Filigorgia schoutedeni ( Stiasny, 1939 ) Sánchez, 2007
|
publication ID |
https://doi.org/10.1080/00222930701237315 |
|
persistent identifier |
https://treatment.plazi.org/id/03AE4A74-FF88-FFC5-FE71-8C83FE02F294 |
|
treatment provided by |
Felipe (2021-08-21 04:45:36, last updated by Plazi 2023-11-04 05:28:44) |
|
scientific name |
Filigorgia schoutedeni ( Stiasny, 1939 ) |
| status |
comb. nov. |
Filigorgia schoutedeni ( Stiasny, 1939) View in CoL com. nov.
Leptogorgia schoutedeni Stiasny 1939, p 106 View in CoL .
Leptogorgia schoutedeni: Grasshoff 1988, p 115 View in CoL .
Why are surface layer sclerites so important?
Since surface sclerites modify colony flexion by preventing compressibility of the outer cortex when they contact each other and help the colony to resist drag forces and flow ( Lewis and Von Wallis 1991), a characteristic on which natural selection can act. Their interaction with the environment has produced a great array of forms and these structures are more variable than polyp or axial sheath sclerites ( Sánchez et al. 2003b; Sánchez 2005). It is important to note, however, that the function of surface sclerites can be homoplasious and similar trends exist in many genera (e.g. Figure 12E, G View Figure 12 ). Nonetheless, surface sclerites can be helpful for supra familial classification and provide a better understanding of octocoral phylogeny in general.
It has been demonstrated that colonial characters such as branching and overall colony form are highly homoplasious, providing multiple analogous forms within the family Gorgoniidae , which should not be considered as characters for classification (e.g. whip like, sea fan, pinnate, etc.). For example, Bayer (1953) first proposed convergent evolution for gorgonian colony architectures such as sea fans ( Pacifigorgia spp. and Gorgonia spp. , Figure 2 View Figure 2 ) and sea leaves ( Phycogorgia spp. and Phyllogorgia spp. , Figure 2 View Figure 2 ). Bayer’s hypothesis for sea fans has been corroborated with mitochondrial ( Sánchez et al. 2003b; Sánchez 2004) and nuclear DNA sequences ( Aguilar and Sánchez 2007a, 2007b). Pinnate morphologies such as Pseudopterogorgia spp. and Pinnigorgia spp. seemed to have evolved their branching morphologies separately (e.g. Figure 1 View Figure 1 ). Surface layer sclerites under the resolution of SEM provided a good character to differentiate gorgoniid clades with very similar external characters.
The molecular contribution
The corroboration of Filigorgia as a genus apart from Leptogorgia is another example where recent molecular results have improved our understanding of some morphological characters ( Fukami et al. 2004). The validation of the genus Filigorgia is also supported with previous molecular studies. Mitochondrial DNA sequences from the mut-S homolog gene (MSH1) show additional classification problems within the Gorgoniidae (A. LePard and S. France, personal communication). Although focusing on Leptogorgia , they found that supposed Leptogorgia ‘‘outgroups’’ such as Pseudopterogorgia , Pacifigorgia , and Eugorgia were intermingled with various clades of Leptogorgia , which clearly show that some of the African fauna from Leptogorgia sensu lato are not reciprocally monophyletic. Williams and Lindo (1997) provided a detailed comparison of spindles of Leptogorgia spp. with several gorgoniid genera showing that scaphoid sclerites are not the best character to differentiate gorgoniid genera due to their high similarity, particularly with Indo-Pacific species of Pseudopterogorgia . Although it was not a goal of this paper, it was clearly noticed that Pacific and Atlantic Pseudopterogorgia spp. surface sclerites were not homologous (e.g. Figures 3A View Figure 3 versus 4E), and deserve further study.
Finally, it is important to note that some genera assigned to the Gorgoniidae need careful revision (hopefully both taxonomical and molecular) in order to be unambiguously assigned to this family, such as in the case of Hicksonella and Rumphella ( Grasshoff and Alderslade 1997) , as well as Adelogorgia and Eunicella , whose sclerites are not likely homologous to the species presented here. In addition, there are a number of plexaurid genera such as Swiftia ( Goldberg 2001) and Plexaurella that, with recent molecular analyses ( Sánchez et al. 2003b; Wirshing et al. 2005; McFadden et al. 2006), have been found to resemble some gorgoniids.
Aguilar C, Sanchez JA. 2007 a. Phylogenetic hypotheses of gorgoniid octocorals according to ITS 2 and their predicted RNA secondary structures. Molecular Phylogenetics & Evolution. Forthcoming.
Aguilar C, Sanchez JA. 2007 b. Molecular morphometrics: contribution of ITS 2 sequences and predicted RNA secondary structures to octocoral systematics. Bulletin of Marine Science. Forthcoming.
Bayer FM. 1953. Zoogeography and evolution in the octocorallian family Gorgoniidae. Bulletin of Marine Science of the Gulf and Caribbean 3: 100 - 119.
Fukami H, Budd AF, Paulay G, Sole' - Cava A, Chen CA, Iwao K, Knowlton N. 2004. Conventional taxonomy obscures deep divergence between Pacific and Atlantic corals. Nature 427: 832 - 835.
Goldberg WM. 2001. The sclerites and geographic distribution of the gorgonian Swiftia exserta (Coelenterata: Octocorallia: Holaxonia). Bulletin of the Biological Society of Washington 10: 100 - 109.
Grasshoff M. 1988. The genus Leptogorgia (Octocorallia: Gorgoniidae) in West Africa. Atlantide Report 14: 91 - 147.
Grasshoff M, Alderslade P. 1997. Gorgoniidae of Indo-Pacific reefs with descriptions of two new genera (Coelenterata: Octocorallia). Senckenbergiana Biologica 77: 23 - 25.
Lewis JC, Von Wallis E. 1991. The function of surface sclerites in gorgonians (Coelenterata Octocorallia). Biological Bulletin 181: 275 - 288.
McFadden CS, France SC, Sanchez JA, Alderslade P. 2006. A molecular phylogenetic analysis of the Octocorallia (Cnidaria: Anthozoa) based on mitochondrial protein-coding sequences. Molecular Phylogenetics & Evolution 41 (3): 513 - 527.
Sanchez JA, McFadden CS, France SC, Lasker HR. 2003 b. Molecular phylogenetic analyses of shallow-water Caribbean octocorals. Marine Biology 142: 975 - 987.
Sanchez JA. 2004. Evolution and dynamics of branching colonial form in marine modular Cnidarians: gorgonian octocorals. Hydrobiologia 530: 283 - 290.
Sanchez JA. 2005. Systematics of the bubblegum corals (Paragorgiidae: Octocorallia: Cnidaria) with description of new species from New Zealand and the Eastern Pacific. Zootaxa 1014: 1 - 72.
Stiasny G. 1939. Gorgonaria von Kap Blanco, Senegal und Rio d'Ouro (Aus dem Zoologischen Museum, Amsterdam). Revue Zoologique et Botanique Africaine 32: 285 - 322.
Williams GC, Lindo KG. 1997. A review of the Octocorallian genus Leptogorgia (Anthozoa: Gorgonidae) in the Indian Ocean and subantarctic, with description of a new species and comparisons with related taxa. Proceedings of the California Academy of Sciences 49: 499 - 521.
Wirshing HH, Messing CG, Douady CJ, Reed J, Stanhope MJ, Shivji MS. 2005. Molecular evidence for multiple lineages in the gorgonian family Plexauridae (Anthozoa: Octocorallia). Marine Biology 147: 497 - 508.
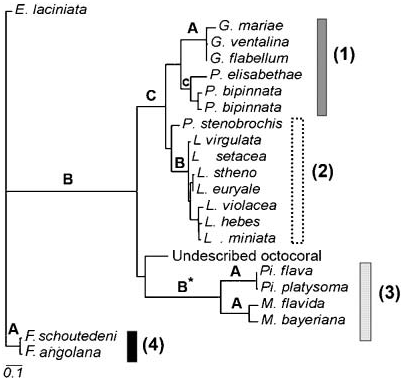
Figure 1. Phylogenetic hypothesis (maximum likelihood estimated phylogram) of Atlantic gorgoniids, modified from Aguilar and Sánchez (2007a, Figure 4A). The ‘‘Undescribed octocoral’’ refers to the new genus and species described below. Above node letters: A, support corresponding to values.90 in 100 bootstrap maximum likelihood replicates, 1000 bootstrap values in maximum parsimony together with values over 0.9 posterior probabilities of Bayesian inference; B, support corresponding to values.90 in 100 bootstrap maximum likelihood replicates but lesser values for maximum parsimony or Bayesian inference (,70 or 0.70, respectively); C, support corresponding to values,70 in 100 bootstrap maximum likelihood replicates but higher values for maximum parsimony or Bayesian inference (,90 or 0.90, respectively). Character mapping: grey bars, gorgoniids with scaphoid sclerites (Clade 1); open bars-dotted, gorgoniids with capstan-derived sclerites (Clade 2); grid bars, gorgoniids with asymmetrical spiny sclerites (Clade 3); black bars, gorgoniids with long and spiny spindles (Clade 4). Scale in substitutions per site. *Pinnigorgia and Muriceopsis are also supported by a complete compensatory base change in their RNA secondary structure, a rare evolutionary event (see Aguilar and Sánchez 2007a).
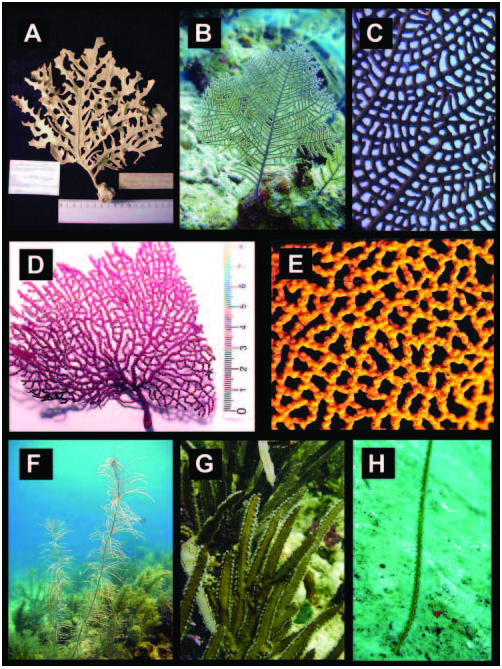
Figure 2. Diverse species of gorgoniids. (A) Phyllogorgia dillatata (Brazil, USNM 5252); (B) Gorgonia mariae (Carrie Bow Cay, Belize); (C) G. ventalina (San Salvador, Bahamas); (D) Pacifigorgia elegans (Brazil, USNM 50953); (E) P. agassizi (Colombia, Invemar collection); (F) Pseudopterogorgia sp. (Carrie Bow Cay, Belize); (G) Pterogorgia anceps; (H) Leptogorgia stheno (Bocas del Toro, Panama´).
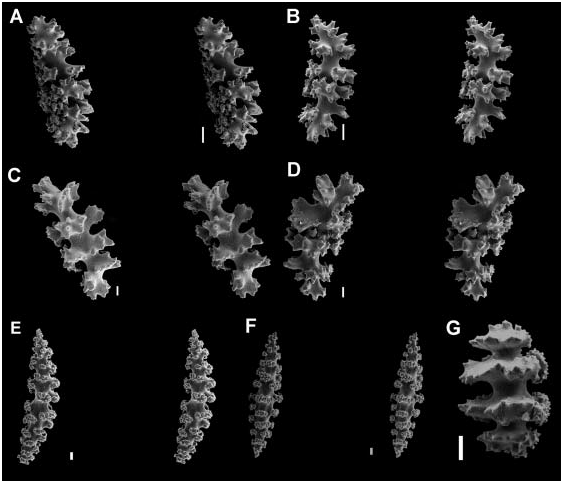
Figure 3. SEM stereopairs and/or images of sclerites from the surface coenenchyme layer of species of gorgoniids with scaphoids sclerites (Clade 1). (A) Pseudopterogorgia acerosa (Tobago), stereopair; (B) Gorgonia mariae Bayer, stereopair (USNM 1007505); (C) Olindogorgia macgravi (Bayer); (D) Pterogorgia anceps (personal collection); (E, F) Pterogorgia citrina (USNM 1007406). Scale bars: 0.01 mm (A, C, E, F); 0.003 mm (B); 0.04 mm (D).
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |
Filigorgia schoutedeni ( Stiasny, 1939 )
| Sánchez, Juan A. 2007 |
Leptogorgia schoutedeni:
| Grasshoff M 1988: 115 |
Leptogorgia schoutedeni
| Stiasny G 1939: 106 |