Helicopsis, Fitzinger, 1833Helicopsis, ASACHARACTERISTIC REPRESENTATIVE OF THE, ASACHARACTERISTIC REPRESENTATIVE OF THE
|
publication ID |
https://doi.org/10.1093/zoolinnean/zlaa156 |
|
persistent identifier |
https://treatment.plazi.org/id/03A687CE-FFCB-0E72-FC64-88C7FDACF9ED |
|
treatment provided by |
Plazi |
|
scientific name |
HelicopsisHelicopsis |
| status |
|
SYSTEMATICS OF HELICOPSIS
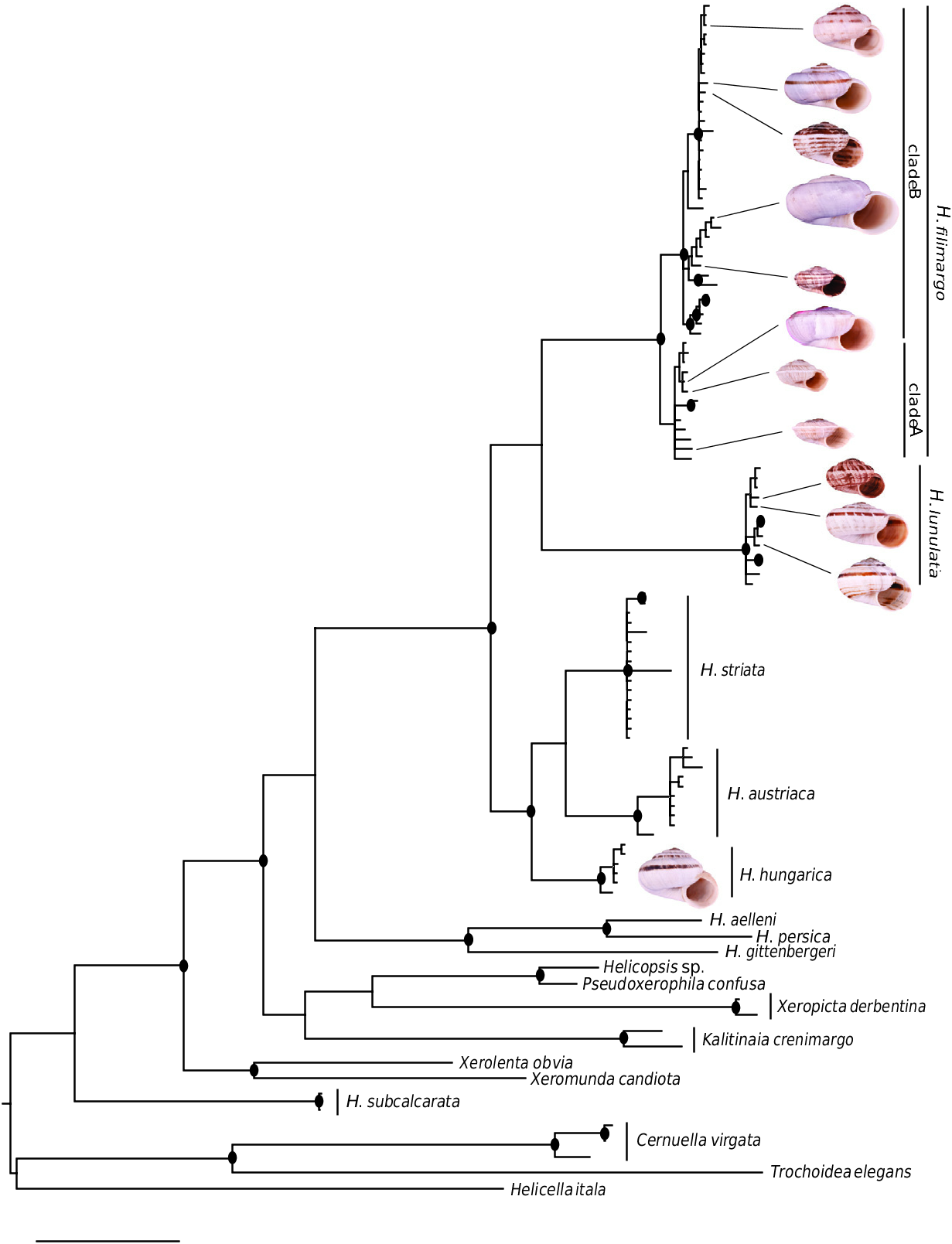
Our phylogenetic analysis of 16S rDNA sequences ( Fig. 2 View Figure 2 ; Supporting Information, Fig. S1 View Figure 1 ) confirms that Helicopsis forms a clade with Xerolenta , Xeromunda , Xeropicta and Pseudoxerophila ( Hausdorf & Bössneck 2016; Neiber et al., 2017) and shows that also Kalitinaia Hudec & Lezhawa, 1967 from the Caucasus region belongs in this clade named Helicopsini. The delimitation of Helicopsis was considered questionable, because it is characterized only by plesiomorphic character states ( Hausdorf, 1996). Actually, the molecular phylogeny based on mitochondrial 16S rDNA sequences ( Fig. 2 View Figure 2 ; Supporting Information, Fig. S1 View Figure 1 ) revealed that H. subcalcarata from Turkey and Helicopsis sp. from Cyprus, which is closely related to H. cypriola (Westerlund, 1889) , do not belong to Helicopsis . Helicopsis subcalcarata is sister to the remaining Helicopsini and, thus, a separate genus is required for this species. Helicopsis sp. from Cyprus is closely related to the eastern Mediterranean genus Pseudoxerophila . Amore detailed analysis of the relationships of these species is in preparation.
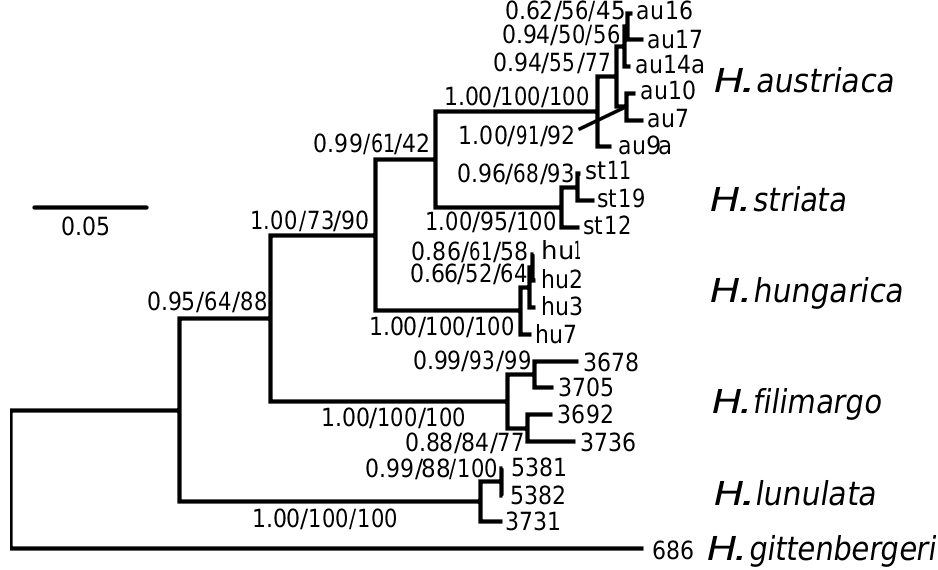
Helicopsis gittenbergeri from Greece formed together with H. aelleni and H. persica from Iran the well-supported sister-group of the Helicopsis species from Eastern and Central Europe [as in the phylogenetic analysis of Hausdorf & Bössneck (2016)]. The monophyly of the Eastern and Central European Helicopsis species , as well as the monophyly of the Central European Helicopsis group including H.striata , H. austriaca and H. hungarica , are well supported in the 16S rDNA tree ( Fig. 2 View Figure 2 ; Supporting Information, Fig. S1 View Figure 1 ) and the tree based on the concatenated COI, 12S rDNA and 16S rDNA sequences ( Fig. 3 View Figure 3 ). The populations from Eastern Europe, which are the focus of this study, form two highly supported clades in these trees, for which the oldest names are H. lunulata (Krynicki, 1833) and H. filimargo (Krynicki, 1833) . The analyses of the concatenated sequences show that H. filimargo is sister to the Central European clade, while H. lunulata is sister to all other Central and Eastern European species ( Fig. 3 View Figure 3 ).
The H. lunulata clade includes mainly populations with depressed conical shells previously identified as H. striata . However, individuals with disc-like shells, identified as H. instabilis based on shell characters and originating from the Lviv region, the type locality of this nominal species, also belonged to this clade. The same is true for the disc-like specimens from Romania. Finally, specimens also identified as H. ( filimargo ) arenosa from Yelanets Steppe Nature Reserve in the Mykolaiv region belong to this clade. Balashov (2016: 471, fig. 264) thought that the specimens at this locality represent sympatric populations of H. striata and H. filimargo arenosa . However, both, specimens identified as H. striata and specimens identified as H. filimargo arenosa belong to the same clade. Beside specimens from Romania, Ukraine and adjacent Russia, an individual of an introduced population from north-eastern Germany (see Zettler et al., 2006) also belongs in this clade. The disc-like shells of this population agree with shells described as Helicella ? depulsa from the surroundings of Varna in Bulgaria by Pintér (1969), which is similar to H. instabilis . Helicella ? depulsa has been classified as a subspecies or a synonym of Xerolenta obvia ( Welter-Schultes, 2012) . Specimens from the surroundings of Varna have to be examined to clarify the identity of the nominal taxon.
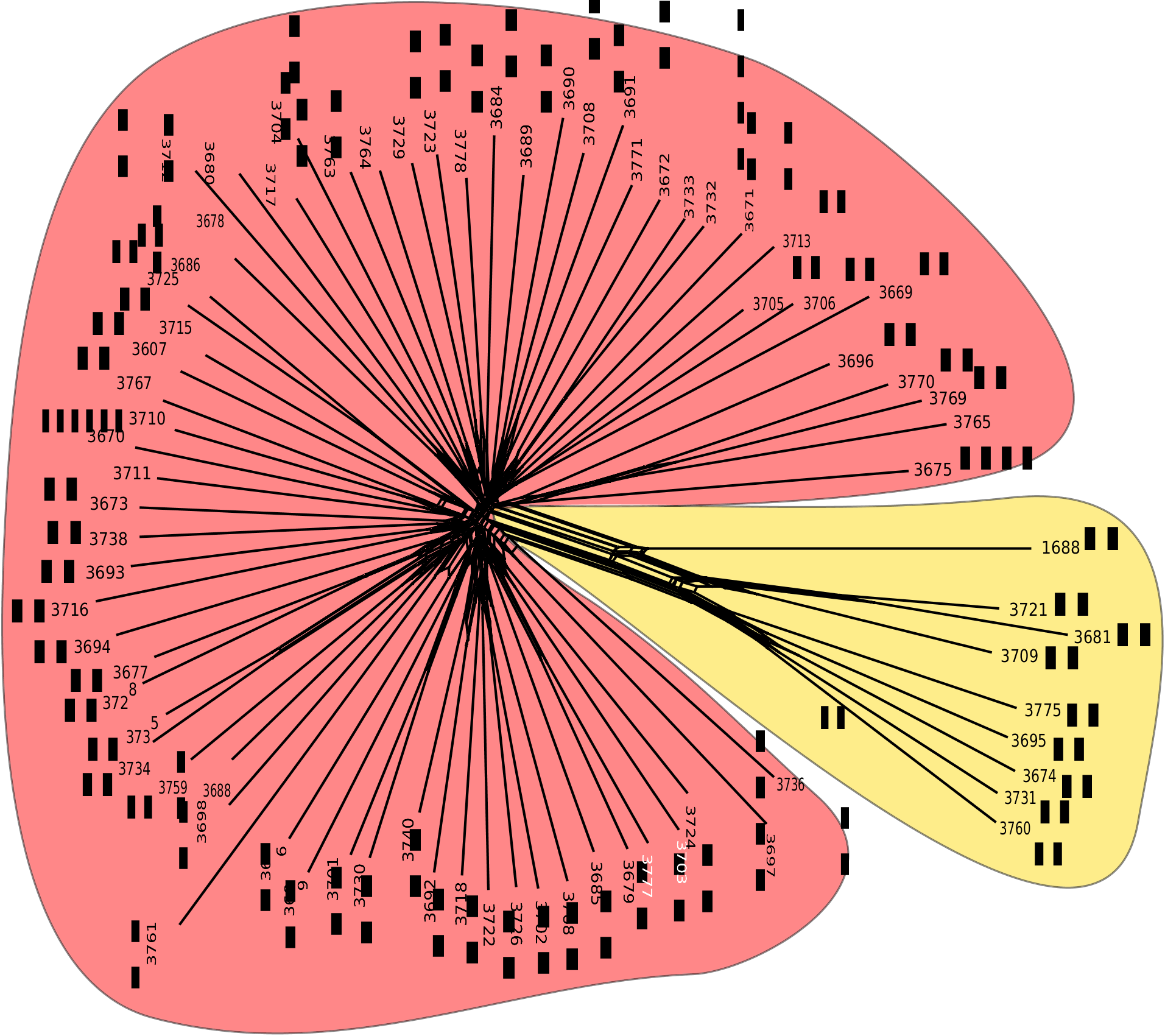
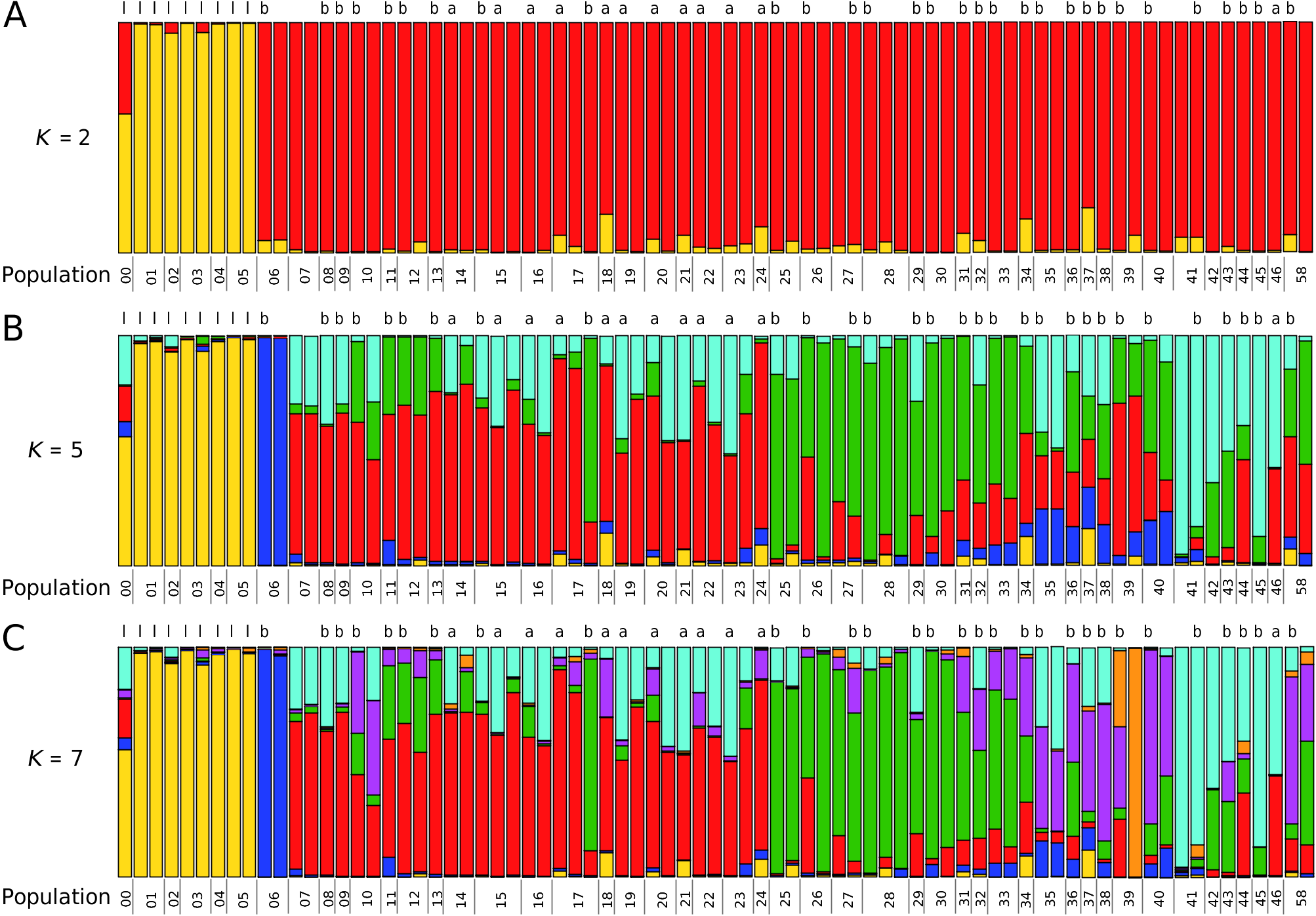
The clustering of the various forms that share the discussed mitochondrial haplotype clade in the network based on the nuclear AFLP markers ( Fig. 4 View Figure 4 ) and in a STRUCTURE analysis of these data ( Fig. 5 View Figure 5 ) demonstrated that they actually represent a single species. This species is distinct from H. striata , with which most populations of this clade were identified. Helicopsis striata is more closely related with the Eastern Alpine H. austriaca and H. hungarica from the Pannonian Basin, than with the Helicopsis species from Eastern Europe ( Fig. 2 View Figure 2 ; Supporting Information, Fig. S1 View Figure 1 ) and is apparently restricted to the Northern steppes and the northwestern Pannonian Basin in Central Europe.
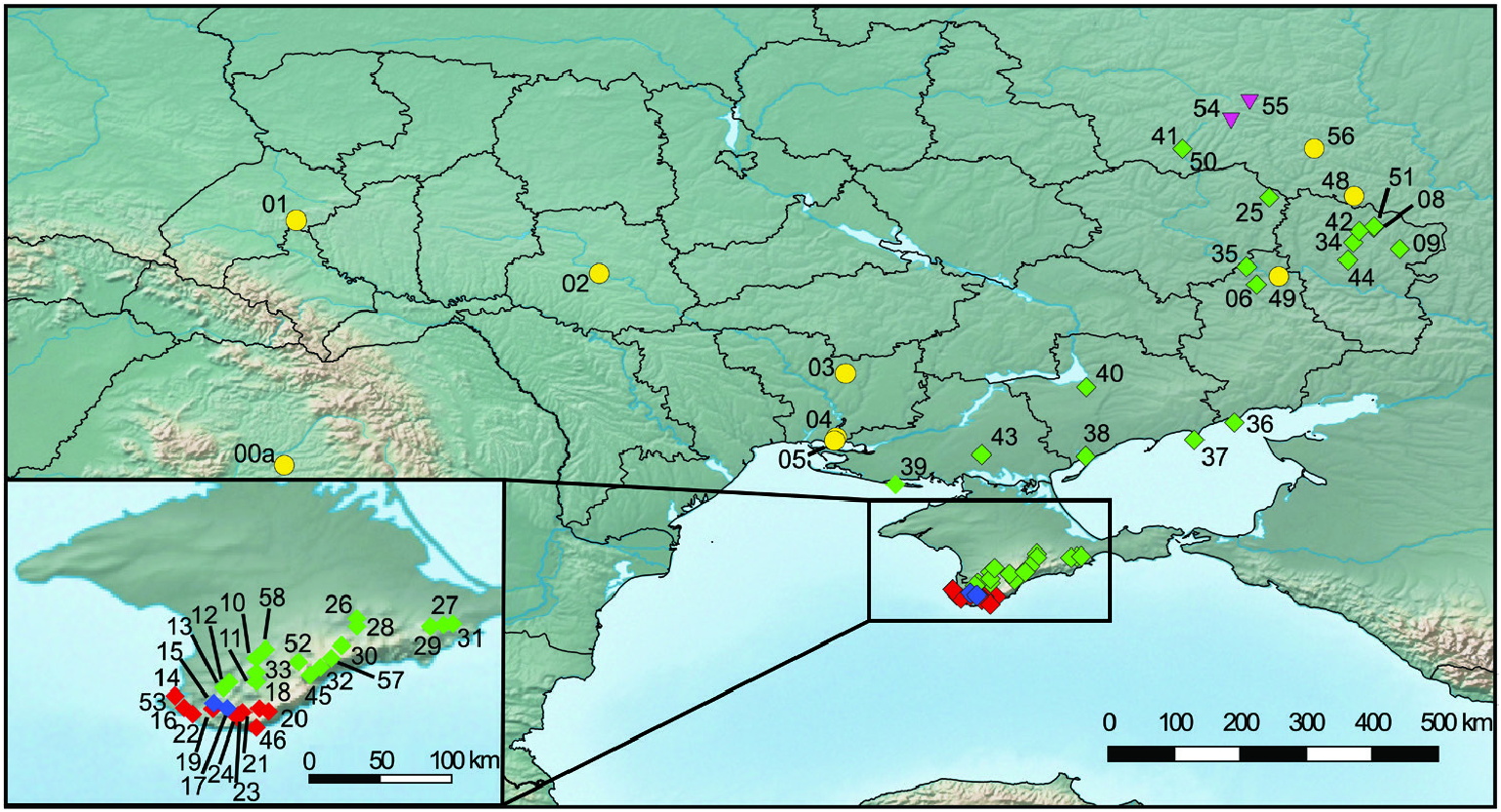
There are several names for forms that belong to the discussed species. The most often used name is H. instabilis (Rossmässler, 1838) , given for a disc-like form from Lviv in Western Ukraine, but also used for disc-like forms from Romania. However, the oldest and, thus, valid name of the species is H. lunulata (Krynicki, 1833) , given for a depressed conical form from Odessa [lectotype see Hausdorf (1990: pl. 2 fig. 4); paralectotype see Sysoev & Schileyko (2009: fig. 113A)]. Our records show that H. lunulata occurs in Transylvania, in the Podolian Upland and the Black Sea Lowland and in the Donetsk Upland and the adjacent Central Russian Upland ( Fig. 1 View Figure 1 ; Supporting Information, Table S1 View Table 1 ), from where a sequence belonging to the H. lunulata clade has already been reported by Sychev & Snegin (2016) from Zasosna. Unfortunately, no AFLP data were available from these eastern specimens. Thus, we cannot completely exclude the possibility that these specimens belong to H. filimargo with a mitochondrial introgression, although there is no evidence for this. There are records of H. instabilis and H. striata from Moldova ( Balashov et al., 2013b) and Bulgaria ( Damjanov & Likharev, 1975), indicating that H. lunulata is probably also distributed in these countries. However, it has to be checked whether all populations identified as H. instabilis so far actually belong to H. lunulata . Beside populations with disc-like shells corresponding to H. instabilis , there are also populations with depressed conical shells in Transylvania that are often classified as an endemic species, H. cereoflava ( Grossu, 1983; Welter-Schultes, 2012). Considering the large intraspecific variability observed in H. lunulata , it is possible that all Transylvanian populations of Helicopsis belong to this species. However, typical H. cereoflava should be studied genetically to prove this hypothesis.
The H. filimargo clade is found in Ukraine east of the Dnieper River, from Crimea and the adjacent eastern part of the Black Sea Lowland to the Donetsk Upland and the Central Russian Upland ( Fig. 1 View Figure 1 ). This group comprises even more diverse morphological forms than H. lunulata . It includes, beside the nominal species listed from Crimea and the Black Sea Lowland, H. filimargo , H. arenosa and H. retowskii and the recently described supposed endemics from the Donetsk Upland, H. luganica , H. martynovi and H. subfilimargo , plus some populations classified as H. striata from eastern Ukraine.
Helicopsis filimargo and H. arenosa have also been recorded from Dobrogea in Romania ( Grossu, 1983) and the latter species was also found in Bulgaria ( Damjanov & Likharev, 1975). However, given the lack of diagnostic morphological characters distinguishing H. filimargo and H. lunulata , it has to be checked genetically whether the populations from Romania and Bulgaria actually belong to H. filimargo or whether they represent forms of H. lunulata .
The H. filimargo clade is subdivided into two geographically restricted clades in the mitochondrial tree ( Fig. 2 View Figure 2 ; Supporting Information, Fig. S1 View Figure 1 ). One occupies most of the range, whereas the other is restricted to southernmost Crimea ( Fig. 1 View Figure 1 ). There are a few populations along the mountains where both haplotypes occurred together. The specimens from southernmost Crimea are concentrated in one cluster of the network based on nuclear AFLP markers ( Fig. 4 View Figure 4 ). However, there are also specimens from the rest of the range with the other mitochondrial subclade among them. This indicates that differentiation between the two subgroups is also evolving in the nuclear genome, but is not yet advanced. This is also corroborated by the STRUCTURE analyses ( Fig. 5 View Figure 5 ). Thus, we do not classify them as subspecies.
The STRUCTURE analyses shows little admixture between H. filimargo and H. lunulata ( Fig. 5 View Figure 5 ), supporting the species status of the two taxa. The only exception was the specimen from the introduced population in north-eastern Germany. However, this specimen is older and not preserved in 100% alcohol, so that the slightly different AFLP pattern might be an artefact resulting from DNA degradation. The specific distinctness of H. filimargo and H. lunulata is also corroborated by the isolation by distance analysis that shows that the distances between individuals of the two taxa are larger than expected based on their geographical distances and the relationship of genetic distances with geographical distances found within the two taxa ( Fig. 7 View Figure 7 ).
An individual from Teleshovka in the Belgorod region in the Central Russian Upland represented another clade in the mitochondrial tree ( Fig. 2 View Figure 2 ; Supporting Information, Fig. S1 View Figure 1 ), which can be identified as H. hungarica based on sequences of the species from Hungary and Austria ( Duda et al., 2018). Actually, a sequence of the same haplotype group has already been reported as H. striata from Gubkin in the Belgorod Region by Sychev & Snegin (2016) and identified as H. hungarica by Duda et al. (2018). The specific identity of the specimens from the Central Russian Upland with the Pannonian H. hungarica should be confirmed with non-mitochondrial genetic markers.
Our results, based on mitochondrial DNA sequences ( Figs 1–3 View Figure 1 View Figure 2 View Figure 3 ) and nuclear AFLP markers ( Figs 4–7 View Figure 4 View Figure 5 View Figure 6 View Figure 7 ), demonstrate that the native populations from Ukraine and adjacent regions can be classified into two species, H. lunulata and H. filimargo . This result resembles the suggestion of Balashov (2016) to classify the Ukrainian Helicopsis populations into two species, H. striata and H. filimargo . However, the delimitation and the distribution of the species in the two classifications are different. Although H. lunulata includes mainly populations with depressed conical shells formerly assigned to H. striata , it also comprises populations with disc-like shells formerly assigned to H. instabilis , which was included in H. filimargo by Balashov (2016). Moreover, Balashov (2016) classified some H. lunulata as H. filimargo arenosa and supposed that there are syntopic co-occurrences of H. striata and H. filimargo . We have not found syntopic co-occurrences of H. lunulata and H. filimargo , although we found some overlap of the ranges of the two species in the Donetsk Upland and the adjacent Central Russian Upland so that co-occurrences are possible.
geographical distance (log-transformed)
PHYLOGEOGRAPHYOF HELICOPSIS ASACHARACTERISTIC REPRESENTATIVE OF THE STEPPE FAUNA
The geographical origin of Helicopsis is unclear. Given that the clade including the Central and Eastern European species is sister to a clade comprising species from Greece and Iran, and the relationships with other genera from the eastern Mediterranean and the Caucasus ( Fig. 2 View Figure 2 ; Supporting Information, Fig. S1 View Figure 1 ), an origin on the Balkan Peninsula is most likely. The nested position of the Central European clade among the Pontic species indicates an eastern origin of the Central European species ( Fig. 3 View Figure 3 ).
As in many other steppe-inhabiting organisms ( Kajtoch et al., 2016), the differentiation pattern of the Central and Eastern European Helicopsis species reflects the geographic structuring of the steppe belt in Europe in Northern steppes in Central Europe, steppes of the Pannonian Basin and steppes in the Pontic region. Whereas the geographic structure of the steppe belt is reflected in most vagile species at the level of intraspecific lineages ( Kajtoch et al., 2016), the separation of the regions resulted in a differentiation of species in different regions in Helicopsis . With regard to specific differentiation in subregions of the steppe belt, Helicopsis is more similar to subterranean mole rats ( Németh et al., 2013) and southern birch mice ( Cserkész et al., 2016) than to other steppe invertebrates investigated so far.
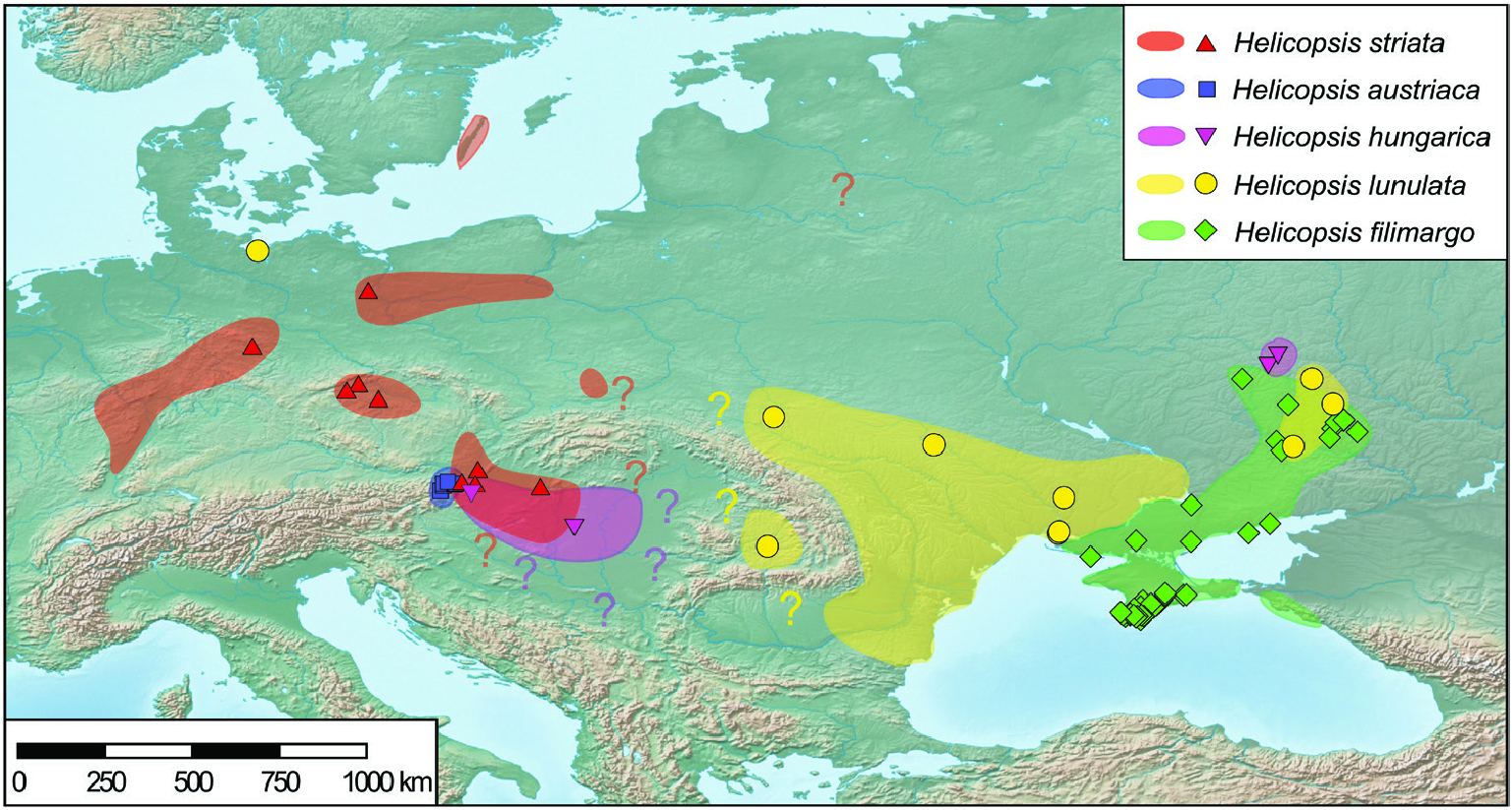
The Northern steppes in Central Europe and the steppes of the Pannonian Basin are inhabited by the Central European clade including H. hungarica , H. austriaca and H. striata ( Fig. 8 View Figure 8 ). Helicopsis striata is widespread in the Northern steppes, but overlaps with H. hungarica in the north-western Pannonian Basin ( Hudec, 1966; Duda et al., 2018). Helicopsis austriaca is endemic to the eastern fringe of the Alps at the margin of the Pannonian Basin ( Duda et al., 2018). Until recently, H. hungarica was thought to be endemic to the Pannonian Basin. However, Sychev & Snegin (2016) published a mitochondrial sequence of a specimen identified as H. striata from Gubkin in the Belgorod region in the Central Russian Upland, which was identified as H. hungarica by Duda et al. (2018). We here report an additional record of this species from Teleshovka in the Belgorod region. Duda et al. (2018) discussed three scenarios for the origin of H. hungarica . We consider an origin of the species in the Pannonian Basin more likely than in the Central Russian Upland because it forms a well-supported clade with the Central European H. striata and H. austriaca and not with H. filimargo and H. lunulata ( Fig. 2 View Figure 2 ; Supporting Information, Fig. S1 View Figure 1 ), which currently occupy the eastern part of the range of Helicopsis ( Fig. 8 View Figure 8 ). We have not found H. hungarica populations in Ukraine. Thus, the distributional gap between the Pannonian Basin and the Central Russian Upland seems to be real. However, Helicopsis was widespread in central and northern Ukraine in the Pleistocene ( Kunitsa, 2007), where it is currently absent ( Balashov, 2016; Balashov et al., 2018b). Sychev et al. (2015) reported Helicopsis from an almost 3000-year-old soil horizon near Gubkin in the Belgorod region. Thus, it is possible that H. hungarica expanded eastwards during the Pleistocene and later went extinct in the large area between the Pannonian Basin and the Central Russian Upland. However, the fossil shells cannot be reliably identified at the species level and it is also possible that the Pleistocene Helicopsis populations from central and northern Ukraine belonged to H. lunulata and formed the connection between its main range in western and southern Ukraine with the nowadays isolated occurrences in the Donetsk Upland and the Central Russian Upland. An alternative explanation for the isolated occurrence of H. hungarica haplotypes in the Central Russian Upland might be an introduction with mining equipment for the extensive iron-ore quarries near Gubkin. However, this hypothesis does not explain the presence of fossil Helicopsis in that region and the occurrence of the species in natural steppe communities. The specific identity of the specimens from the Central Russian Upland with the Pannonian H. hungarica should be confirmed with non-mitochondrial genetic markers to ensure that the H. hungarica haplotypes are not the result of an introgression. There is no evidence for the final scenario of Duda et al. (2018) that the present distribution of H. hungarica in the Carpathian Basin derives from a hitherto unknown southern refuge. The southern range border of H. hungarica is unknown. It is possible that the species extends southwards into the western Balkan Peninsula. However, there is no necessity to postulate a southern refugium for the survival of the species, given that H. austriaca obviously survived at asimilar latitude.
The Pontic region is characterized by H. filimargo and H. lunulata ( Figs 1 View Figure 1 , 8 View Figure 8 ). Helicopsis filimargo is found east of the Dnieper River and especially in Crimea, whereas H. lunulata occupies the part west of the Dnieper. It is not clear whether the isolated populations of H. lunulata in the Donetsk Upland and the adjacent Central Russian Upland are the remains of a formerly continuous range (see above) or whether they are the result of transport by humans. The former hypothesis is supported by the fact that H. lunulata occupies only undisturbed natural steppes in the Donetsk Upland, whereas H. filimargo can occupy transformed habitats. The geographical boundaries of H. striata and H. lunulata are unclear. It has to be checked whether the populations from south-eastern Poland, classified as H. striata ( Stępczak, 2004) , belong to that species or to H. lunulata .
Stewart et al. (2010) suggested that the ranges of some steppe species are currently contracted in interglacial refugia. This is the case for H. striata that was more widespread in Central Europe westwards to England during the glacials ( Jaeckel, 1962; Ložek, 1964), but is currently restricted to some of the Northern steppe fragments and the north-western part of the Pannonian Basin. However, not all steppe species are contracting in the interglacials. This is shown by significantly negative Fu’s F s statistic indicating population expansion in H. filimargo . The highest mitochondrial haplotype diversity, and a high diversity with regard to nuclear AFLP markers, were found in Crimea. Helicopsis filimargo probably originated in Crimea and expanded from there into the Black Sea Lowland and the Donetsk Upland. Fu’s F s statistic was not significant in H. lunulata , which is patchily distributed across Ukraine. However, we cannot exclude that the lack of significance was the result of the small sample size.
PHYLOGEOGRAPHYOF HELICOPSIS ASACHARACTERISTIC REPRESENTATIVE OF THE STEPPE FAUNA
The geographical origin of Helicopsis is unclear. Given that the clade including the Central and Eastern European species is sister to a clade comprising species from Greece and Iran, and the relationships with other genera from the eastern Mediterranean and the Caucasus ( Fig. 2 View Figure 2 ; Supporting Information, Fig. S1 View Figure 1 ), an origin on the Balkan Peninsula is most likely. The nested position of the Central European clade among the Pontic species indicates an eastern origin of the Central European species ( Fig. 3 View Figure 3 ).
As in many other steppe-inhabiting organisms ( Kajtoch et al., 2016), the differentiation pattern of the Central and Eastern European Helicopsis species reflects the geographic structuring of the steppe belt in Europe in Northern steppes in Central Europe, steppes of the Pannonian Basin and steppes in the Pontic region. Whereas the geographic structure of the steppe belt is reflected in most vagile species at the level of intraspecific lineages ( Kajtoch et al., 2016), the separation of the regions resulted in a differentiation of species in different regions in Helicopsis . With regard to specific differentiation in subregions of the steppe belt, Helicopsis is more similar to subterranean mole rats ( Németh et al., 2013) and southern birch mice ( Cserkész et al., 2016) than to other steppe invertebrates investigated so far.
The Northern steppes in Central Europe and the steppes of the Pannonian Basin are inhabited by the Central European clade including H. hungarica , H. austriaca and H. striata ( Fig. 8 View Figure 8 ). Helicopsis striata is widespread in the Northern steppes, but overlaps with H. hungarica in the north-western Pannonian Basin ( Hudec, 1966; Duda et al., 2018). Helicopsis austriaca is endemic to the eastern fringe of the Alps at the margin of the Pannonian Basin ( Duda et al., 2018). Until recently, H. hungarica was thought to be endemic to the Pannonian Basin. However, Sychev & Snegin (2016) published a mitochondrial sequence of a specimen identified as H. striata from Gubkin in the Belgorod region in the Central Russian Upland, which was identified as H. hungarica by Duda et al. (2018). We here report an additional record of this species from Teleshovka in the Belgorod region. Duda et al. (2018) discussed three scenarios for the origin of H. hungarica . We consider an origin of the species in the Pannonian Basin more likely than in the Central Russian Upland because it forms a well-supported clade with the Central European H. striata and H. austriaca and not with H. filimargo and H. lunulata ( Fig. 2 View Figure 2 ; Supporting Information, Fig. S1 View Figure 1 ), which currently occupy the eastern part of the range of Helicopsis ( Fig. 8 View Figure 8 ). We have not found H. hungarica populations in Ukraine. Thus, the distributional gap between the Pannonian Basin and the Central Russian Upland seems to be real. However, Helicopsis was widespread in central and northern Ukraine in the Pleistocene ( Kunitsa, 2007), where it is currently absent ( Balashov, 2016; Balashov et al., 2018b). Sychev et al. (2015) reported Helicopsis from an almost 3000-year-old soil horizon near Gubkin in the Belgorod region. Thus, it is possible that H. hungarica expanded eastwards during the Pleistocene and later went extinct in the large area between the Pannonian Basin and the Central Russian Upland. However, the fossil shells cannot be reliably identified at the species level and it is also possible that the Pleistocene Helicopsis populations from central and northern Ukraine belonged to H. lunulata and formed the connection between its main range in western and southern Ukraine with the nowadays isolated occurrences in the Donetsk Upland and the Central Russian Upland. An alternative explanation for the isolated occurrence of H. hungarica haplotypes in the Central Russian Upland might be an introduction with mining equipment for the extensive iron-ore quarries near Gubkin. However, this hypothesis does not explain the presence of fossil Helicopsis in that region and the occurrence of the species in natural steppe communities. The specific identity of the specimens from the Central Russian Upland with the Pannonian H. hungarica should be confirmed with non-mitochondrial genetic markers to ensure that the H. hungarica haplotypes are not the result of an introgression. There is no evidence for the final scenario of Duda et al. (2018) that the present distribution of H. hungarica in the Carpathian Basin derives from a hitherto unknown southern refuge. The southern range border of H. hungarica is unknown. It is possible that the species extends southwards into the western Balkan Peninsula. However, there is no necessity to postulate a southern refugium for the survival of the species, given that H. austriaca obviously survived at asimilar latitude.
The Pontic region is characterized by H. filimargo and H. lunulata ( Figs 1 View Figure 1 , 8 View Figure 8 ). Helicopsis filimargo is found east of the Dnieper River and especially in Crimea, whereas H. lunulata occupies the part west of the Dnieper. It is not clear whether the isolated populations of H. lunulata in the Donetsk Upland and the adjacent Central Russian Upland are the remains of a formerly continuous range (see above) or whether they are the result of transport by humans. The former hypothesis is supported by the fact that H. lunulata occupies only undisturbed natural steppes in the Donetsk Upland, whereas H. filimargo can occupy transformed habitats. The geographical boundaries of H. striata and H. lunulata are unclear. It has to be checked whether the populations from south-eastern Poland, classified as H. striata ( Stępczak, 2004) , belong to that species or to H. lunulata .
Stewart et al. (2010) suggested that the ranges of some steppe species are currently contracted in interglacial refugia. This is the case for H. striata that was more widespread in Central Europe westwards to England during the glacials ( Jaeckel, 1962; Ložek, 1964), but is currently restricted to some of the Northern steppe fragments and the north-western part of the Pannonian Basin. However, not all steppe species are contracting in the interglacials. This is shown by significantly negative Fu’s F s statistic indicating population expansion in H. filimargo . The highest mitochondrial haplotype diversity, and a high diversity with regard to nuclear AFLP markers, were found in Crimea. Helicopsis filimargo probably originated in Crimea and expanded from there into the Black Sea Lowland and the Donetsk Upland. Fu’s F s statistic was not significant in H. lunulata , which is patchily distributed across Ukraine. However, we cannot exclude that the lack of significance was the result of the small sample size.
Balashov IA, Son MO, Coada V, Welter-Schultes F. 2013 b. An updated annotated checklist of the molluscs of the Republic of Moldova. Folia Malacologica 21: 175 - 181.
Balashov IA. 2016. Fauna Ukrainy. Tom 29. Mollyuski. Vypusk 5. Stebelchatoglazye (Stylommatophora). Kiev: Naukova Dumka.
Balashov I, Vasyliuk O, Shyriaieva D, Shvydka Z, Oskyrko O, Marushchak O, Stetsun H, Bezsmertna O, Babytskij A, Kostiushyn V. 2018 b. Terrestrial molluscs in the dry grasslands of the Dnipro Upland (central Ukraine): new records, rare species and conservation potential. Vestnik Zoologii 52: 3 - 12.
Cserkesz T, Rusin M, Sramko G. 2016. An integrative systematic revision of the European southern birch mice (Rodentia: Sminthidae, Sicista subtilis group). Mammal Review 46: 114 - 130.
Damjanov SG, Likharev IM. 1975. Fauna na Bulgariya. 4. Sukhozemni okhlyuvi (Gastropoda terrestria). Sofia: Bulgarska Akademiya na Naukite.
Duda M, Haring E, Bieringer G, Eschner A, Mrkvicka A, Mason K. 2018. Taxonomic reassessment of Helicopsis austriaca Gittenberger, 1969 and its relationships to H. striata (OF Muller, 1774) and H. hungarica (Soos & H. Wagner, 1935) (Eupulmonata: Helicoidea). Journal of Molluscan Studies 84: 432 - 450.
Grossu AV. 1983. Gastropoda Romaniae 4. Suprafam.: Arionacea, Zonitacea, Ariophantacea si Helicacea. Bucharest: Editura Litera.
Hausdorf B. 1990. Zur Kenntnis einiger Arten der Gattung Helicopsis Fitzinger aus Griechenland und der Turkei (Gastropoda: Hygromiidae). Archiv fur Molluskenkunde 120: 57 - 71.
Hausdorf B. 1996. Helicopsis aelleni n. sp. from northern Iran, with remarks on Helicopsis Fitzinger 1833 (Gastropoda: Pulmonata: Hygromiidae). Archiv fur Molluskenkunde 126: 65 - 71.
Hausdorf B, Bossneck U. 2016. Helicopsis persica n. sp. from northern Iran (Gastropoda: Geomitridae). Zootaxa 4066: 194 - 200.
Hudec V. 1966. Zur Problematik des Vorkommens der Schnecken Helicopsis striata (Mull.) und H. hungarica (Soos H. Wagner) im Karpatenbecken. Biologia 3: 161 - 176.
Jaeckel SGA. 1962. Erganzungen und Berichtigungen zum rezenten und quartaren Vorkommen der mitteleuropaischen Mollusken. In: Brohmer P, Ehrmann P, Ulmer G, eds. Die Tierwelt Mitteleuropas 2 (1, Erg.). Leipzig: Quelle and Meyer, 25 - 294.
Kajtoch L, Cieslak E, Varga Z, Paul W, Mazur MA, Sramko G, Kubisz D. 2016. Phylogeographic patterns of steppe species in eastern Central Europe: a review and the implications for conservation. Biodiversity and Conservation 25: 2309 - 2339.
Kunitsa NA. 2007. Priroda Ukrainy v Plejstocene (po dannym malakofaunisticheskogo analiza). Chernovtsi: Ruta.
Lozek V. 1964. Quartarmollusken der Tschechoslowakei. Rozpravy Ustredniho Ustavu Geologickeho 31: 1 - 374, 32 pls.
Neiber MT, Razkin O, Hausdorf B. 2017. Molecular phylogeny and biogeography of the land snail family Hygromiidae (Gastropoda: Helicoidea). Molecular Phylogenetics and Evolution 111: 169 - 184.
Nemeth A, Homonnay ZG, Krizsik V, Csorba M, Pavlicek T, Hegyeli Z, Hadid Y, Sugar S, Farkas J, Csorba G. 2013. Old views and new insights: taxonomic revision of the Bukovina blind mole rat, Spalax graecus (Rodentia: Spalacinae). Zoological Journal of the Linnean Society 169: 903 - 914.
Pinter L. 1969. Neue Mollusken aus Bulgarien (Gastropoda: Helicidae). Acta Zoologica Academiae Scientiarum Hungaricae 15: 91 - 96.
Stepczak K. 2004. Helicopsis striata (O. F. Muller, 1774). In: Glowacinski Z, Nowacki J, eds. Polska czerwona ksiega zwierzat. Bezkregowce. Krakow: Instytut Ochrony Przyrody Polskiej Akademii Nauk.
Stewart JR, Lister AM, Barnes I, Dalen L. 2010. Refugia revisited: individualistic responses of species in space and time. Proceedings of the Royal Society B: Biological Sciences 277: 661 - 671.
Sychev AA, Snegin EA, Shapovalov AS, Ponomarenko EV, Chendev YG. 2015. K voprosu o strukture fauny nazemnykh molljuskov zapovednogo uchastka Yamskaya step' v pozdnem golotsene. Vestnik Tomskogo gosudarstvennogo universiteta, Biologija 2: 146 - 164.
Sychev AA, Snegin EA. 2016. K probleme sistematiki roda Helicopsis (Gastropoda: Pulmonata: Hygromiidae) na territorii Vostochnoy Evropy. Ruthenica 26: 175 - 189.
Sysoev AV, Schileyko AA. 2009. Land snails and slugs of Russia and adjacent countries. Sofia & Moscow: Pensoft.
Welter-Schultes F. 2012. European non-marine molluscs, a guide for species identification. Gottingen: Planet Poster Editions.
Zettler ML, Jueg U, Menzel-Harloff H, Gollnitz U, Petrick S, Weber E, Seemann R. 2006. Die Land- und Susswassermollusken Mecklenburg-Vorpommerns. Schwerin: Obotritendruck.
Figure 1. Sampled Helicopsis populations in Ukraine, the adjacent Central Russian Upland and Romania and distribution of mitochondrial haplotype clades: H. lunulata (yellow dots), H. hungarica (magenta triangles) and H. filimargo (clade A, red diamonds, clade B, green diamonds and mixed population of clades A and B, blue diamonds).
Figure 2. Bayesian 50% majority-rule consensus tree of Helicopsis and related groups based on sequences of the mitochondrial 16S rDNA. Nodes supported by posterior probabilities ≥ 0.95 are marked by a dot. For information about sequenced specimens and detailed support values, see Supporting Information, Figure S1 and Table S1.
Figure 3. Bayesian 50% majority-rule consensus tree of European Helicopsis based on concatenated sequences of the mitochondrial COI, 12S rDNA and 16S rDNA. Support values at the nodes correspond to Bayesian posterior probabilities (left), maximum likelihood (middle) and maximum parsimony (right) bootstrap values. Extraction voucher numbers are given at the tips of the tree. For information about sequenced specimens, see Supporting Information, Table S1.
Figure 4. Neighbour-net network of Helicopsis from Ukraine based on Jaccard distances obtained from AFLP data. Coloration of clusters corresponds to the STRUCTURE solution for K = 2. Red corresponds to H. filimargo, yellow to H. lunulata. DNA voucher numbers for specimens are given at the tips of the network. Coloured dots at the tips of the network correspond to cluster assignments (on majority-rule basis) of specimens in the STRUCTURE solutions for K = 5 (inner) and K = 7 (outer). For locality data and the distribution of clusters, see also Supporting Information, Table S1 and Figure 6, respectively.
Figure 5. Assignment of specimens of Helicopsis from Ukraine to clusters resulting from the admixture analysis of AFLP data with STRUCTURE. A, solution for K = 2. B, solution for K = 5. C, solution for K = 7. Numbers below the plots refer to populations (see Fig. 6; Supporting Information, Table S1). Letters above the plots refer to mitochondrial clades: a, H. filimargo (clade A); b, H. filimargo (clade B); l, H. lunulata.
Figure 7. Relationships between Jaccard distances between individuals of Helicopsis filimargo and H. lunulata based on AFLP data and logarithmized geographical distances. Black circles and red triangles: distances between individuals belonging to H. filimargo and H. lunulata, respectively; green crosses: distances between individuals belonging to different species; black and red broken lines: regression lines fitted within species; green broken line: regression line fitted on the within-group distances only (i.e. the black circles and red triangles taken together); green solid line: regression line fitted on all distances together; blue lines: centres of the betweengroups geographical distances.
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |