Kingsleya attenboroughi, Pinheiro, Allysson P. & Santana, William, 2016
|
publication ID |
https://doi.org/ 10.11646/zootaxa.4171.2.9 |
|
publication LSID |
lsid:zoobank.org:pub:0FDD38FF-ED01-4AC1-8581-62D7FAF017FB |
|
DOI |
https://doi.org/10.5281/zenodo.6058367 |
|
persistent identifier |
https://treatment.plazi.org/id/22C2A532-20C0-46D6-857A-6EDEDDB0099C |
|
taxon LSID |
lsid:zoobank.org:act:22C2A532-20C0-46D6-857A-6EDEDDB0099C |
|
treatment provided by |
Plazi |
|
scientific name |
Kingsleya attenboroughi |
| status |
sp. nov. |
Kingsleya attenboroughi View in CoL n. sp.
( Figs. 1 View FIGURE 1. A, B C–F; 2A, B; 3A–D; 4A–D)
Holotype: male, cw 46mm, cl 29.2mm, Brazil, Ceará state, Barbalha, Arajara district , 07°20’07.6”S, 39°23’58.8”W, 17.iv.2016 ( MZUSP 34626 View Materials ) GoogleMaps . Paratypes (7 specimens): Brazil, Ceará state, Barbalha, Arajara district, Córrego do Arajara , 04.v.2016 , 1 male cw 51.2mm, cl 31mm (MZUSP 34627); 1 female cw 41.5mm, cl 26.7mm (MZUSP 34628); 1 male cw 40mm, cl 26.1mm, 1 female cw 35mm, cl 23mm (INPA 2208); 2 males cw 36.8mm, cl 24mm and cw 36mm, cl 24mm, 1 female cw 37.7mm, cl 24mm (LACRUSE 0221).
Comparative material. Fredius reflexifrons (Ortmann, 1897) : Brazil, Amapá, Município de Laranjal , 16.i.2012, 1 male ( INPA 2125 View Materials ) . Pará, Rio do Peixe Boi , 01°11’30”S, 47°18’54”W, 03.iii.1995, E. Matos & A. Henriques Jr coll., 1 male ( INPA 851 View Materials ) GoogleMaps . Amazonas, Manaus, Reserva do Km 41, 02°26’56”S, 59°46’13”W, 1 male ( INPA 889 View Materials ) GoogleMaps . Ceará, Viçosa do Ceará, Fonte do Caranguejo , 03º33’43.2”S, 41º5’09.6”W, 24.vi.2004, M. Pereira coll., 1 male ( INPA 1382 View Materials ) GoogleMaps . Kingsleya castrensis Pedraza, Martinelli-Filho & Magalhães, 2015 : Brazil, Pará, Caverna Pedra da Cachoeira , 03°19’14.8”S, 52°19’53.1”W, R. Pinto-da-Rocha coll., 20.vi.2012, male paratype cw 50.9mm, cl 30mm ( MZUSP 26394 View Materials ) GoogleMaps . Brazil, Pará, Altamira , 03°19’14.8”S, 52°19’53.1”W, R. Pinto-da-Rocha coll., 24.iii.2012, male paratype cw 32.7mm ( MZUSP 25943 View Materials ) GoogleMaps . Kingsleya gustavoi Magalhães, 2005 : Brazil, Pará, Rio Itacaiúnas, Projeto Carajás , 17.vii.1988, 2 male paratypes cw 37mm, cl 23.3mm and cw 19.5mm, cl 12.2mm ( MZUSP 9699 View Materials ) . Pará, Canaã dos Carajás, Gruta NV 06, Andrade & Amoni coll., 22–28.v.2005, C. Magalhães det., 2 males ( MZUSP 16702 View Materials ) . Kingsleya junki Magalhães, 2003 : Brazil, Pará, Rio Xingú, Vitória do Xingú , 02°53’S, 52°01’W, R. Santos, C. Maciel & J.O. Dias coll., 03.xii.2000, male holotype cw 33mm, cl 20.8mm ( MPEG 777 View Materials ) GoogleMaps . Kingsleya latifrons (Randall, 1840) : Suriname, Anapaika Village, Rio Lawa, B. Malkin coll., 26.xi.1963, C. Magalhães det., 2 males, 2 females (MZUSP 1887). Brazil, Pará, Rio Trombetas , Cachoeira Porteira, V. P. Daniel et al. coll., 10.iv.1985, C. Magalhães det., 1 male, 1 female ( MZUSP 7008 View Materials ) . Brazil, Amazonia, Imbó, Rio Uatumã , Cachoeira Morena, F. Efrem et al. coll., 07.x.1987, 4 males ( MZUSP 11673 View Materials ) . Kingsleya ytupora Magalhães, 1986 : Brazil, Pará, Rio Trombetas , Cachoeira Porteira, C. Magalhães et al. coll., 2–10.x.1985, 1 male paratype cw 48mm, cl 28.5mm, 2 female paratypes, cw 64.5mm, cl 38mm and cw 55.6mm, cl 33mm ( MZUSP 7009 View Materials ) .
Description. Carapace ellipsoid, much wider than long, widest medially, dorsally smooth, slightly convex anteriorly, regions poorly defined. Two distinct gastric pits on metagastric region. Cervical grooves deep, narrow, slightly divergent anteriorly, fading proximally, distal end almost reaching anterolateral margin. Intestinal, cardiac regions indistinct. Postfrontal lobules inconspicuous; median groove indistinct. Carapace between front, postfrontal lobules smooth, weakly concave. Front upper border smooth, angulate, slightly bilobed in dorsal view, median notch faint; lower border carinated, slightly sinuous in frontal view, not visible in dorsal view. Superior orbital margin smooth, lower orbital margin crenualted; exorbital angle low, somewhat acute; orbital cavity with several thick setae near exorbital angle. Inner orbital tooth well developed, broadly triangular, partially occluding orbital cavity. Anterolateral margin of carapace with very distinct depression just behind exorbital angle, followed by a set of minute teeth increasing in size from the anterior to posterior portion; posterolateral margin smooth, barely defined.
Epistome narrow, deeply grooved transversally, upper margin straight, lower margin forming a mesial triangular tooth, deflexed, crenulated. Subhepatic region smooth; pterystogomial region with scarce pubescence bordering bucal frame.
Endopod of maxilliped III with outer margin of ischium almost straight, subretangular, slightly wider than merus; mesial margin setose, crista dentata with small, subquadrate, corneous teeth. Merus shorter than ischium, wider as long, inner margin almost straight, with a small proximal lobe covered with several long setae; outer margin curved distally. Inner surface of palp covered with very few, small setae; outer margin of propodus, dactylus densely covered with long setae, carpus with few long setae. Third maxilliped exopod vestigial, about one fifth of ischium lateral margin length. Aperture of efferent branchial channel wide, subquadrate, lacking setae.
Chelipeds heterochelous in both males, females, more pronounced in males, similarly armed, right P1 usually largest (holotype left cheliped largest). Merus subtriangular in cross section; upper, mesial margins distinctly crenulated; inferior lateral margin with row of distinct small tubercles, not reaching the proximal, distal margins; distal margin arched, smooth laterally, crenulated mesially. Carpus with inner margin indented proximally, with prominent median spine, smooth distally; outer margin rounded, smooth. Propodus massive, upper, mesial, lateral, ventral surfaces smooth. Movable, fixed fingers with lateral, mesial, dorsal surfaces with punctations arranged in longitudinal subparallel lines; movable finger distinctly curved downwards. Fingers noticeable gaping, tips crossing distally; cutting edges of fingers with small, massive teeth. P2–5 slender, similar in shape, decreasing in size very slightly from P2 to P5. P2–5 meri upper margin with sparse row of minute spines; dactyli punctuate in all surfaces, with five longitudinal rows of sharp spines, 3 dorsal, 2 ventral; increasing in size distally.
Thoracic sternum longer than broad. Sternal sutures 4/5 to 7/8 very distinct, ending just before reaching median line of thoracic sternum. Episternites 4–6 subtriangular, episternite 7 shorter, truncated in males, rounded in females. Episternites 4–7 fringed with short setae, longer in females. Sterno-abdominal cavity deeply excavated, shallower in females; sternites 5 to 8 densely covered medially with distinct long setae, sternite 4 with few long setae in males, females with sparse setae mostly on sternites 1–4; tubercle of abdominal holding system reduced in males, almost imperceptible in females, placed near sternal suture 5/ 6 in males,. Male, female abdomen with 6 free somites. Male abdomen tapering distally from third to sixth somite; telson triangular; margins, including telson, densely setose, setae short. Female abdomen rounded, sixth somite longer, wider, telson broadly triangular. Penis distinctly long, larger proximally, tapering distally, emerging near coxosternal condyle articulation.
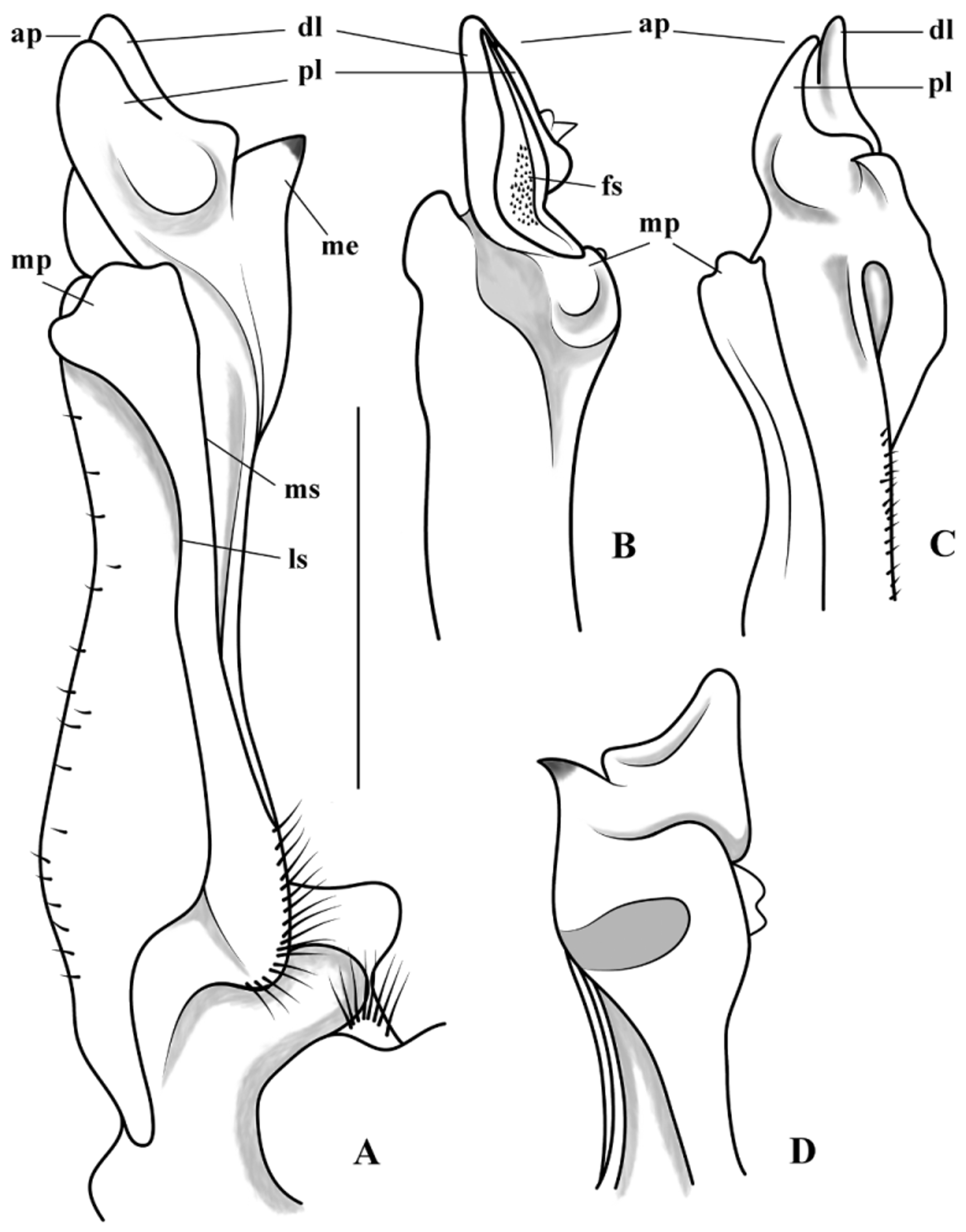
G1 almost straight, broadened distally, with very shallow curvature on abdominal surface in mesial view, with well-developed mesial process. Marginal suture almost straight, displaced to mesial side distally, with several long setae proximally. Lateral suture deep, approximately 2/3 of gonopod length from proximal portion. Marginal process short, broad, subquadrate in mesial view, not projecting distally beyond field of apical spines, distal notch in latero-abdominal surface. Mesial process short, subtriangular, proximal portion straight, distal portion protruded into sharp conical spine curved in anteromesial direction, mesial process separated by a deep incision from apical plate. Apical plate with two large, subtriangular lobes, separated from each other by distinct groove in mesial, sternal views, proximal lobe with conspicuous semicircular protuberance in mesial, sternal views. Sperm channel opening near the base of apical plate in sternal view. Apical spine field well developed, curved proximally, with a patch of minute spines concentrated medially, longitudinally directed along caudal side of apical plate, delimited by mesial, lateral borders of distal lobe of apical plate, distally closed.
G2 slightly sinuous, almost as long as G1, distinctly slender, distal 3/4 tapered; tip flattened, with numerous short spinules on sternal surface.
Type locality and distribution. Known so far only from the type locality in Arajara district, Barbalha Municipality, Ceará state, Brazil, 07°20’07.6”S, 39°23’58.8”W.
Ecological notes. The specimens were collected at the Córrego do Arajara, a small stream in the peri-urban region of the Arajara district. The stream has deforested margins surrounded by farms and corrals and is located in a slope area of pluvio-nebular forest, evergreen, about 750 m above sea level, with representatives from the Atlantic and Amazonian forests ( Sales et al. 1998). The stream was shallower than 1 m and 4 to 5 m wide, perennial, and with medium energy, with bottoms of consolidated sandstone blocks and clear water ( Fig. 5A View FIGURE 5. A, B ). The specimens were found between rocks and in small crevices of about 20 to 50 cm deep ( Fig. 5B View FIGURE 5. A, B ). Burrows were not found on the margins or in the riverbed. Crabs were more active at night and were probably hiding and inactive during the day.
Etymology. This new species is named in honor of the English naturalist Sir David Attenborough, a devoted naturalist and communicator of science that promotes environmental education and protection, including in the Chapada do Araripe, from where this species appears to be endemic.
Remarks. Kingsleya attenboroughi n. sp. can be clearly included in Kingsleya due to several characters of the G1. The apical plate of the G1 is bilobed with lobes partially overlapping; the marginal process distally enlarged, not surpassing the apical field of spines; the mesial process distinctly separated from the apical plate and standing out from the cephalic surface of the stem; and the field of apical spines distally divided by a terminal notch ( Fig. 4A–D View FIGURE 4. A – D ) ( Magalhães & Türkay 2008; Pedraza & Tavares 2015).
Kingsleya attenboroughi View in CoL n. sp. closely resembles K. gustavoi Magalhães, 2005 View in CoL , until now the easternmostly distributed species of the genus. Both species have a gonopod with a subtriangular apical plate with two lobes, with distal lobe larger, a mesial processes ending distally in strong spine, a well-developed field of apical spines, and a mesial process clearly separated by a deep incision from the apical plate ( Figs. 3A View FIGURE 3. A – D –H). Kingsleya attenboroughi View in CoL n. sp. nevertheless differs from K. gustavoi View in CoL and other congeners in having: 1) a short apical plate, with anteriorly directed lobes and angulated proximally (apical plate longer and with anterolaterally directed lobes and enlarged proximally, forming a distinct rounded prominence in K. gustavoi View in CoL ; Figs. 3B View FIGURE 3. A – D , F, 4A, D); 2) proximal lobe of the apical plate with conspicuous semicircular protuberance in mesial and sternal views (proximal lobe of the apical plate partially folded over the mesial crest of the field of apical spines in K. gustavoi View in CoL ; Figs. 3A, D View FIGURE 3. A – D , G, E, H, 4A, C); 3) marginal process protruded, projecting mesially in sternal view, subquadrate in mesial view (mesial process prominent, but not projecting mesially in sternal view, subtriangular in mesial view; Figs. 3A View FIGURE 3. A – D , E, 4C); 4) field of apical spines narrow, tapering distally, with small spines concentrated proximally (field of apical spines more opened, with small spines evenly distributed along the field; Figs. 3D View FIGURE 3. A – D , H, 4B).
Apart from the differences in the G1, Kingsleya attenboroughi View in CoL n. sp. can also be differentiated from K. gustavoi View in CoL by a set of small differences observed in the paratypes (MZUSP 9699): 1) the front lower border not visible in dorsal view (front lower border projected anteriorly and visible in dorsal view in K. gustavoi View in CoL ); 2) anterolateral margin of the carapace straight just after the exorbital angle in frontal view (anterolateral margin of the carapace slightly sinuous just after the exorbital angle in frontal view in K. gustavoi View in CoL ); 3) anterolateral margin of the carapace with a set of minute teeth increasing in size from the anterior to posterior portion (anterolateral margin of the carapace with a set of minute teeth of equal size in K. gustavoi View in CoL ).
The discovery of the new species is evidence of our lack of knowledge on this important group and emphasizes the need of conserving these unique environments and their fauna. These remnants of humid forests isolated in the Caatinga biome, which is characterized by desert-like vegetation and is exclulsive to northeastern Brazil, have been suffering under anthropogenic pressures mainly related to land development and the use of water resources, factors that restrict the habitat of the new species to small portions near the source of streams. The new species is already at significant risk of extinction, taking as base the IUCN criteria B2b, which qualifies the species as endangered (EN) (IUCN, 2012).
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |
Kingsleya attenboroughi
| Pinheiro, Allysson P. & Santana, William 2016 |
K. gustavoi Magalhães, 2005
| Magalhaes 2005 |