Halopteris polymorpha ( Billard, 1913 ), 1888
|
publication ID |
https://doi.org/ 10.5281/zenodo.1196007 |
|
DOI |
https://doi.org/10.5281/zenodo.14163722 |
|
persistent identifier |
https://treatment.plazi.org/id/03A487AE-FF81-2C12-FC73-8874FDE66C08 |
|
treatment provided by |
Plazi |
|
scientific name |
Halopteris polymorpha ( Billard, 1913 ) |
| status |
|
Halopteris polymorpha ( Billard, 1913) View in CoL
Figs 1A View Fig , 2A View Fig , 3 View Fig A-K; Tables 1 View Table 1 , 2 View Table 2 ; Appendix 1
Plumularia polymorpha Billard, 1913 (pro parte): 24, figs 14A, 15. – Van Soest, 1976: 89.
non Plumularia polymorpha Billard, 1913 (pro parte): 24, fig. 14B, C.
Thecocarpus polymorphus – Bedot, 1921: 9. – Von Schenck, 1965: 928.
Heterotheca polymorpha – Stechow, 1923: 15.
Halopteris polymorpha View in CoL – (?) Pennycuik, 1959: 178. – Schuchert, 1997 (pro parte): 64, fig. 20A, C-F. – Ryland & Gibbons, 1991: 530, fig. 4. – Di Camillo et al., 2008: 1592.
non Halopteris polymorpha View in CoL – Vervoort, 1966: 132, fig. 35. – Millard & Bouillon (pro parte), 1973: 83, fig. 10F-H, J; 1974: 9. – Millard, 1975: 354, fig. 112G-L; 1977: 107; 1978: 193; 1980: 132. – Hirohito, 1983: 62, fig. 31. – Bouillon et al., 1995: 49. – Schuchert, 1997 (pro parte): 64, figs 20B, 21-23. – Watson, 2000: 46, fig. 35. – Ansín Agís et al., 2001: 167, fig. 70. – Preker, 2001: 154. – Kirkendale & Calder, 2003: 169. – Preker, 2005: 49. – Preker & Lawn, 2005: 342. – Ansín Agís et al., 2009: 53. – Preker & Lawn, 2010: 120; 2012: 45, fig. 7.
non Antennella polymorpha – Vervoort, 1941: 218.
non Antennella secundaria – Vervoort, 1967: 42, fig. 12 [not Antennella secundaria ( Gmelin, 1791) ].
non Plumularia nuttingi Billard, 1911: 66 , fig. 8 – Van Soest, 1976: 89.
non Plumularia buskii – Thornely, 1904: 120. – Ritchie, 1910: 832. – Thornely, 1916: 150. – Nutting, 1927: 221.
non Plumularia buski – Hartlaub, 1901: 374, pl. 22 figs 22, 32, 36. – Billard, 1913: 21, fig. 11, pl. 1 fig. 15. – Redier, 1966: 90, pl. 2 figs 1 & 3, pl. 3 fig. 1 (incorrect subsequent spelling).
non Heterotheca buski – Hirohito, 1974: 30, fig. 14 (incorrect subsequent spelling).
non Halopteris buskii – Vervoort & Vasseur, 1977: 72, fig. 31. – Rees & Vervoort, 1987 (pro parte): 119, fig. 25A-B. – Ryland & Gibbons, 1991: 527, fig. 2. – Bouillon et al., 1995: 49. – Migotto, 1996: 48, fig. 9F-H. – Preker, 2001: 154; 2005: 48.
non Halopteris buski – Rees & Thursfield, 1965: 160. – Hirohito, 1983: 61; 1995: 244, fig. 82 (incorrect subsequent spelling).
Material examined: MHNG-INVE-97937; Indonesia, Bali, Padangbai, Jepun shipwreck, -8.52819° 115.51478°, 20 m, coll. H. R. Galea; 06.10.2016; several sterile plumes, up to 3.7 cm high; 16 S sequence MF784530 View Materials . – MNHG-INVE-97951; Indonesia, Bunaken National Marine Park , Manado Tua I., Negeri , 1.61684° 124.70140°, 10 m, coll. G. Allard ; 22.11.2010; several plumes, up to 3.7 cm high, some bearing female gonothecae. – HRG-0421; Indonesia, Bunaken National Marine Park , off Bunaken I., Leukan 2, 1.59989° 124.76697°, 10 m, coll. G. Allard ; 21.11.2010; 6 stems up to 3 cm high, one of which bears female gonothecae, and three others carrying male gonothecae. – CDC002 About CDC ; Indonesia, Bali, Tulamben, Liberty shipwreck, -8.27417° 115.59265°, 18 m, coll. C. G. Di Camillo; 21.10.2008; two sterile cormoids, 14 and 21 mm high. – CDC003 About CDC ; Indonesia, Ambon, Laha I., -3.69221° 128.12310°, 10-15 m, coll. C. G. Di Camillo; 14.10.2008; a 17 mm high cormoid and a 12 mm high fragment, both infertile. – CDC004 About CDC ; Indonesia, Bunaken National Marine Park, Raymond’s Point, 1.62713° 124.73363°, 40-50 m, coll. C. G. Di Camillo; 01.09.2003; two sterile fragments, 7 and 16 mm high, likely from different cormoids. – CDC005 About CDC ; Indonesia, Bunaken National Marine Park, Mandolin’s Point, 1.61095° 124.73257°, 20 m, coll. C. G. Di Camillo; 12.02.2005; a 27 mm high cormoid bearing a gonotheca, probably male .
Additional material: MNHN H. L.1309; Indonesia, Rote Island, Buka Bay, -10.87333 123.01833, 34 m, Siboga Stn. 299; a 1.1 cm high sterile cormoid belonging to the syntype of Plumularia polymorpha Billard, 1913 .
Diagnosis: Halopteris with cormoids reaching heights of up to 3.7 cm, with monosiphonic, unbranched stems, divided homomerously into rather long internodes bearing a hydrotheca, a lateral apophysis, and up to 9 nematothecae [1 mesial, a pair of laterals, an axillar one, and generally 2-3 (though up to 5 possibly present) above hydrotheca]. Cladia alternately arranged along stem, heteromerously divided into internodes; hydrothecate internodes slightly shorter than their ahydrothecate counterparts, carrying a hydrotheca and its up to 4 associated nematothecae (1 mesial, a pair of laterals, and occasionally an axillar one); ahydrothecate internodes with 1-2 nematothecae. Hydrothecae conical and shallow; lateral nematothecae with either lowered, emarginated or sinuated margin adaxially, not surpassing hydrothecal rim, borne on inconspicuous apophyses. Female gonotheca broadly ovoid, with apical, large, rounded aperture perpendicular to long axis of the theca, and closed by glass-watch-shaped operculum; 2-3 basal nematothecae. Male gonotheca smaller than female, ovoid, without noticeable aperture, with 2 basal nematothecae. Cormoids yellow throughout in life.
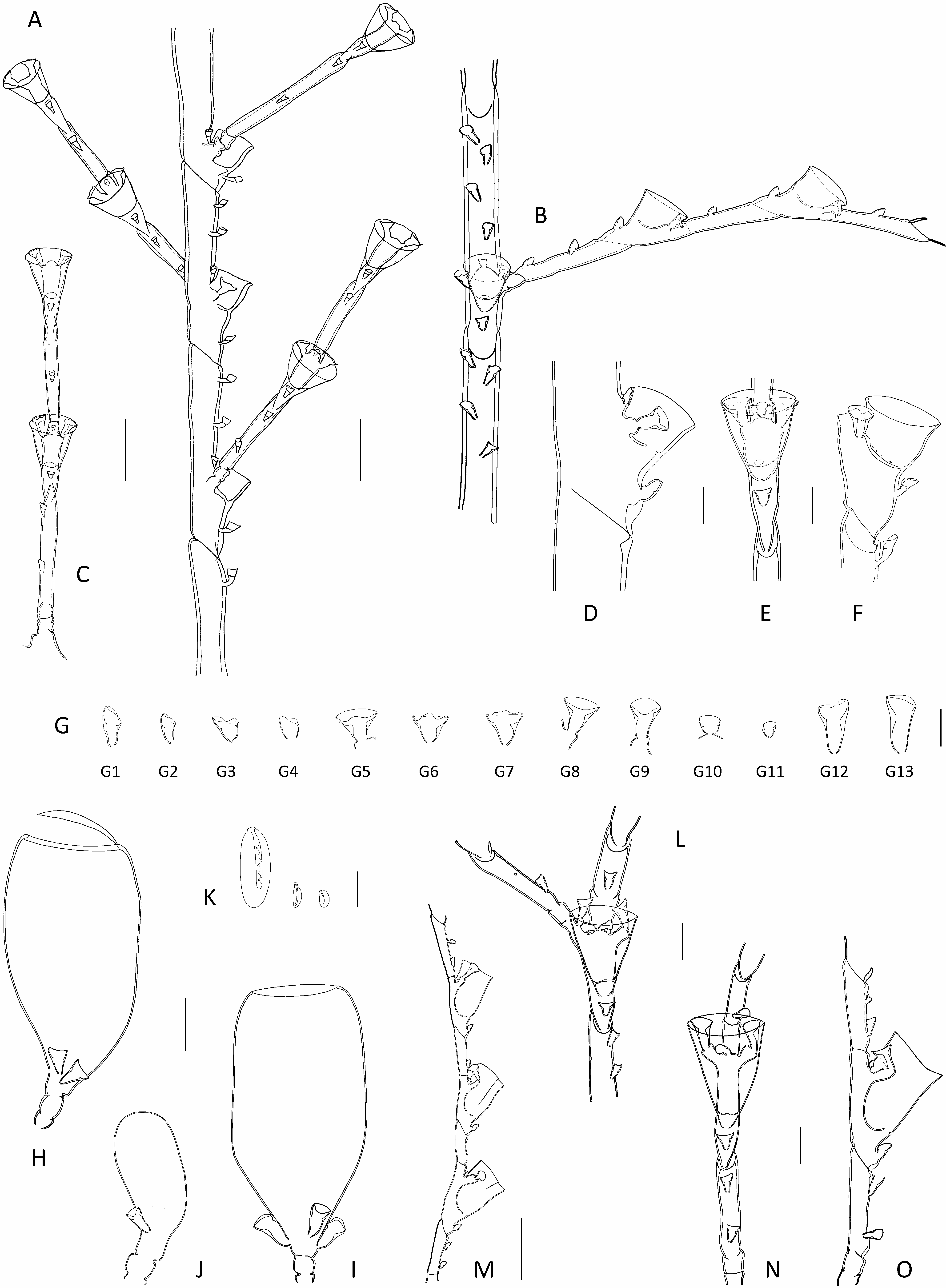
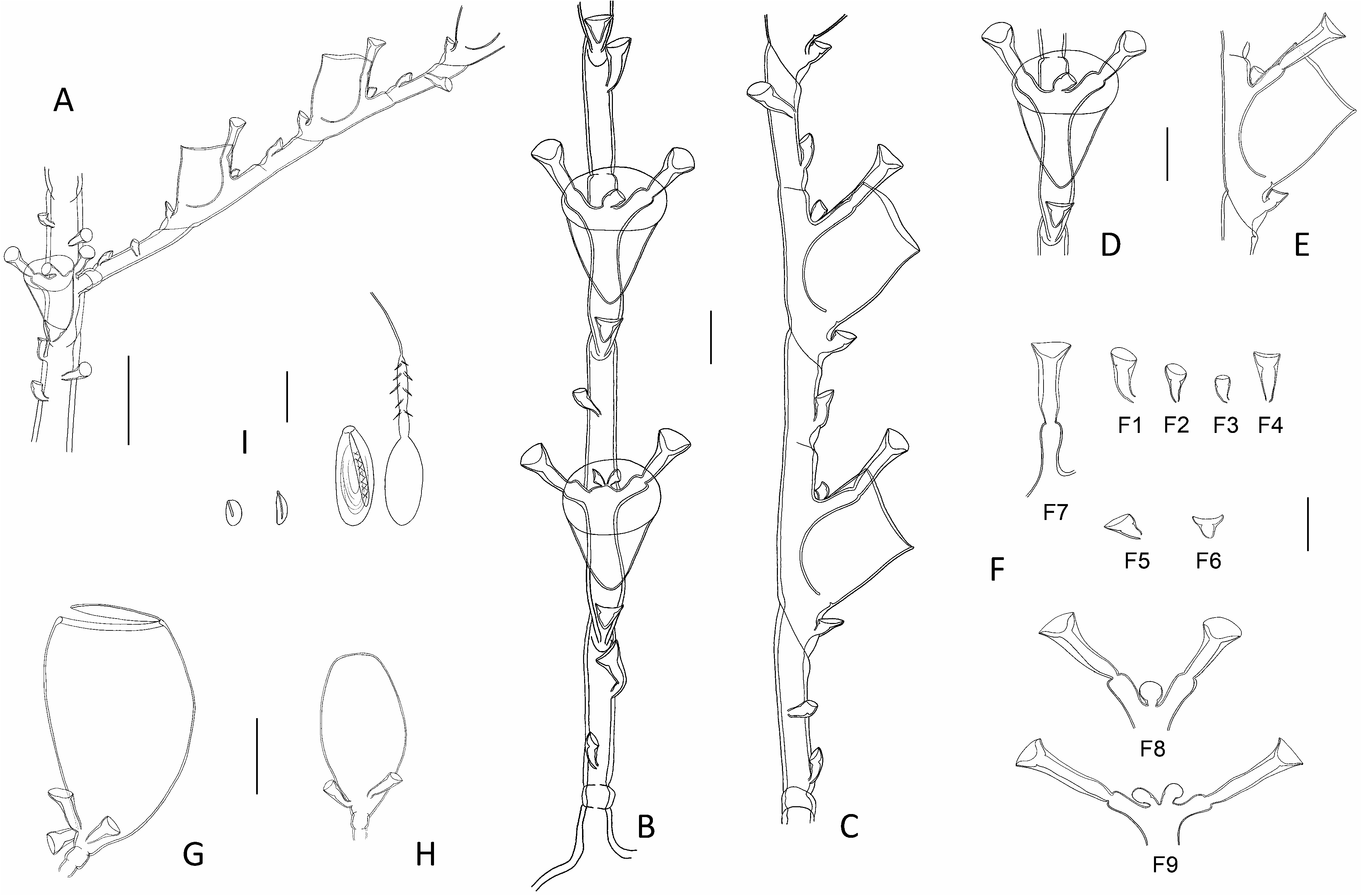
Description: Colonies composed of a varied number of cormoids arising from creeping, branching stolon, not carrying nematothecae. Cormoids erect (though flaccid when out of liquid), up to 3.7 cm high. Cauli simple, monosiphonic ( Figs 1A View Fig , 2A View Fig ), composed of an up to 1 cm long, ahydrothecate, proximal part above origin from stolon, and a much longer, distal part carrying both hydrothecae and hydrocladia. Basal part usually divided by up to 4 transverse nodes into segments of varied length, bearing a number of nematothecae (up to 34 observed) arranged into two parallel, closelyset rows; distalmost segment delimited from the remainder of caulus through a deeply-cut, oblique node. Stem above basal part longer, homomerously segmented into up to 37 internodes trough oblique constrictions of the perisarc ( Fig. 3A View Fig ); internodes long, bearing one hydrotheca in their lower third, a number of nematothecae, and an apophysis lateral to the hydrotheca supporting a cladium (two apophyses are usually present in the proximal most internode, and these support a pair of cladia) ( Fig. 3B View Fig ); nematothecae: one mesial, a pair of laterals, one axillar, as well as usually 2-3 (though occasionally 1-5 possibly present) above the hydrotheca, arranged in two closely-set rows. Cladia, except for the proximal most that can be paired, alternately-arranged along caulus; up to 5 mm long, usually less so; each composed of a short, proximal, athecate, quadrangular segment, followed by a succession of ahydrothecate and hydrothecate internodes, delimited through a heteromerous segmentation; ahydrothecate internodes with proximal node transverse and distal node oblique; the reverse in hydrothecate internodes. First ahydrothecate internode quite long, and longer than its subsequent counterparts, bearing constantly two superior nematothecae in a single row; remaining ahydrothecate internodes provided with commonly 1, or rarely 2, nematothecae. Hydrothecate internodes, up to 7 (usually 4-6) per cladium, relatively short, with a hydrotheca confined to most of their length ( Fig. 3B View Fig ), and up to four nematothecae: one mesial, a pair of laterals and, occasionally, an axillar one ( Fig. 3E View Fig ). Hydrothecae cup-shaped and shallow, walls slightly divergent, rim circular, entire ( Fig. 3D, F View Fig ). All nematothecae of the colony bithalamic and movable; mesial ones short, triangular in frontal view, rim of upper chamber with deep, adaxial emargination ( Fig. 3G 3 View Fig , 4 View Fig ); laterals short, not surpassing the hydrothecal rim ( Fig. 3D, F View Fig ), and mounted on very short apophyses, conical in shape, aperture wide, margin of upper chamber of variable shape: variously lowered to emarginated to sigmoid adaxially ( Fig. 3G View Fig 5-9 View Fig View Fig View Fig View Fig View Fig 9 ); cauline ( Fig. 3G View Fig 1 View Fig ) and cladial ( Fig. 3G View Fig 2 View Fig ) nematothecae long, with tall basal and shallow upper chambers, rim scooped adaxially; axillar nematothecae conical to broadly ovoid, rather inconspicuous due to their comparatively smaller size and thinner perisarc ( Fig. 3G View Fig 10, 11). Hydranths with 15-16 filiform tentacles; in life, whole colony of a distinctive yellow tinge ( Fig. 1A View Fig ). Colonies dioecious. Gonothecae borne on both stems and cladia, inserted singly beside the base of a hydrotheca through a short, lateral apophysis, and mounted on single quadrangular pedicel; female large, broadly ovoid, tapering below, and there provided with 2-3 basal nematothecae; aperture distal, perpendicular to long axis of the theca, large and circular, with conspicuously thickened rim, closed by a watch-glass-shaped operculum ( Fig. 3H, I View Fig ); male comparatively smaller than female, ovoid, tapering below, without distinct aperture, provided basally with a couple of nematothecae ( Fig. 3J View Fig ). Cnidome ( Fig. 3K View Fig ) composed of 3 types of microbasic mastigophores: large, elongated-ovoid [(21.9-22.6) × (8.2-8.5) μm, in nematophores, as well as scattered in the coenosarc]; small, banana-shaped [(6.9-7.5) × ca. 2.4 μm, in tentacles]; small, ovoid capsules [(4.5-5.1) × (2.8- 2.9) μm, scattered in the coenosarc].
Dimensions: See Table 1 View Table 1 .
Remarks: The ordinary cauline internodes bear usually 2-3 nematothecae above their corresponding hydrothecae (60.8% and 34.8%, respectively, n=115), though exceptionally as few as 1, or as much as 4 or even 5, may occur (1%, 1.7% and 1.7%, respectively, n=115). The basalmost cauline internodes, supporting pairs of cladia, bear an increased number of nematothecae, usually 4-5. The ordinary cladial ahydrothecate internodes bear generally 1 and, less frequently, 2 nematothecae (92% and 8%, respectively, n=110).
Behind each cauline hydrotheca, there is large foramen for the passage of a nematophore, itself protected by a bithalamic nematotheca; the latter is occasionally lost, but the constant presence of the foramen in all internodes indicates the pre-existence of an axillar nematotheca. Conversely, the cladial hydrothecae bear only occasionally single axillar nematothecae, but their basal foramina are inconspicuous.
Terminal stolonization is quite common in the available samples, but no branched cladia have been observed.
The bulk of the syntype material of H. polymorpha ( Billard, 1913) is housed in the collection of NBC but, due to important ongoing renovation works, could not be re-examined for the purpose of the present study (Koos van Egmond, pers. comm.).
However, the lectotype (from Siboga Stn. 80) – designated and well-illustrated by Schuchert (1997) – is distinctive through its long cauline and cladial ahydrothecate internodes, and its rather shallow hydrothecae, provided with short, conical lateral nematothecae borne on inconspicuous apophyses. The present material fully agrees with these, allowing an updated and more comprehensive account on H. polymorpha to be done.
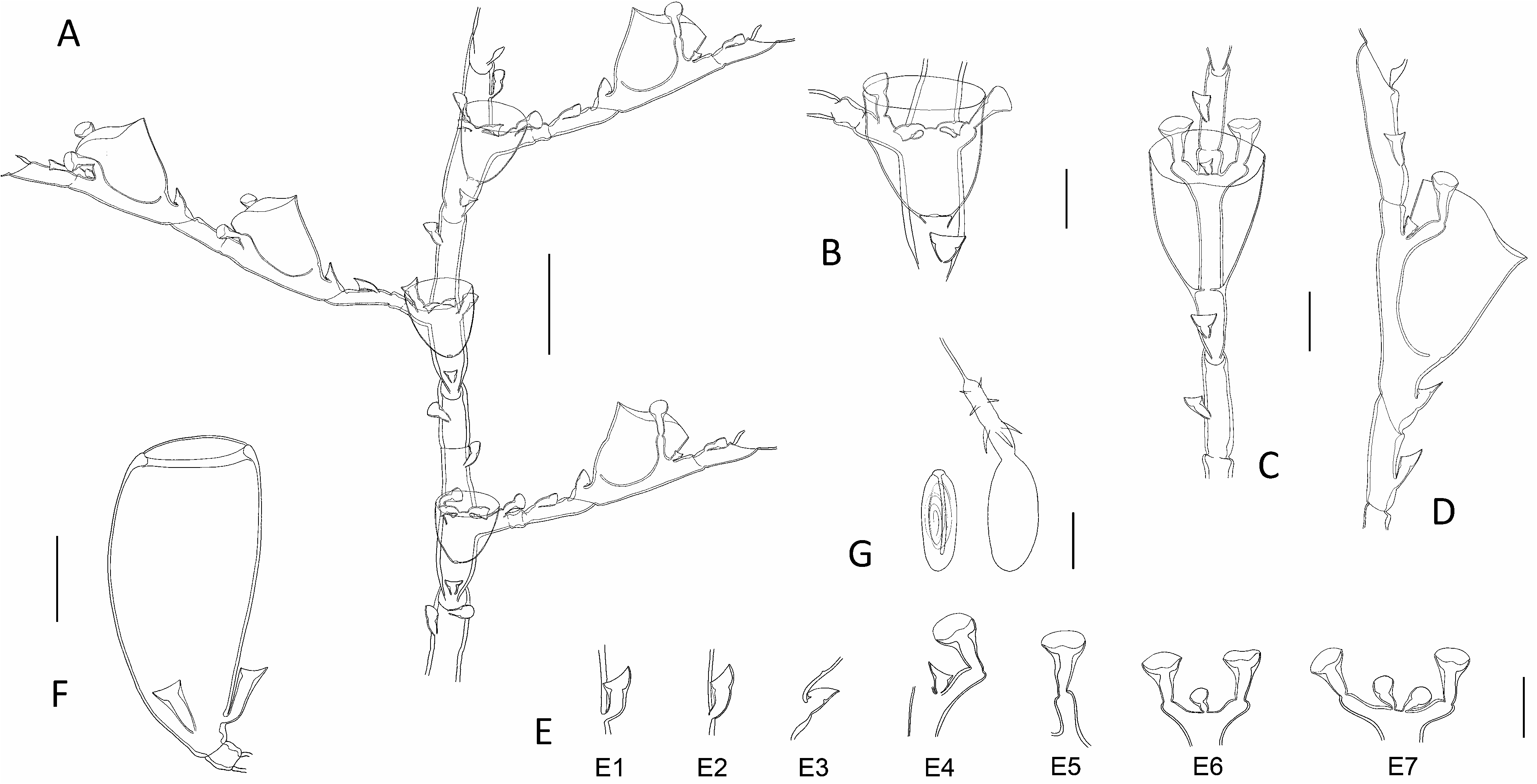
A microslide (H.L.1309), stored in MNHN and containing a sterile cormoid from Siboga Stn. 299, was re-examined. The caulus is homomerously segmented, with the exception of its distalmost part, where transverse nodes intervene; each internode is moderately long and comprises a hydrotheca in its lower half, a lateral apophysis and up to 7 nematothecae (1 mesial, a pair of laterals, a pair of axillar, as well as 1-2 superior ones, a certain distance one above the other, and slightly displaced laterally to one another) ( Fig. 3L View Fig ). The hydrocladia are heteromerously segmented; the 1st ahydrothecate internode is longer than its subsequent counterparts, and bears generally 2 nematothecae (although 3 were noted in one instance); the ordinary ahydrothecate internodes are of a rather varied length and carry 1-2 nematothecae ( Fig. 3M View Fig ); the hydrothecate internodes bear a hydrotheca and its 4 associated nematothecae (1 mesial, a pair of laterals, and an axillar one) ( Fig. 3N View Fig ). All nematothecae, including the axillar ones, are bithalamic. The hydrothecae are deep and almost cylindrical, and their lateral nematothecae are mounted on well-developed apophyses, and scarcely reach the hydrothecal rim ( Fig. 3O View Fig ). Its gonothecae remain to be discovered.
Accordingly, it results that the material from Siboga Stn. 299 is morphologically different from the lectotype of H. polymorpha (compare Fig. 3 View Fig A-F and 3L-O; see also Table 1 View Table 1 ), a finding that contrasts with earlier views expressed by both Billard (1913) and Schuchert (1997). The former material obviously belongs to a different, possibly an as yet unnamed species, whose comprehensive description requires additional, fertile material.
The 3rd morphotype belonging to the syntype of H. polymorpha , from Siboga Stn. 77, is presently the less documented (the available data are summarized in Table 4 View Table 4 herein). According to Billard (1913), its cladia, besides the proximal most, quadrangular segment, begin with an ahydrothecate internode provided with only one nematotheca, followed by a succession of “mostly” homomerously-segmented internodes bearing “most often” a single nematotheca. The rather deep hydrothecae, provided with lateral nematothecae borne on well-developed apophyses, combined with the structure of the hydrocladia, suggest – with little doubt – that the material from Siboga Stn. 77 is specifically different from both the lectotype of H. polymorpha and the material in hand described above, thus contradicting – again – the opinions expressed by both Billard (1913) and Schuchert (1997).
Halopteris nuttingi ( Billard, 1911) was synonymized with H. polymorpha by Schuchert (1997), an opinion not shared here. However, we agree with him that its inclusion in the synonymy of H. buskii ( Bale, 1884) , proposed by Billard (1913), is not justified as they have morphologically different gonothecae. According to Billard (1913), H. nuttingi [as Plumularia buski (sic!)] and H. polymorpha could be confidently distinguished through the shape of the upper chamber of their lateral nematothecae: globular with distinctly emarginated ad- and abaxial walls in the former, and conical with slight adaxial emargination in the latter (it was stated above that the complete panoply of shapes displayed by the latter also include a sinuated rim or an adaxial emargination). In Schuchert’s (1997) view, this character is unreliable, arguing that in other halopteridids, e.g. Antennella quadriaurita Ritchie, 1909 , “it is notoriously variable”. However, since then, it has been suggested that the former concept of A. quadriaurita likely includes a complex of species ( Galea, 2013: 29), and it has been demonstrated, for instance, that at least one “morphotype” represents a distinct, well-characterized species ( Galea & Ferry, 2015: 237). Moreover, besides the distinctive shape of the lateral nematothecae, the number and position of their counterparts confined to the cauline internodes distal to hydrotheca is different in H. nuttingi (see Billard, 1913). Last but not least, according to both Billard (1911, fig. 8; 1913, fig. 11) and Schuchert (1997, fig. 21C), this nominal species has shorter cladial ahydrothecate internodes and deeper hydrothecae compared to H. polymorpha .
Additionally, it should be stressed that H. nuttingi was created based on a syntype, as it results from Billard’s [1913, as Plumularia buski (sic!) Bale, 1884] work, but not from his original account ( Billard, 1911). Moreover, according to the former publication, it is very likely that the syntype contains a mix of species, as Billard mentions (p. 22) ahydrothecate cladial internodes either short or long, and provided with one or two nematothecae. However, a neotype (sic!) for H. nuttingi has been designated (Coel. 5241) by Schuchert (1997: 64), who also provided reliable illustrations of it (N.B.: This material should be best referred to as the lectotype). Besides Billard’s (1911, 1913) record of H. nuttingi , at least two others seem to occur in the literature, viz. Redier [1966: 90, pl. 2 figs 1 & 3, pl. 3 fig. 1; as P. buski (sic!)] and Watson (2000: 46, fig. 35C, E; pro parte as H. polymorpha ).
Literature records of H. polymorpha are a matter of debate, due to several main factors: 1) the lack of formal descriptions, or descriptions too succinct, sometimes not accompanied by illustrations, a situation mainly occurring in older literature; 2) only sterile material was available, thus generating confusion with H. buskii ( Bale, 1884) ; 3) the artificial inclusion in the synonymy of Billard’s (1913) species of a variety of hydroids displaying a large panoply of morphological features, the specific name “ polymorpha ” being obviously misleading. Schuchert (1997) and Ansín Agis et al. (2001, 2009) provided extensive lists of synonyms for this taxon (a compilation is given in the synonymy above), though only a few prove reliable in light of the present observations.
For instance, the material studied by Di Camillo et al. (2008) and re-examined herein, belongs to the present species.In addition, the Fijian record by Ryland & Gibbons (1991: 530) is also in agreement with it, since it displays the distinctively long cladial ahydrothecate internodes, relatively shallow hydrothecae, lateral nematothecae (with flared upper chamber) borne on inconspicuous apophyses, as well as the occasional presence of axillar nematothecae behind the cladial hydrothecae (it is assumed that their cauline counterparts were overlooked by the authors). Although neither formally described, nor illustrated, the Queensland record by Pennycuik (1959) is reportedly said similar with Billard’s fig. 14A, presently known as representing the lectotype of H. polymorpha .
Besides these few records, many others clearly deviate morphologically from the lectotype. Among them, there are morphotypes characteristically forming either tall (> 4 cm high) or small-sized (<2 cm high) cormoids. Specimens with tall stems were described, for instance, in materials from South Africa ( Millard, 1975), Zanzibar ( Rees & Vervoort, 1987; as H. buskii ), and the Seychelles ( Millard & Bouillon, 1973). Millard’s (1975) material is, obviously, a mix of species: one with very deep, almost tubular hydrothecae (her fig. 112K), while the other (her fig. 112L) corresponds morphologically to the redescription of the lectotype of H. buskii provided by Schuchert (1997). Conversely, the specimens from Zanzibar and the Seychelles (part of the latter reexamined herein) belong to an as yet undescribed species, Halopteris millardae (see below).
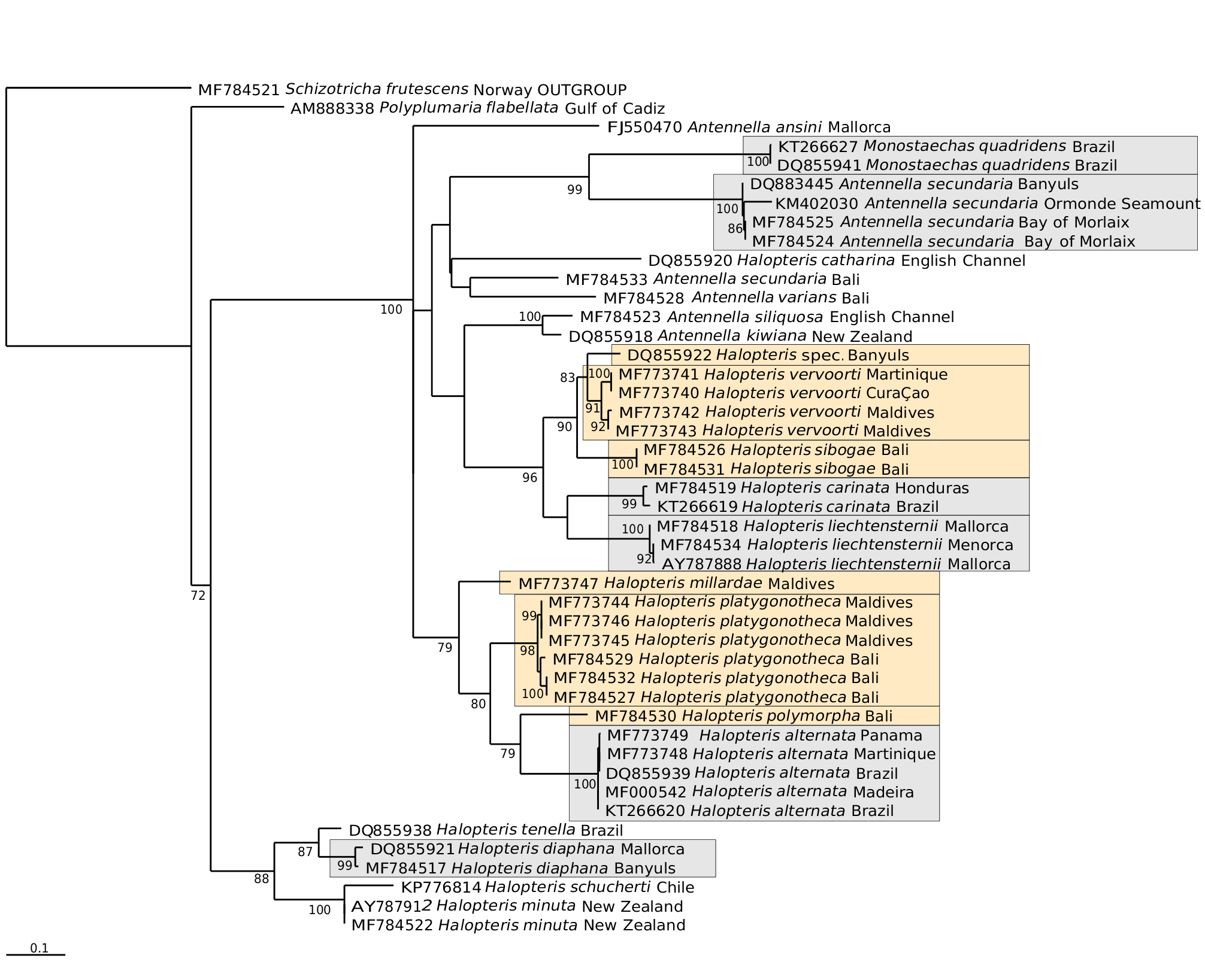
On the other hand, among the materials with smallsized cormoids, several morphological groups could be distinguished. First, there are specimens whose hydrothecae distinctly display sinuated margins ( Vervoort & Vasseur, 1977), and these belong to the new species, Halopteris australis , described below. Second, there are materials whose hydrothecae possess an even rim, but further divide into a subgroup with homomerouslysegmented cladia (e.g. Vervoort, 1966; Hirohito, 1983) and another one displaying a heteromerous division into internodes [e.g. Vervoort (1967), as Antennella secundaria ; Hirohito (1974), as Heterotheca buski (sic!); Ryland & Gibbons (1991), as H. buskii ; Hirohito (1995), as H. buski (sic!); Preker & Lawn (2010, 2012)]. Some specimens, among the materials with heteromerous cladia, are thought to belong to H. vervoorti Galea, 2008 (see below under this species), while the taxonomic status of the remaining ones is uncertain in light of the available data (see Tables 2 View Table 2 and 4 View Table 4 ). The reexamination of extant specimens, the availability of newly-collected materials, as well as modern, molecular approaches are expected to gradually solve the intricacies of this species group.
A sample provisionally identified as H. polymorpha , originating from the Mediterranean, was first used in a molecular phylogeny by Leclère et al. (2007).The voucher specimen used in that work (MHNG-INVE-30117, data in Appendix 2) was re-examined for the purpose this study. The single cormoid is sterile and thus not reliably identifiable. It resembles H. vervoorti , notably in having pairs of axillar nemathothecae associated to the cauline hydrothecae. More and especially fertile material is needed for a correct identification, as it likely belongs to an as yet undescribed species, according to the 16S data ( Fig. 9 View Fig 9 ; DQ855922 View Materials ).
Halopteris polymorpha , as presently understood, can be separated from its congeners [see list in Schuchert (2015)] through a series of morphological features. The following hydroids can be excluded a priori from the comparison, on the account of a series of diagnostic traits which separate them easily from the species discussed here:
1) the fascicled habit of their stems [occasional in H. campanula ( Busk, 1852) , common in H. valdiviae ( Stechow, 1923) ];
2) their cladia arranged in opposite pairs [ H. catharina ( Johnston, 1833) , H. clarkei ( Nutting, 1900) , H. enersis Galea, 2006 , H. gemellipara Millard, 1962 , H. geminata ( Allman, 1877) , H. opposita ( Mulder & Trebilcock, 1911) , H. plagiocampa ( Pictet, 1893) , H. prominens Vervoort & Watson, 2003 ];
3) their gutter-shaped hydrothecae [ H. everta ( Mulder & Trebilcock, 1909) ], or 4) their hydrothecae divided by internal septa [ H. diaphragmata ( Billard, 1911) , H. jedani ( Billard, 1913) ], or
5) provided with either an abaxial cusp ( H. rostrata Millard, 1975 ) or
6) a longitudinal carina ( H. carinata Allman, 1877 );
7) the presence of two pairs of lateral nematothecae ( H. infundibulum Vervoort, 1966 ).
As to the remaining species, their differences to H. polymorpha are summarized in Appendix 1.
Among them, according to the phylogenetic tree shown in Fig. 9 View Fig 9 , H. polymorpha comes close to H. platygonotheca Schuchert, 1997 . Besides notable differences in their respective female gonothecae (pear-shaped in the former, and conspicuously laterally-flattened in the latter), their trophosomes display several common characters: 1) their stem internodes are long and provided with several nematothecae distal to the hydrothecae [commonly 2-3 (but up to 5 possible) in H. polymorpha , and from 1 (sample MHNG-INVE-97943) to 1-3 (sample HRG- 1288) in H. platygonotheca ]; 2) the occurrence (regular in the former and occasional in the latter) of an axillar nematotheca behind the cauline hydrothecae; 3) their cladial ahydrothecate internodes are long; 4) the apophyses supporting their lateral nematothecae are inconspicuous, and the thecae themselves do not reach the hydrothecal rim.
Taken together, the previous supposed morphological variability of, and the implicit difficulty in establishing a specific limitation in H. polymorpha , are now solved through the discovery of additional records in perfect agreement with the lectotype designated by Schuchert (1997). It is concluded that Billard’s (1913) species is well-characterized and morphologically homogenous, as illustrated with the present material belonging to various geographically-distant Indonesian populations.
Distribution: Scattered records from Indonesia, viz. off Kalimantan ( Billard, 1913, Siboga Stn. 80), Bali and Ambon (present study), Bunaken National Park ( Di Camillo et al., 2008; present study). Also occurring in Fiji ( Ryland & Gibbons, 1991). A doubtful record from Queensland, Australia ( Pennycuik, 1959).
| H |
University of Helsinki |
| S |
Department of Botany, Swedish Museum of Natural History |
| I |
"Alexandru Ioan Cuza" University |
| G |
Conservatoire et Jardin botaniques de la Ville de Genève |
| C |
University of Copenhagen |
| MNHN |
Museum National d'Histoire Naturelle |
| L |
Nationaal Herbarium Nederland, Leiden University branch |
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |
Halopteris polymorpha ( Billard, 1913 )
| Galea, Horia R., Gioia Di Camillo, Cristina, Maggioni, Davide, Montano, Simone & Schuchert, Peter 2018 |
Halopteris buskii
| Preker M. 2001: 154 |
| Migotto A. E. 1996: 48 |
| Bouillon J. & Massin C. & Kresevic R. 1995: 49 |
| Ryland J. S. & Gibbons M. J. 1991: 527 |
| Vervoort W. & Vasseur P. 1977: 72 |
Plumularia polymorpha Billard, 1913
| Van Soest R. W. M. 1976: 89 |
Heterotheca buski
| Hirohito & Emperor of Japan 1974: 30 |
Antennella secundaria
| Vervoort W. 1967: 42 |
Halopteris polymorpha
| Preker M. & Lawn I. D. 2012: 45 |
| Preker M. & Lawn I. D. 2010: 120 |
| Preker M. & Lawn I. 2005: 342 |
| Kirkendale L. & Calder D. R. 2003: 169 |
| Preker M. 2001: 154 |
| Watson J. E. 2000: 46 |
| Bouillon J. & Massin C. & Kresevic R. 1995: 49 |
| Hirohito & Emperor of Japan 1983: 62 |
| Millard N. A. H. 1975: 354 |
| Vervoort W. 1966: 132 |
Halopteris buski
| Hirohito & Emperor of Japan 1995: 244 |
| Hirohito & Emperor of Japan 1983: 61 |
| Rees W. J. & Thursfield S. 1965: 160 |
Halopteris polymorpha
| Pennycuik P. R. 1959: 178 |
Antennella polymorpha
| Vervoort W. 1941: 218 |
Heterotheca polymorpha
| Stechow E. 1923: 15 |
Thecocarpus polymorphus
| Bedot M. 1921: 9 |
Plumularia nuttingi
| Billard A. 1911: 66 |
Plumularia buskii
| Nutting C. C. 1927: 221 |
| Thornely L. R. 1916: 150 |
| Thornely L. R. 1904: 120 |
Plumularia buski
| Redier L. 1966: 90 |
| Billard A. 1913: 21 |
| Hartlaub C. 1901: 374 |