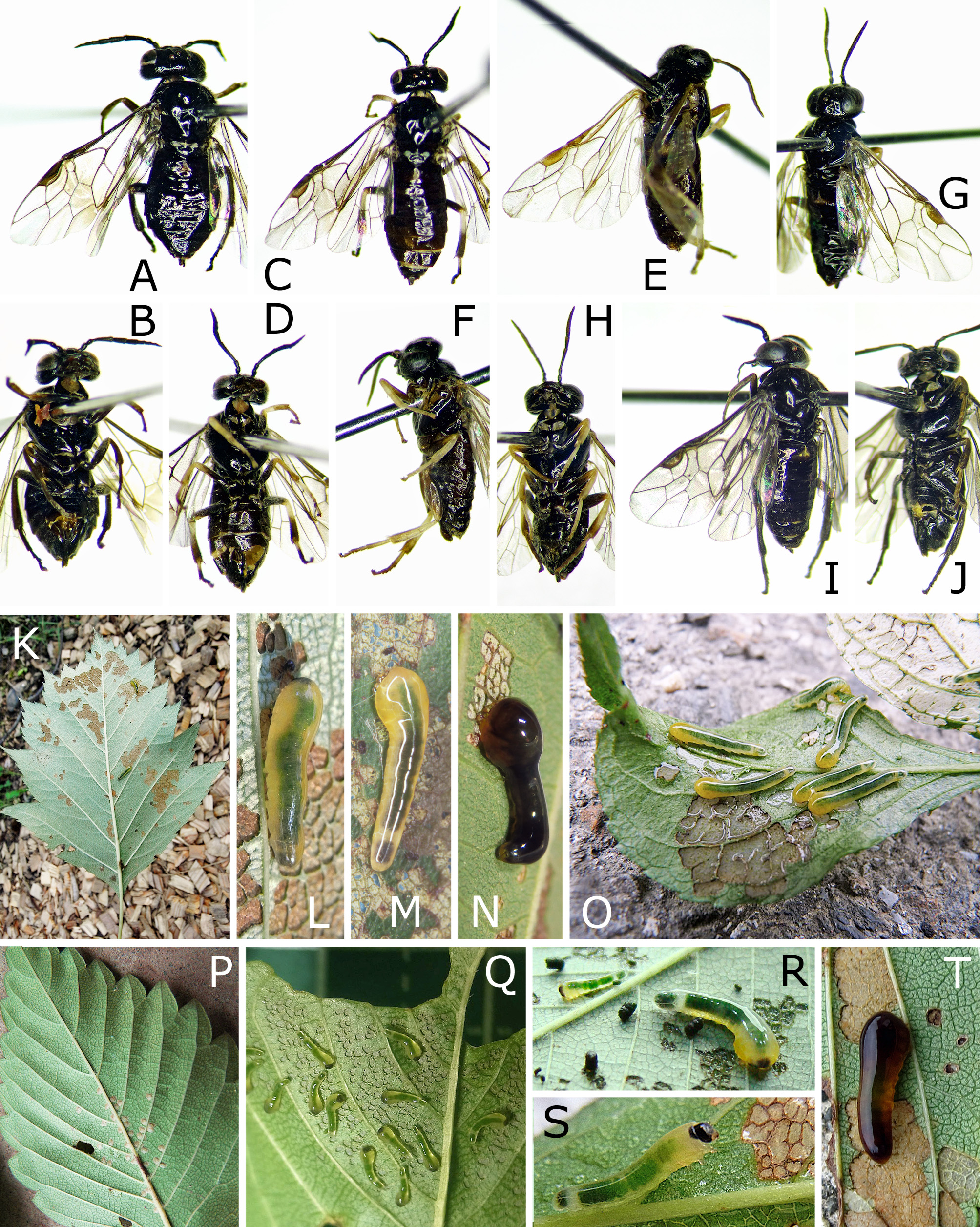
FIGURE 1. Female adults (A–J) and immature stages (K–T). A, B, Caliroa cerasi, female, Sapporo. C, D, K, L, C. bibaiensis: C, D, female, holotype; K, L, late instar larvae on Crataegus chlorosarca, Bibai, 6 August 2009. E–H, M, C. oishii: E, F, female, holotype or paratopotype of C. oishii; G, H, female, holotype of C. quercivora; M, late instar larva on Quercus crispula, Shintoku, 28 August 1993. I, J, N, C. ibukii: I, J, female, holotype; N, final feeding-instar larva, 6 July 2018. O, C. staphyleae, late instar larvae on Staphylea bumalda, Nakagawa, 9 July 2012. P–S, C. zelkovae: P, eggs in Zelkova serrata, Nakagawa, 30 June 2010; Q, young larvae on Z. serrata, Nakagawa, 26 June 2014; R, middle and late instar larvae on Z. serrata, Nakagawa, 30 June 2010; S, late instar larva on Prunus domestica, Nakagawa, 8 July 2012. T, C. nire, late instar larva on Ulmus pumila, holotype, Shintoku, 21 August 2011. A, C, E, G, I, dorsal or dorsolateral view. B, D, F, H, J, ventral or ventrolateral view. K–M, T, photographed by H. Hara. N–S, photographed by S. Ibuki.
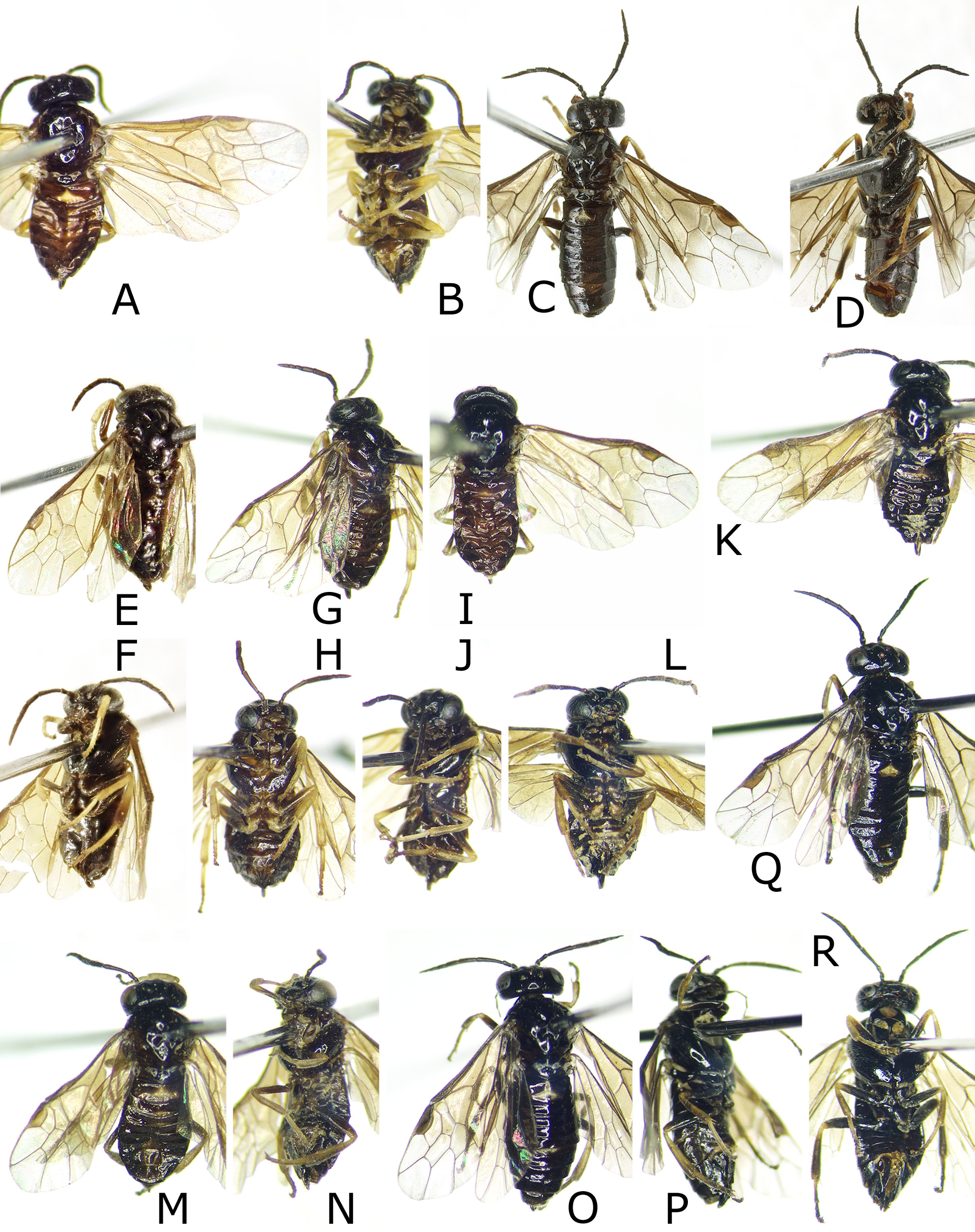
FIGURE 2. Female adults. A, B, Caliroa vaccini, paratype. C, D, C. ouensis, holotype. E, F, C. aizankei, holotype. G–J, C. staphyleae: G, H, lectotype of C. staphyleae; I, J, holotype or paratype of C. staphylea. K–P, C. zelkovae: K, L, lectotype of C. zelkovae; M, N, holotype of C. zelkova; O, P, Nakagawa, host Prunus domestica. Q, R, C. nire, holotype.A, C, E, G, I, K, M, O, Q, dorsal or dorsolateral view. B, D, F, H, J, L, N, P, R, ventral or ventrolateral view.
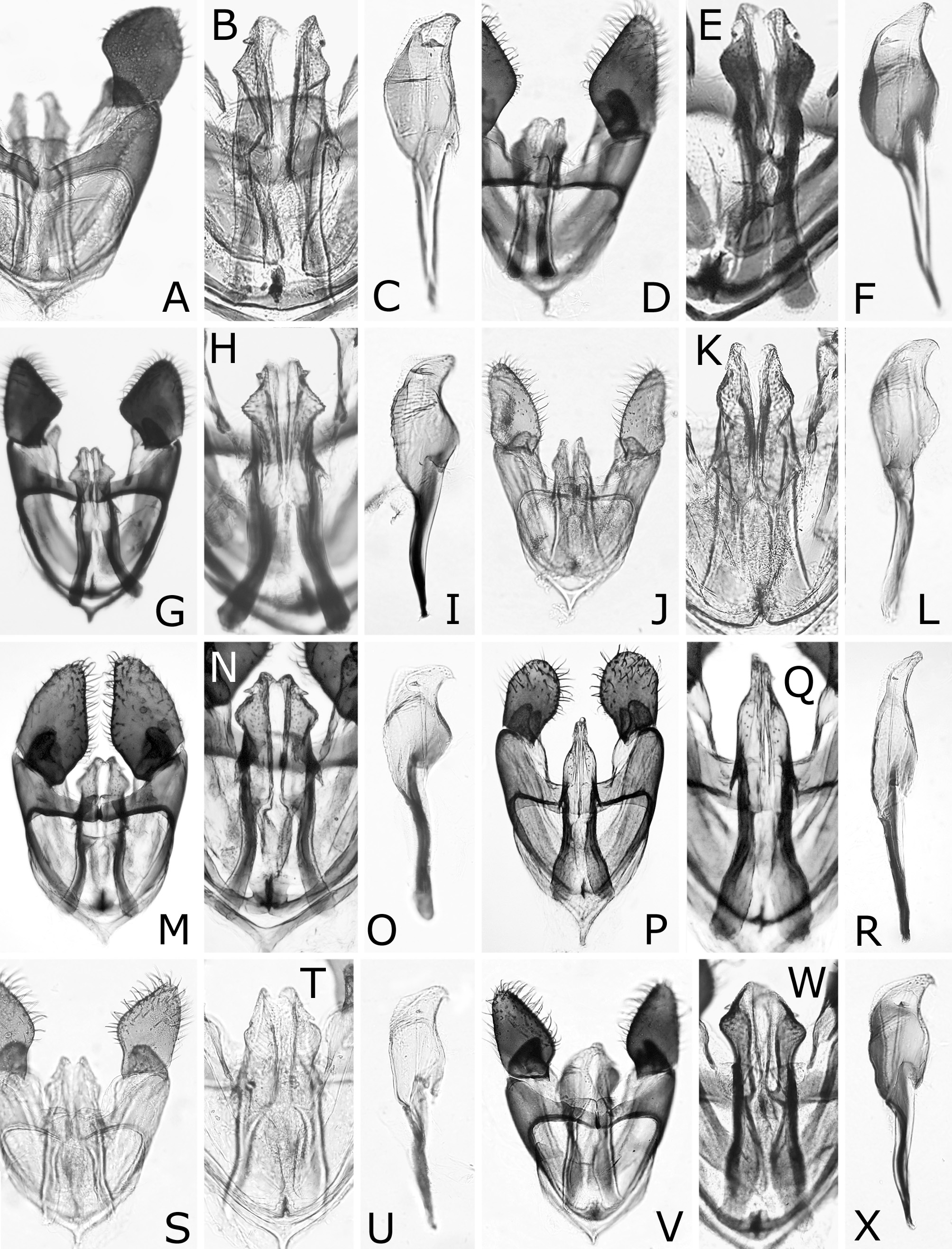
FIGURE 3. Heads (A–M), antennae (N–W) and hind tarsus (X). A, K, L, V, Caliroa zelkovae: A, K, female, Nakagawa, host Ulmus davidiana var. japonica; L, V, female, lectotype. B–D, N, X, C. cerasi, female: B–D, N, Shimizu; X, Sapporo. E, O, C. bibaiensis, female, holotype. F, P, C. oishii, female, holotype or paratopotype. G, Q, C. ibukii, female, holotype. H, R, C. vaccini, female, paratype. S, C. ouensis, female, holotype. I, T, C. aizankei, female, holotype. J, U, C. staphyleae, female: J, paralectotype; U, lectotype. M, W, C. nire, female, holotype. A, B, dorsal view. C, anterolateral view. D–M, frontal view. N–W, inner or outer lateral view. X, anteroventral view. P, Q, U, X, reversed.
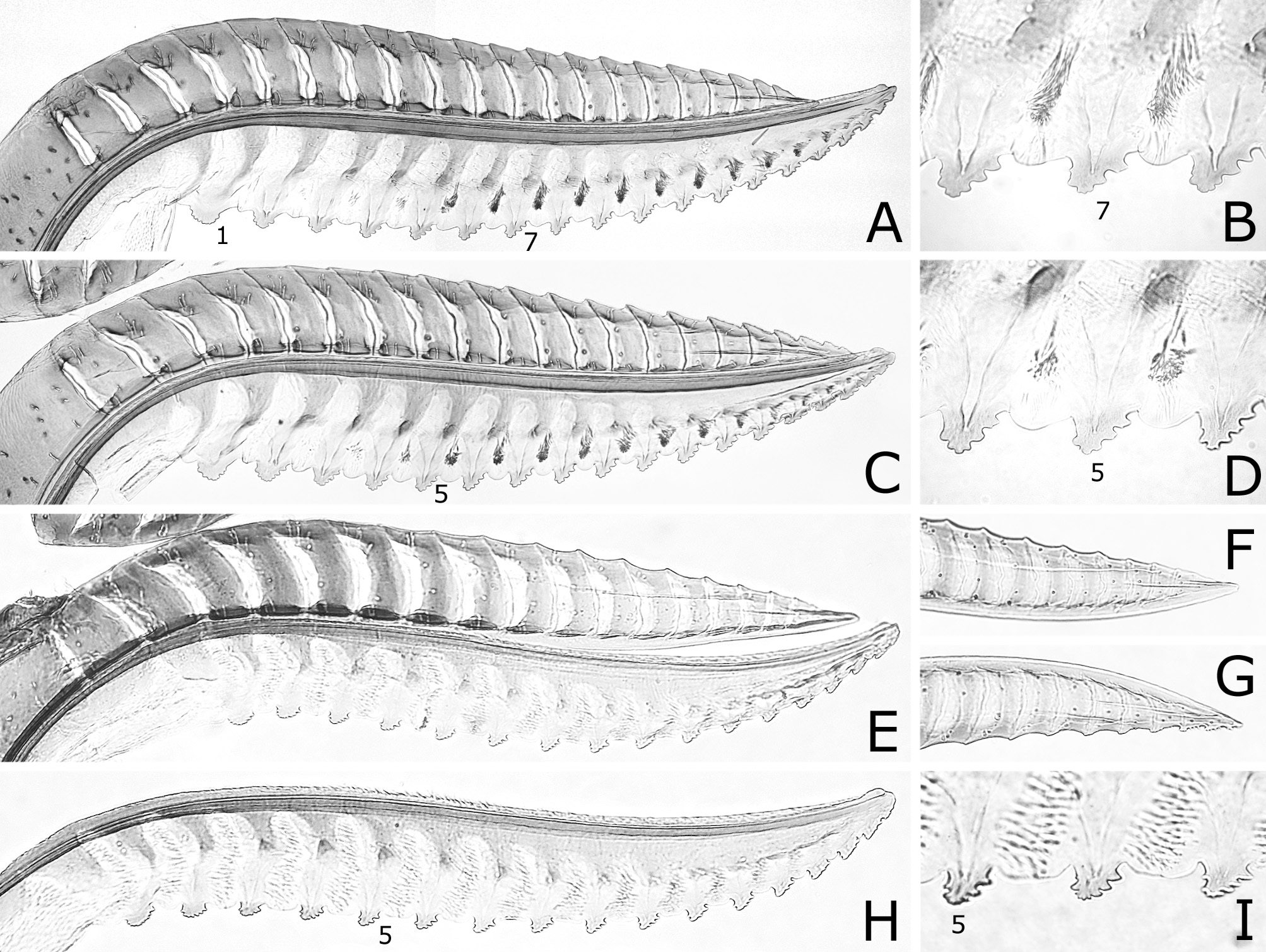
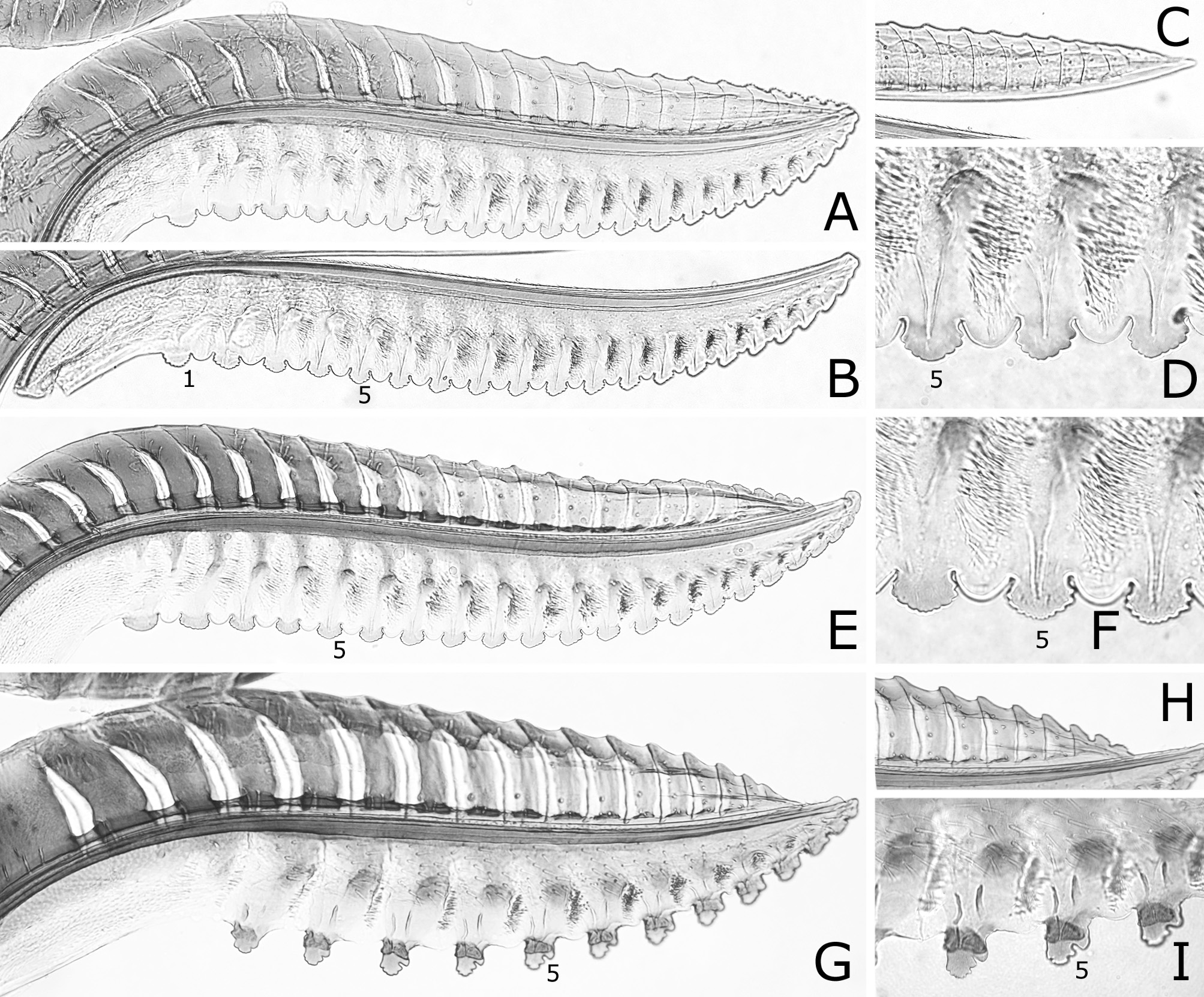
FIGURE 6. Lances and lancets (A, C, E, H), middle ventral parts of lancets (B, D, I) and apices of lances (F, G). A–D, Caliroa cerasi: A, B, Sapporo; C, D, Germany. E–I, C. bibaiensis: E, holotype; F–I, paratype. 1, 5, 7, first (most basal), fifth, seventh serrula. B–D, E, H, I, reversed.
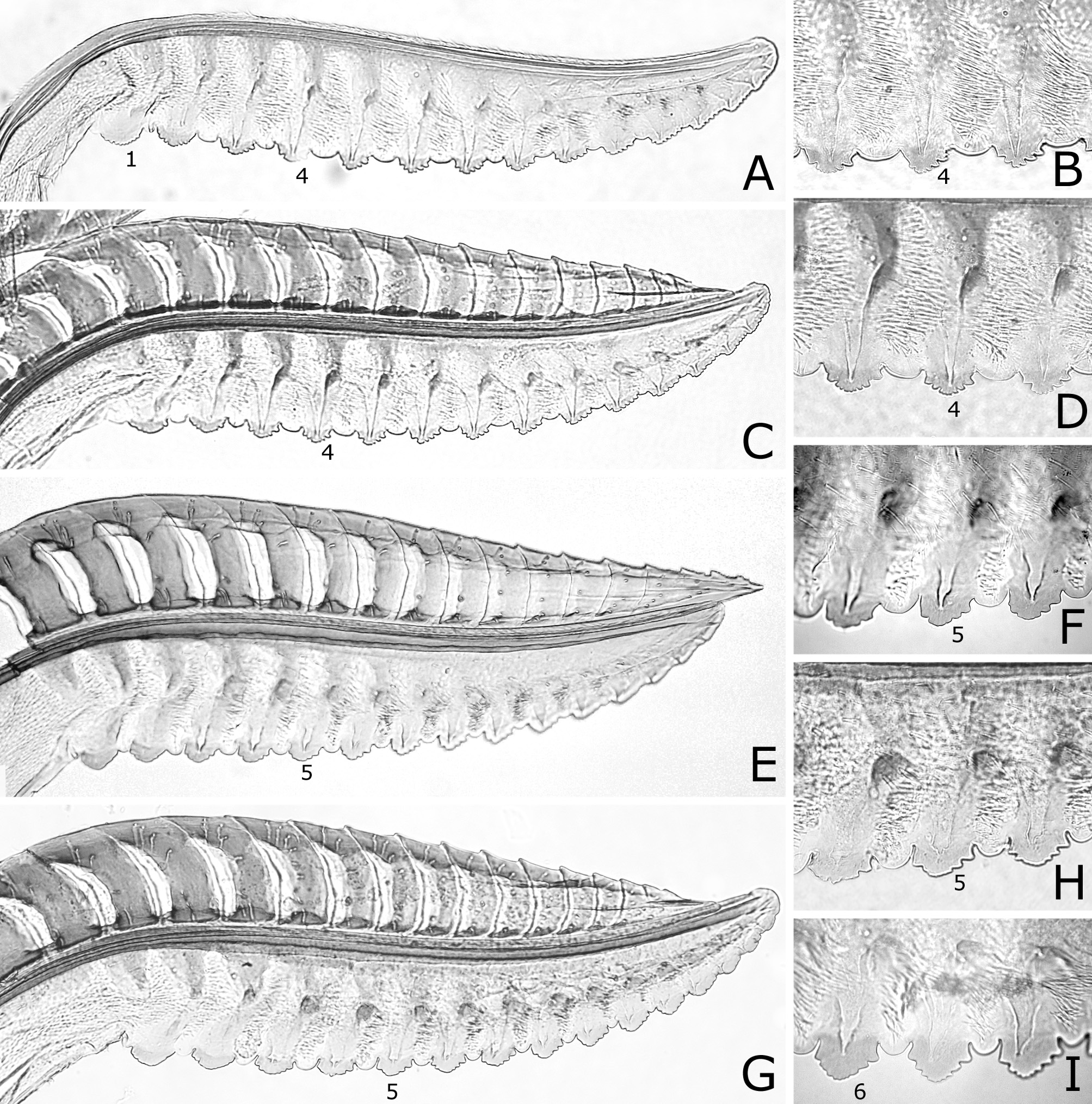
FIGURE 7. Lances and lancets (A, B, E, G), apices of lances (C, H) and middle ventral parts of lancets (D, F, I). A–F, Caliroa oishii: A–D, holotype or paratopotype of C. oishii; E, F, holotype of C. quercivora. G–I, C. ibukii, holotype. 1, 5, first (most basal), fifth serrula.A, G, I, reversed.
FIGURE 8. Lances and lancets (A, C, E, G) and middle ventral parts of lancets (B, D, F, H, I). A–D, Caliroa vaccini: A, B, paratype; C, D, holotype or paratype. E–H, C. ouensis: E, F, holotype; G, H, paratype, Mt. Zao. I, C. annulipes, Austria. 1, 4, 5, 6, first (most basal), fourth, fifth, sixth serrula.A, B, E, reversed.
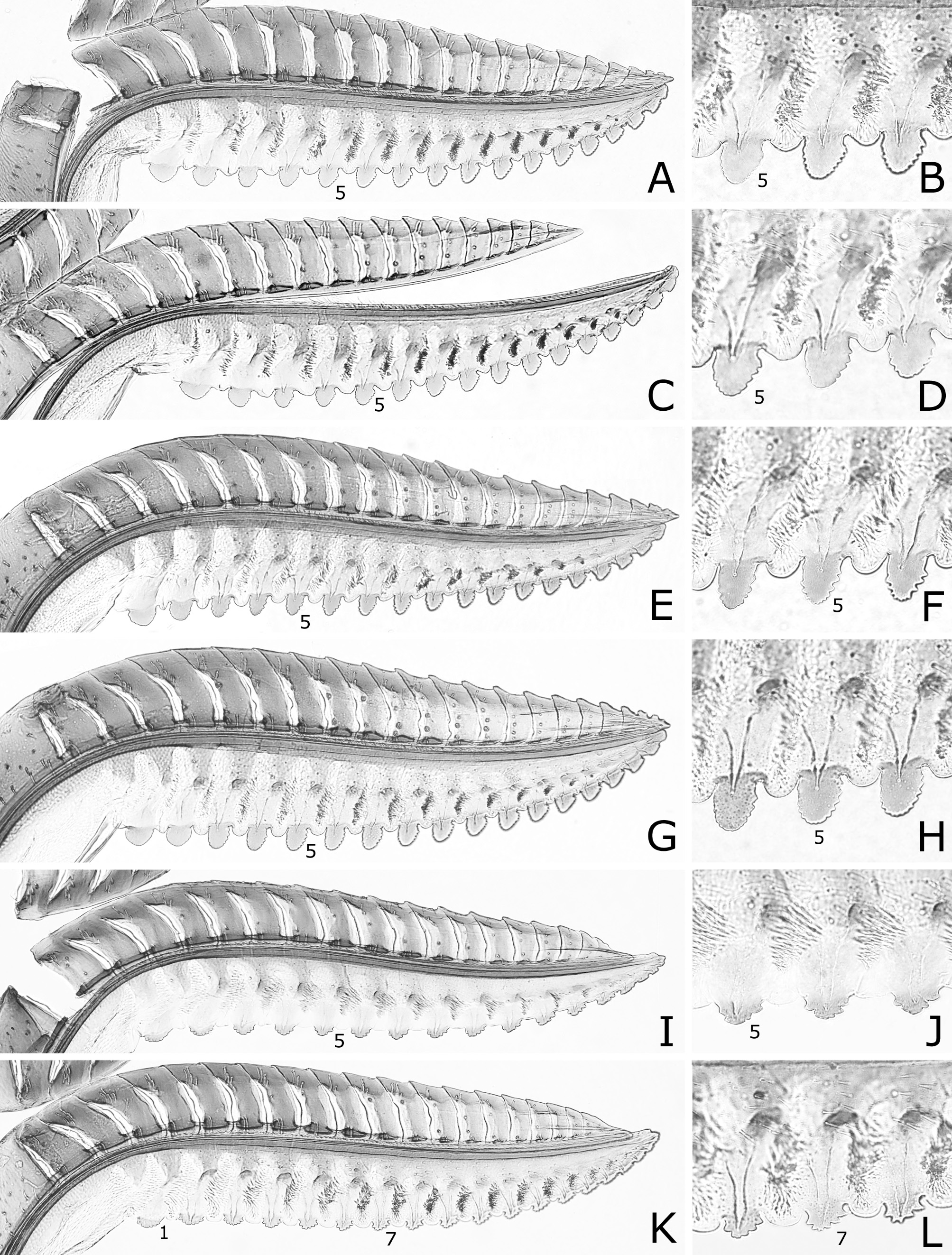
FIGURE 9. Lances and lancets (A, C, E) and middle ventral parts of lancets (B, D, F). A, B, Caliroa aizankei, holotype. C–F, C. staphyleae: C, D, lectotype of C. staphyleae; E, F, holotype or paratype of C. staphylea. 5, fifth serrula. E, F, reversed.
FIGURE 10. Lances and lancets (A, C, E, G, I, K) and middle ventral parts of lancets (B, D, F, H, J, L). A–H, Caliroa zelkovae: A, B, lectotype of C. zelkovae; C, D, holotype of C. zelkova; E, F, Nakagawa, host Ulmus davidiana var. japonica; G, H, Nakagawa, host Prunus domestica. I, J, C. matsumotonis, Higashine. K, L, C. nire, holotype. 1, 5, 7, first (most basal), fifth, seventh serrula. G, H, reversed.
FIGURE 11. Male genitalia in ventral view (A, D, G, J, M, P, S, V), penis valves in dorsal view and lateral view, left dorsal (B, C, E, F, H, I, K, L, N, O, Q, R, T, U, W, X). A–C, Caliroa cerasi, Finland. D–F, C. oishii, Shintoku. G–I, C. ibukii, paratype. J–L, C. vaccini, paratype. M–O, C. ouensis, paratype. P–R, C. staphyleae, Nakagawa. S–X, C. zelkovae: S–U, Hobara; V–X, Nakagawa, host Prunus domestica.
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
Caliroa Costa, 1859
| Hara, Hideho & Ibuki, Shinichi 2020 |
Periclistoptera
| Ashmead, W. H. 1898: 255 |
Eriocampoides
| Konow, F. W. 1890: 233 |
Caliroa
| Koch, F. & Smith, D. R. 2011: 445 |
| Taeger, A. & Blank, S. M. & Liston, A. D. 2010: 364 |
| Lacourt, J. 1999: 54 |
| Vasu, V. 1998: 286 |
| Wei, M. 1998: 26 |
| Zhelochovtsev, A. N. & Zinovjev, A. G. 1988: 57 |
| Smith, D. R. 1971: 12 |
| Lorenz, H. & Kraus, M. 1957: 111 |
| Takeuchi, K. 1952: 56 |
| Costa, A. 1859: 59 |