Netrobalane canopus
|
publication ID |
https://doi.org/ 10.11646/zootaxa.2893.1.1 |
|
persistent identifier |
https://treatment.plazi.org/id/03A587EC-277D-9153-31C9-6B02E2E85FE1 |
|
treatment provided by |
Felipe |
|
scientific name |
Netrobalane canopus |
| status |
|
Netrobalane canopus View in CoL (Trimen 1864 (1862–1866)) ( Figures 29–33 View FIGURE 29 View FIGURE 30 View FIGURE 31 View FIGURE 32 View FIGURE 33 )
This species is distributed from the eastern coast of South Africa, North to Sudan and then West to Nigeria ( Evans 1937, Larsen 2005) and Mt Tonkoui in Côte d’Ivoire (Larsen pers. comm. 2010), and was first described from South Africa (Kaffraria and Natal) by Trimen (1864 in Trimen 1862 –1866). In Kenya it is widespread, but seldom common. Sevastopulo (1974) found it rare in the Makadara Forest, Shimba Hills. In the forests around Nairobi, the caterpillars are quite easy to find, but the adults cannot be considered more than occasional. In Tanzania, Kielland (1990) associates it with riverine thickets, forest margins and open forest to 1700m, common in the west of the country, but less so in the east.
Adult behaviour
Males perch quite high up on trees at the edge of forest clearings, usually at about 4m. They will select an exposed twig or branch from which to defend a territory, or will rest beneath a leaf, but always with the wings spread. Cordeiro (1990) writing about the butterflies of Lake Manyara, Tanzania provides the following description of territorial behaviour “ Males of N. canopus are very territorial, occupying territories in open habitats throughout the groundwater forest. Each male perches on a favoured leaf of a branch that extends far out from the canopy of an understory tree, often at approximately 2m from the ground. For five consecutive days I observed three males that shared territories on different branches of the same tree, also occupied by a male Graphium leonidas . They did not interfere with G. leonidas (or vice versa) but would fight amongst themselves for hours on end. First one male would fly into the territory of another and then an attack commenced whereupon the intruder was "tussled" within the air. During such aerial "battles", both would enter the territory of a third male, resulting in all three attacking each other. After several minutes of contest, one male would return to its perch. After further and short aerial combats, the other two would also retreat to their perches .”
Trimen (1862 –66, 1889) quotes Mr. J.H. Bowker that N. canopus is "A curious flyer, rapidly opening and shutting the wings (like a bird), with a sharp creaking or buzzing noise." We have no observations of such behaviour, which could be connected with courtship as suggested by Trimen (1889).
Noting that bird droppings can be categorised into those that are deposited as a solid rather cylindrical lump, and those of a more liquid nature that spread out on a leaf surface to produce a dark patch with an irregular white area around it, Preston–Mafham & Preston–Mafham (1988) suggest that the white skippers which rest on leaves with their wings spread ( Eagris lucetia and N. canopus ) mimic the latter type.
Food plants
The normal food plants around Nairobi, and probably generally in upland Kenya are Hibiscus calyphyllus and Dombeya burgessiae , the former more frequently than the latter. Incidentally, non-flowering H. calyphyllus can often be recognised in the woods around Nairobi by the eriophyid leaf galls on the leaves, which are found on this species, but not on Pavonia urens or D. burgessiae .
At the coast I have found N. canopus caterpillars on Grewia glandulosa , and also found shelters, probably of this species, on G. ectasicarpa at Arabuko–Sokoke Forest. The caterpillar from G. glandulosa subsequently fed for three weeks on G. similis in Nairobi before pupating and dying as a pupa; G. similis is not used as a food plant around Nairobi.
In Kakamega Forest I found a second instar caterpillar on a white-flowered variety of Pavonia urens which I believe was this species but it subsequently died. The mauve-flowered variety of Pavonia urens is not used as a food plant around Nairobi.
Dollman (unpublished) was one of the first to rear this species, finding the caterpillars, but never the adults, at Solwezi, Zambia, feeding on “chitumpu”, i.e. Syzygium guineense (N.D. Riley in Dollman unpublished) and more rarely on “muresha” (unidentified), from October to April. As discussed under C. pillaana , the identification of S. guineense is likely to be an error, and this food plant record should not be accepted without confirmation. Subsequent published records are given in Table 6.
Life history
G.C. Clark (in Dickson & Kroon 1978) provides an excellent and detailed illustrated life history of this interesting species, which matches well with my own observations. Henning et al. (1997) include a photograph of the final instar caterpillar, similar to Figure 32 View FIGURE 32 . With regard to the early stages there are significant similarities between C. pillaana and N. canopus .
Ovum
The ovum is 0.94mm (n=4) in diameter and may be laid on either the upper surface or under surface of the leaf, away from the margin. It is not obviously ribbed, but there are 28 very fine ribs (Clark in Dickson & Kroon 1978). The ovum is covered with about 20 long linear scales, matching those on the end of the abdomen of the female. The scales are arranged flat around the edge of the ovum; one third of each scale is white and stuck to the ovum, and the other two thirds project above the egg and are grey-brown. These scales are about 0.1mm wide and 2.5mm long. The general appearance is similar to that of the ovum of Abantis paradisea ( Figure 47 View FIGURE 47 ), but the scales on the ova of N. canopus do not extend over the leaf at the base. The caterpillars eat a hole in the upper part of the egg to eclose; one such hole measured 0.9 x 0.8mm.
Leaf shelters
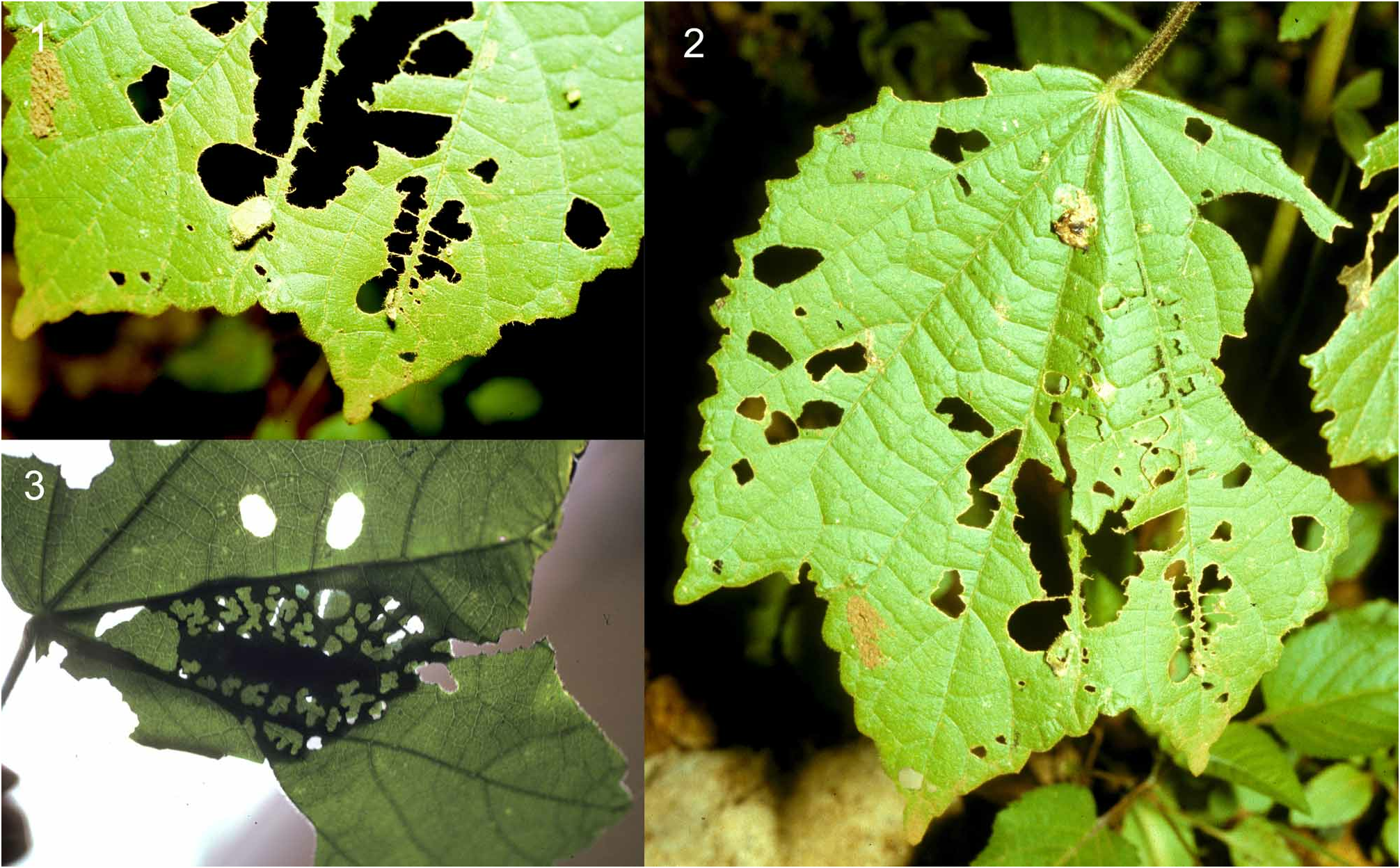
The first and second shelters are similar to those of other members of the Tagiadini : one-cut ovals cut from the leaf lamina, and turned over onto the leaf upper surface hinged on the bridge around a vein. Stage 1 shelters are small, 4.5–5.0 x 2.5–3.0mm. The third shelter, however, is very different. A large oval (c. 48 x 25 mm), pointed at the distal end, is cut from the leaf except for the bridge at the base consisting a main vein. This flap is then swung across either on top of, or more usually under the leaf, without being turned over, to form a shelter between it and the flap ( Figure 30 View FIGURE 30 , and 31.2). This has the advantage of making the shelter top surface the same as the leaf upper surface, which is much less conspicuous than other shelters in which the flap is turned over. It also means that the caterpillar does not have to pull two of the food plant leaves together which are large, quite tough and well spaced. This final shelter is subsequently modified by eating notches from the edges of the flap, and rows of holes along the line of the shelter, except for a central strip where the caterpillar rests ( Figure 31.3 View FIGURE 31 ). The whole shelter is lined with silk so that none of the holes provide access to the shelter, and the flap is held in place by tough strands of silk up to 5 mm long. This arrangement of holes in the shelter is unique in my experience of the African fauna, but parallels the habits of a group of South American genera which includes Quadrus and Ouleus ( Cock 1996) . The caterpillars of the West African genus Melphina (Hesperiinae) feed on broad leaved plants and make a flap with a very irregular margin, but do not perforate the shelter in this way. The purpose of the holes and notches is not known, but my guess is that it serves to break up the outline of the shelter so that visually hunting predators do not recognise it.
Caterpillar
There are probably at least six, perhaps seven instars, but after the second instar, the size of the head capsules start to run into or overlap between instars, so that it is not possible to generalise. Instars 2 onwards are similar ( Figure 32 View FIGURE 32 ). Caterpillars of this species often die when being reared, and don't seem to settle well in captivity. They will transfer between Hibiscus calyphyllus and Dombeya burgessiae , and feed on either (but often subsequently die). It seems that my rearing conditions were less than ideal, and this may have slowed development and increased the number of instars.
This species takes longer than usual to complete ecdysis; I suspect this is because the base of the head capsule (in common with Caprona pillaana and probably Abantis spp. ) is very narrow. I have noted instar durations of 9 days (L3), 8 days (L4), and 23 days (L5).
Final instar ( Figure 32 View FIGURE 32 ). The head measures 3.3 (range 3.0–3.4, n=7) x 3.2mm (range 3.0–3.3, n=7) wide x high (without hairs). Entirely dark brown, heavily reticulate; hairs up to 0.9mm long on dorsal half of head ( Figure 32 View FIGURE 32 ); those on apex extending laterally are dark, remainder white apart from a brown band across the face; similarly to C. pillaana , the hairs on the dorsal and lateral parts of the head are divided and subdivided, whereas those on the face are short, branched and recumbent; those around the mouthparts longer and some unbranched, directed downwards. T1 transverse, shiny black plate; scattered pale hairs, stellate at apex; divided in dorsal line and with a crease near posterior margin; extending about half way down the side of the segment; a small raised transparent subdorsal bump. Body dull khaki-green, yellowish on anal segments; covered in yellow dots and spots each with a pale hair which is stellate near apex, about five rows of four on each side, but irregular ( Figure 32 View FIGURE 32 ).
The caterpillar painted in dorsal view by Dollman (unpublished) is similar, but Dollman could not attempt to show the branching hairs which he described.
Early instars. The head of the penultimate instar (Ln–1) measures 2.5 (range 2.0–2.9, n=9) x 2.3mm (range 2.0–2.7, n=9) wide x high. The hairs on the head are up to 0.75mm long, but otherwise, a smaller version of the final instar. The second instar head measures 0.8 x 0.8 (n=2) mm wide x high, and is similar to the later instars.
Instar 1. Based on a caterpillar collected 5 May 1988 on Hibiscus calyphyllus , transplanted into my garden in Kyuna Estate, Nairobi (88/36). Head black, finely rugose, shiny; chordate, 0.60 x 0.56mm (n=3) wide x high; scattered white stalked stellate hairs. Length 2.5mm; brownish; with a transverse ring of stalked stellate hairs on each segment.
Pupa
An n–2 instar caterpillar (88/19) collected in a stage 3 shelter on H. calyphyllus in Ololua Forest, Nairobi 6 Mar 1988 pupated on 1 April, when the following description was prepared. Pupa 14mm. Frontal projection 1mm, upturned, parallel-sided, bifurcate at apex; black above; dull yellow below; spiracle T1 protrudes as a tube, 0.5mm width, apex black. Rest of pupa dull yellow with black markings. Head: on head a circle open ventrally and dorsally joined to base of frontal projection; around base of frontal projection, linked to central black spot dorsal to frontal projection; spot above eye, nearly joined by narrow diffuse line across top of head; eye with stripe down the middle, ends curved posteriorly; spots on anterior margin of the line over the eye in the middle and at ventral end; dot between these last and circle joined to frontal projection; ventrally between eyes a W with base anterior; short line on posterior margin of eye. Thorax: wing cell and veins 1–4; a pair of converging streaks in space 1A; tip of proboscis (between wing cases) slightly darkened; spot at base of wings; row of five lines between T1 spiracles, that in dorsal position longest, extending posteriorly; narrow black anterior margin to thorax across the front of these five lines; just before the posterior margin of thorax a strong dorsolateral spot close to one on base of wing dorsum; a dorsal transverse arc on posterior margin of thorax; dorsal area of thorax with indistinct white spots. Abdomen markings fully developed on A5–A8: row of four quadrate spots across dorsum on posterior margin, linked by fine line on margin; laterally a semicircle around each pale brown spiracle with base on posterior margin; ventral to this scattered white dots; on A1–A4 the dorsal spots are widely spaced and the lateral semicircle absent; A8 dorsal spots merge to solid dorsal line; A9 a short transverse dorsal bar; A10 a dorsal V pointing posteriorly; base of cremaster a pair of close parallel lines on dorsum to posterior margin of A10, where the lines turn outwards along margin and loop back to cremaster laterally; ventrally a ¾ circle has open section anterior and joins lateral line just described; within the ¾ circle two spots; cremaster black; dorsally on abdomen 3–4 irregular rows of white spots, but ventrally no white spots.
Like the caterpillars, the pupae also suffer quite high mortality, often for no apparent reason. On average pupation lasts 17.2 days.
Natural enemies
Dickson & Kroon (1978) record a tachinid, Thecocarcelia sp. , from the final instar caterpillar in South Africa. In Ngong Forest I found a fresh eulophid pupa in an otherwise empty stage 1 shelter on Dombeya burgessiae (12.xi.1988, 88/115). This white pupa subsequently turned black and an adult wasp emerged after eight days. I have also found a 5mm ichneumonid cocoon in an empty third stage shelter on Hibiscus calyphyllus in Ololua Forest, which emerged between 10 and 13 days after collection (5 May 1988, 88/39). Neither has been identified as yet.
Discussion
The biology described and illustrated here, including the leaf shelters, is very close to that of the Asian genus Odontoptilum based on the life history of O. angulatum as described by Bell (1923b) and shown by Bascombe et al. (1999) and Tan (2008b). The covering of the ovum of O. angulatum is hair-like, similar to that of Eagris sabadius ( Figure 3 View FIGURE 3 ), but the food plants Ceiba sp. , Hibiscus tiliaceus , Microcos paniculata , Urena lobata (Malvaceae) and Allophylus cobbe (Sapindaceae) ( Bell 1923b; Bascombe et al. 1999; Tan 2008b; Kehimkar 2008), caterpillar, leaf shelter, including the unusual stage 3 shelter, and pupa could all be of a Netrobalane species.
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.