Sabethes (Peytonulus) shannoni ( Lane & Cerqueira, 1942 )
|
publication ID |
https://doi.org/10.11646/zootaxa.5082.3.4 |
|
publication LSID |
lsid:zoobank.org:pub:C29D8D06-0D80-4661-8E4B-BADEF178506C |
|
DOI |
https://doi.org/10.5281/zenodo.5789505 |
|
persistent identifier |
https://treatment.plazi.org/id/038E8F23-5B5B-2628-FF76-48FDFB0EF84B |
|
treatment provided by |
Plazi (2021-12-17 08:53:29, last updated 2024-11-27 18:17:11) |
|
scientific name |
Sabethes (Peytonulus) shannoni ( Lane & Cerqueira, 1942 ) |
| status |
comb. nov. |
Sabethes (Peytonulus) shannoni ( Lane & Cerqueira, 1942) View in CoL , comb. nov.
( Figs.1–4 View FIGURE 1 View FIGURE 2 View FIGURE 3 View FIGURE 4 ) (transfer from genus Wyeomyia )
1942. Wyeomyia (Dendromyia) shannoni Lane & Cerqueira, 1942: 599 . Holotype ♂: Petrópolis (not Mangaratiba, see Marchon- Silva et al. 1996), Rio de Janeiro, Brazil (IOC).
2002. Sabethes (Peytonulus) paradoxus Harbach, 2002 (in Harbach & Howard 2002): 363. Holotype ♂ with associated larval and pupal exuviae and dissected genitalia on separate microscope slides: Darien, Panama (USNM). NEW SYNONYMY.
Wyeomyia (Dendromyia) shannoni View in CoL of Lane 1953: 868, 873, 967–968, 1110 (♀, ♂ G*, taxonomy, distribution, key); Horsfall 1955: 329, 720 (distribution, bionomics); Stone et al. 1959: 87, 354 (catalog, distribution, holotype info.); Cerqueira 1961b: 160 (distribution, bionomics); Belkin et al. 1971: 12, 37, 47, 54, 64 (bionomics, holotype info.); Knight & Stone 1977: 331, 603 (catalog, distribution, holotype info.); Forattini et al. 1986a: 7 (record, bionomics, ecology, collection method), 1986b: 185 (record, bionomics, ecology, collection method); Xavier et al. (1989): 312 (distribution, state checklist); Guimarães et al. 1989: 248–250, 252 (record, bionomics, ecology), 2000d (in part): 6, 8, 10–12, 15 (record, bionomics, ecology).
Wyeomyia shannoni View in CoL of Forattini et al. 1970: 91, 99 (catalog, paratype info.), 1988: 546 (catalog), 1993a: 317 (record, bionomics, ecology), 1993b: 404 (record, bionomics, ecology); Marchon-Silva et al. 1996: 447 (catalog, holotype info., type-locality correction); Dutra et al. 1996: 376 (bionomics, ecology); Guimarães et al. 2000a (in part): 20–21, 23, 25 (bionomics, ecology), 2000b (in part): 754–756, 759 (bionomics); Hutchings et al. 2005a: 26–27 (catalog, distribution, paratype info.); Abreu et al. 2019: 221, suppl. table 2 (record, ecology, medical importance).
Sabethes (Sabethinus) View in CoL sp 2 of Heinemann & Belkin 1978: 193, 195 (records, bionomics).
Wyeomyia shannoni View in CoL (subgenus uncertain) of Motta&Lourenço-de-Oliveira1995:384 (classification,systematic); Guimarães 1997: 131, 283 (catalog, holotype info., distribution); Harbach 2018: 119, 182 (nomenclature, etymology, taxonomy, classification); Silva et al. 2019: 195, 197, 200 (♀, record, distribution, bionomics, taxonomy); Santos et al. 2019: 829 (record, ecology); Brilhante et al. 2020: 100 (distribution, state checklist); Harbach 2021 (species list, classification, taxonomy).
Sabethes (Peytonulus) paradoxus View in CoL of Talaga et al. 2015: 770–771, 775, 779 (record, bionomics, country checklist), 2016: 1139, 1142 (ecology, bionomics, functional morphology), 2017: 6, suppl. tables 1 and 2 (molecular phylogeny, Barcode Index Number); Harbach 2018: 100 (nomenclature, etymology, taxonomy); Harbach, 2021 (species list, classification, taxonomy); Wilkerson et al. 2021 (catalog, distribution).
The immature forms and adult male, including male genitalia, of Sabethes (Peytonulus) shannoni ( Lane & Cerqueira, 1942) View in CoL have already been described and illustrated (as Sa. paradoxus View in CoL ) in detail in Harbach & Howard (2002). Since we were able to examine a larger number of individuals of Sa. shannoni View in CoL from a wider geographical range in Brazil, including the type locality, we add below some morphological characteristics of the male and immature forms of this species to complement the description of the species in Harbach & Howard (2002). Additionally, we redescribe the female, including the description and illustration of characters not included in the original description by Lane & Cerqueira (1942), e.g. the female genitalia.
Adult. Sexes essentially identical in body size and external appearance, exhibiting slight secondary sexual differences in the antennal flagellum and clypeus, and conspicuous sexual differences in the proboscis and genitalia. As highlighted in Harbach & Howard (2002), the adults of Sa. shannoni (as Sa. paradoxus ) do not have the brilliant metallic-colored scaling on the scutum characteristic of Sabethes species , being like Wyeomyia species in general habitus, differing mainly in the absence of prealar setae ( Fig. 1A,B View FIGURE 1 ).
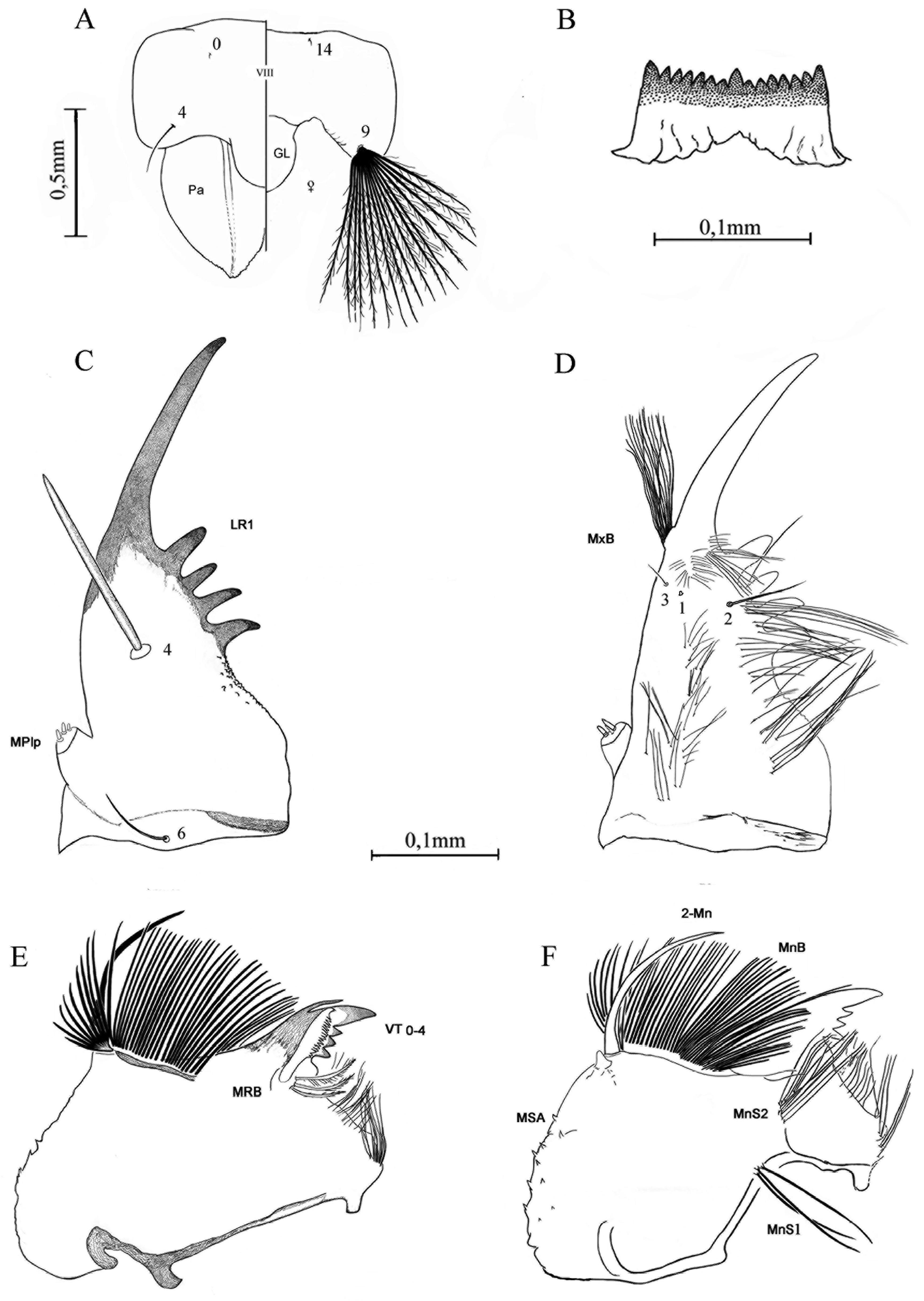
Female. Head: Eyes joined above and below. Occiput with transverse row of short semi-erect scales on back of head, occiput and posterior part of vertex with recumbent scales with metallic greenish blue reflections, scales of anterior area of vertex (near the interocular space) with weak violaceous to darkish blue reflections; postgena with silvery white reflections. Ocular setae moderately long, darkish brown; 2 long, approximated, darkish brown interocular setae. Antenna: Length 1.9–2.2 mm, essentially as long as proboscis; pedicel large, brown, pubescent, mesal side darkish brown with inconspicuous minute setae, sometimes with minute scales among setae; flagellum dark brown, moderately verticillate, with 13 flagellomeres, flagellomere 1 with inconspicuous cluster of dark scales on mesal area, flagellar whorl of flagellomere 1 near apex, flagellar whorls of flagellomeres 2–13 at base with about 10 setae. Clypeus nude, dark brown, ovate, densely pubescent, longer than wide. Proboscis: Length 2–2.2(2.1) mm, nearly as long as antenna, about 0.6–0.85(0.73) length of forefemur; slightly bent upward, distal 0.22–0.27(0.22) slightly flattened and expanded laterally to 2.0–2.5 times width of proximal part of proboscis; dark-scaled dorsally, ventral surface dark with a conspicuous spot of white scales on middle area; 3–8(5) basal labial setae; labella dark with numerous minute pale setae. Maxillary palpus 0.13–0.23(0.18) length of proboscis, dark-scaled dorsally, ventral surface without scales. Thorax: Integument brown; antepronotum with 22–34(29) darkish brown setae, scales on anterior part with violet reflections, scales on mid part with bluish reflections and sometimes also with violet reflections, those on posterior part with pearly white reflections; postpronotum without setae, scales on lower and upper area with pearly white reflection; dorsum with 17–30 darkish brown setae on anterior promontory, supraalar area with 22–31 brown setae; scutellum with 22–29(29) darkish brown setae, 8 on midlobe [3–5(3) long and 3–5(5) short] and 14–21 on each lateral lobe [9–12(11) long and 4–10(10) short]; mesopostnotum with 7–15(15) light brown setae; acrostichal and dorsocentral areas, scutal fossa, and prescutellar area without setae. Dorsum with dull scales of weak greenish blue reflections covering entire scutum, including anterior promontory, scutal fossa, antealar, supraalar and prescutellar areas, and mid and lateral lobes of scutellum. Pleural integument light brown, with 4–6 pale yellow upper proepisternal setae, 2 or 3(2) brown prespiracular setae, 5–7(7) pale yellow lower mesokatepisternal setae and 13–19 pale yellow upper mesepimeral setae; lower proepisternal area, proepimeron, subspiracular and postspiracular areas, upper mesokatepisternal area, prealar knob, lower mesepimeral area, mesomeron, metameron and metepisternum without setae. Pleural scales with pearly white reflections ( Fig. 1A View FIGURE 1 ) on upper proepisternum, proepimeron, subspiracular, postspiracular, upper and lower mesokatepisternal, upper and lower prealar, and lower, posterior, anterior and upper mesepimeral areas; scales absent on lower proepisternum and prespiracular areas, paratergite, mesomeron, metameron and metepisternum. Wing: Length 3.6–4.1 mm; costa and basal part of vein R dark-scaled with weak bluish reflections; subcosta, almost all of veins R and R1 darkscaled, remaining veins brown-scaled; veins M3+4, CuA 1A and mcu crossvein moderately broad and slightly asymmetrical; costa, vein R, basal part of R1, proximal 0.67 of mcu, CuA and 1A with decumbent scales; subcosta, distal 0.67 of veins R1, R2+3, RS, R2, R3, M, M1+2, M1, M2, M3+4, distal 0.33 of mcu and 1A with anterior and posterolateral scales; vein R2 55–75% longer than R2+3. Alula with 4–10(8) setae on distal margin, upper calypter without setae, remigium entirely covered with dark scales with bluish reflections. Halter: Scabellum yellowish, without scales; pedicel and capitellum with dark scales. Legs: Coxae with yellowish integument; upper anterior and outer surface of fore- and midcoxae and outer surface of hindcoxa covered with pearly white scales; forecoxa with yellow setae on anterior (10–14 setae), outer (2–4) and mesal (2–7) surfaces; midcoxa with yellowish setae on outer (2–8 setae) and mesal (6–8, mode 8) surfaces; hindcoxa with yellow setae on outer (5–7, mode 5), posterior (4 or 5, mode 5) and mesal (2–5, mode 4) surfaces. Trochanters entirely pearly white-scaled, except for a small apical patch of conspicuous dark scales on dorsal surface. Femora dark-scaled on dorsal, anterior and posterior surfaces, white-scaled ventrally. Tibiae entirely dark-scaled, sometimes with a continuous stripe or discontinuous small patches of white scales. Tarsi without paddles, totally dark-scaled, except for a weak stripe of white scales on base of hindtarsomere 1 and ventral surface of hindtarsomere 5, which is entirely covered by conspicuous white scales. Unguis simple and dark, foreunguis small, mid- and hindunguis smaller than foreunguis. Abdomen: Densely covered with dark lackluster scales with greenish-blue reflection similar in color to scutal scales on dorsal and upper lateral margins, lower lateral margin and ventral surface with pearly white scales, lateral incisions rounded ( Fig. 1A View FIGURE 1 ). Tergum I with dark lackluster scales on dorsal surface, proximal area without scales, lateral surfaces with pearly white scales. Terga II–VII with dark lackluster scales on dorsal surface, II with diagonal to rounded, pearly white scale-patches on lateral surfaces, III–VI with sharply rounded white scale-patches laterally, VII with slightly rounded pearly white scale-patches laterally. Tergum I with numerous yellow setae basally and few yellow setae on posterior margin; terga II–VI with inconspicuous posterior setae, tergum VII with posterior dark setae. Sternum I without scales, sterna II–VII with pearly white scales; sterna I,II without setae, sterna III–V with few short dark setae on posterior margin, sternum VI with dark setae posteriorly, sternum VII with dark brown setae posteriorly. Genitalia ( Fig. 2 View FIGURE 2 ). Tergum VIII with anterior margin convex and posterior margin slightly concave medially, entirely covered with scales, posterior margin with setae, longest setae inserted immediately before a distal row of setae, scales absent on basal area, but densely mixed with setae on central and posterior areas. Sternum VIII shorter medially, anterior and posterior margins concave, setae inserted exclusively on distal 0.33, except in central area, all but narrow anterior and posterior areas covered with scales, scales mixed with setae mainly on central area. Tergum IX, insula, postgenital lobe and cerci densely spiculate. Tergum IX constricted in middle, broad on either side of midline, bearing 5 setae on posterior margin. Insula broader basally than posteriorly, conspicuous cleft on midline bearing 8 or 9 setae from near base to posterior margin on each side. Postgenital lobe about 1.25 length of cerci, tip essentially straight medially and slightly curved laterally, base twice width at tip, dorsal surface with 8 or 9 setae on dorsocentral line reaching tip, ventral surface with scattered setae on distal 0.5. Cercus arising obliquely in relation to sagittal plane of body, dorsal and ventral surfaces densely covered with minute setae, scales absent, dorsal surface with setae inserted mostly posteriorly, ventral surface with setae restricted to distal 0.5, largest setae inserted mostly in posterior area; 3 spherical spermathecal capsules, one slightly larger than the others.
Male. As previously described in Harbach & Howard (2002: 364) (as Sa. paradoxus ), and similar to the female except for the following characters: Head: Antenna length 1.7–2.1 mm. Clypeus longer than wide, smaller than in female. Proboscis ( Fig. 3A,B View FIGURE 3 ): Length 1.9–2.2 mm, short, distal 0.20–0.29 flattened and greatly expanded laterally, 4.88–5.66 width of proximal part; white-scaling on ventral surface beginning 0.31–0.49 from base and extending to 0.77–0.86(0.77) of expanded distal part; 1–5(3) basal labial setae; labella pale. Maxillary palpus 0.17–0.19 length of proboscis. Thorax: Antepronotum with 10–18 darkish brown setae, anterior promontory with 17–25 darkish brown setae, supraalar area with 20–32 brown setae, scutellum with 20–31 darkish brown setae (7–10 on midlobe, 1–4 long and 3–6 short; 13–21 on each lateral lobe, 3–12 long and 6–10 short), mesopostnotum with 8–12 brown setae. Pleura with 3–5(4) pale yellow upper proepisternal setae, 1–3(2) brown prespiracular setae, 5–7(6) pale yellow lower mesokatepisternal setae and 15–18(16) pale yellow upper mesepimeral setae. Wing: Length 3.3–3.6 mm, vein R 2 0.56–0.67 length of R 2+3, alula with 6–10 scales. Legs: Forecoxal setae present on anterior (7–12 yellow to brown setae), outer (1–4 yellow to light brown) and mesal (3–5 yellow to brown setae) areas, midcoxal setae present on outer (5–7 pale yellow to light brown setae, mode 6) and mesal (3–9 pale yellow to light brown setae, mode 7) areas, hindcoxal setae present in outer (6 or 7 pale yellow to light brown setae, mode 6), posterior (3–5 pale yellow to light brown setae, mode 5) and mesal (4–7 yellow to light brown setae, mode 5) areas. Ventral surface of midtrochanter with conspicuous dark spot of scales and hindtrochanter with fainter dark scales. Genitalia: Sternum IX ( Fig. 2A View FIGURE 2 ) with concave anterior margin, posterior 0.3 roughly triangular. Gonocoxite with a large alveolus in lateral position, above and mesad to tergomesal setae.
Pupa ( Fig. 4A View FIGURE 4 ). As previously described by Harbach & Howard (2002) (as Sa. paradoxus ). Genital lobe of female: Lightly tanned, length about 0.5 length of paddle. Median caudal lobe: Lightly tanned, length about 0.5 length of paddle.
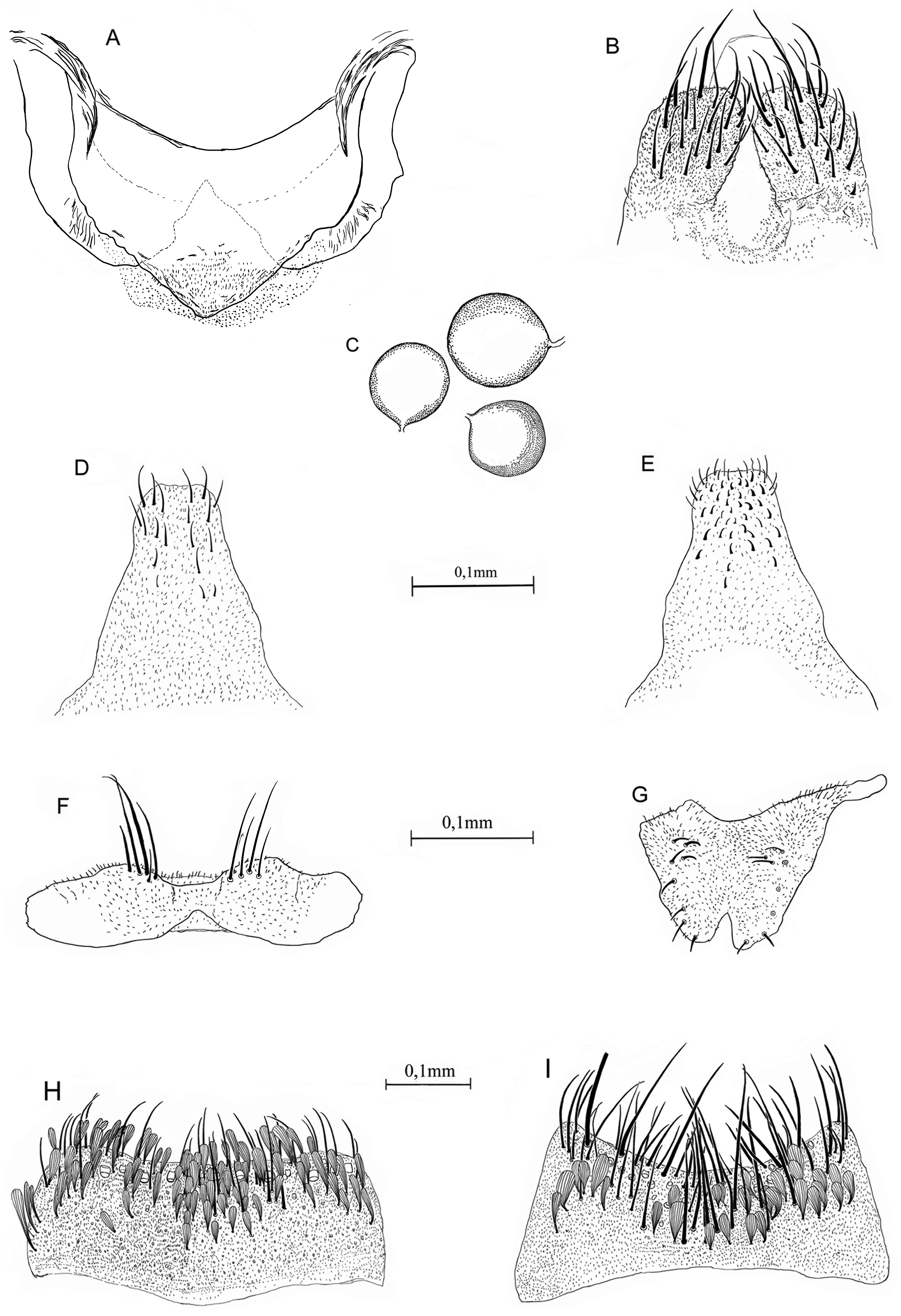
Larva, mouthparts ( Fig. 4B–F View FIGURE 4 ). As previously described by Harbach & Howard (2002) (as Sa. paradoxus ). Dorsomentum: Short, roughly rectangular, with 6–8(7) teeth on either side of median tooth, median and most lateral tooth of either side larger than others, all aligned ( Fig. 4B View FIGURE 4 ). Mandible: Mandibular sweeper 1 setae thicker and less numerous than setae of mandibular sweeper 2; mandibular sweeper 1 inserted on margin of mandible ( Fig. 4E,F View FIGURE 4 ). Maxilla: Maxillary body elongate, laciniarastrum 1 with 4 large, dark teeth similarly developed, about 0.2 length of apical tooth; apical tooth well developed and sclerotized, about 0.66 length of maxillary body, slightly curved mesad; maxillary brush long, filaments slender, about 0.6 length of apical tooth, arising from a pit; maxillary pilose area with spicules scattered and shorter than laciniarastrum 2; setae 1–3-Mx adjacent to each other, inserted apically; seta 4-Mx single, large, stout and pointed apically, about 0.9 length of apical tooth; seta 6-Mx single; maxillary palpus short, fused with maxillary body, with 3 setae at apex ( Fig. 4C,D View FIGURE 4 ).
Systematics. The systematics of Sa. shannoni (as Sa. paradoxus ) was discussed by Harbach & Howard (2002). Concerning the morphology of the immature stages, we additionally found that the dorsomentum of Sa. shannoni (Lane & Cerqueira) is similar to that of Sa. (Pey.) aurescens ( Lutz, 1905) , Sa. (Pey.) undosus ( Coquillett, 1906) and Sa. (Pey.) luxodens Hall, Howard & Harbach, 1999 in having the median and most lateral teeth on a straight line and of similar length ( Howard et al. 1913, 1915; Harbach 1991; Hall et al. 1999). The four large lateral teeth of laciniarastrum 1 are similar in size and resemble those of Sa. (Pey.) soperi Lane & Cerqueira, 1942. Additionally, we found that pupal seta 8-VI is always dorsal in position, thus confirming the description and comments of Harbach & Howard (2002) that this character is a fixed feature of this species. In relation to the adult male, the exceptional expansion of the distal part of the proboscis is only shared with Sa. (Pey.) fabricii Lane & Cerqueira, 1942 . Among all Peytonulus species, only Sa. hadrognathus Harbach, 1995a has the female genitalia described and illustrated. Sabethes shannoni can be easily distinguished from Sa. hadrognathus by possessing an insula with a conspicuous cleft on the midline, postgenital lobe without invagination at the tip and tergum IX narrow in the middle with a larger interlobar space.
Bionomics. As other species of the subgenus Peytonulus , the immature stages of Sa. shannoni often develop in bamboo. In our collections, in the Brazilian Atlantic Rainforest, immature stages of Sa. shannoni were found in cut bamboo ( Fig. 5 View FIGURE 5 ) together with Haemagogus (Conopostegus) leucocelaenus ( Dyar & Shannon, 1924) and Wyeomyia (Wyeomyia) arthrostigma ( Lutz, 1905) . Talaga et al. (2015) found immatures stages (as Sa. paradoxus ) in perforated bamboo internodes ( Guadua latifolia (Bonpl.) Kunth ) in the Amazon Rainforest of Saül, French Guiana. Essentially all available data on the biting behavior of Sa. shannoni (as Wy. shannoni ) has been recorded at sites in the Atlantic Rainforest biome of southeast Brazil, either in primitive jungle ( Forattini et al. 1986a, b, 1993a, b; Guimarães et al. 2000a, b, d; Dutra et al. 1996), protected areas of secondary forest ( Guimarães et al. 1989; Silva et al. 2019; Santos et al. 2019) or fragments of forest ( Abreu et al. 2019). In those areas, Sa. shannoni was found biting inside the woods exclusively during daytime ( Guimarães et al. 2000b), resting on vegetation ( Forattini et al. 1993b) and rarely attacking humans in areas without dense forest ( Guimarães et al. 2000a). Temperature, rather than relative humidity and rainfall, according to Guimarães et al. (2000d), seems to influence the frequency of Sa. shannoni .
Distribution. Sabethes shannoni is recorded from Brazil, Ecuador and French Guiana in South America and from Panama and Nicaragua in Central America ( Wilkerson et al. 2021). The species seems to occur predominantly in the Atlantic Forest biome in Brazil, with isolated records (as Wy. shannoni or Sa. paradoxus ) in the Amazon Rainforest biome ( Brazil, states of Acre and Rondônia; French Guiana, Saint-Laurent-du-Maroni on the northwestern border with Suriname) and the Panamanian Tropical Rainforest ( Panama, province of Darien) ( Lane & Cerqueira 1942; Forattini et al. 1986a; Guimarães et al. 2000d; Harbach & Howard 2002; Talaga et al. 2015; Coleção de Culicidae 2021 ). The northernmost and southernmost records of Sa. shannoni (as Wy. shannoni ) in the Brazilian Atlantic Rainforest biome is Ilhéus, state of Bahia (see material examined) and Paranaguá, state of Paraná ( Santos et al. 2019), respectively. In this Brazilian biome, the most inland record is from Simonésia (state of Minas Gerais), nearly 160 km from the coast ( Abreu et al. 2019).
Material examined. One hundred and twenty-two specimens (95 ♀, 5 ♀ G, 1 ♀ Pe, 2 ♀ LePe, 1 ♀ LeLmpPe, 1 ♀ LePeG, 5 ♂, 5 ♂ G, 6 ♂ LePeG, 1 ♂ LeLmpPeG, including the holotype and allotype). HOLOTYPE ♂ with genitalia dissected and mounted on a microscope slide (no. 2034), BRAZIL: Rio de Janeiro, Petrópolis (not Mangaratiba, corrected by Marchon-Silva et al. 1996), R.C. Shannon coll., April 1938, deposited in CEIOC. ALLOTYPE ♀ (pinned): Rio de Janeiro, Mangaratiba, R.C. Shannon coll., April 1938, deposited in CEIOC. BRAZIL: 1 ♀ deposited in CMN (no. 18180), Acre, Xapuri, R. Franco coll. 19.XII.1937, N.L. Cerqueira det., 05.V.1938; 1 ♀ ( CMN, nº 33477), Bahia, Ilhéus, Ribeirão da Fortuna , human bait, C. Ciardelli coll., I.1944, O.V. Ferreira det., 15.II.1944; 1 ♀ Pe ( CCULI, nº 5500), Espírito Santo, Viana, São Paulo de Cima (-20.290833º S, -40.555556º W), bamboo trap 5 m, A. Falqueto coll., 14. VI.2017, M.A. Motta det.; GoogleMaps 1 ♀ ( CMN, no number), Rio de Janeiro, Cachoeiras de Macacu , Fazenda Martinez , Serviço de Febre Amarela coll., IV.1938; 1 ♂ LePeG ( CCULI), RPPN Reserva Ecológica do Guapiaçu , Trilha Verde GoogleMaps (-22.417416º S, -42.738472º W), cut bamboo, M.S.A.S. Neves and T. Gomes coll., 21.III.2019, M.A. Motta det.; 2 ♂, 5 ♂ LePeG, 1 ♂ LeLmpPeG, 3 ♀ LePe, 1 ♀ LeLmpPe ( CCULI, nº 5191, 5488–5499), M.S.A.S. Neves and T. Gomes coll., 04.IV.2019, A.C. Nascimento-Pereira and M.A. Motta det.; 1 ♀ G ( CCULI, nº 5487), Casimiro de Abreu, Sítio Alto do Bom Gosto GoogleMaps (-22.444600º S, -42.210253º W), human bait, A.C. Nascimento-Pereira coll., 21.III.2018, A.C. Nascimento-Pereira det.; 1 G ♂ ( CMN, no number), Mangaratiba GoogleMaps (-22.959699º S, -44.040599º W), R.C. Shannon coll., IV.1938, 1 ♀ ( CMN, no number), R.C. Shannon and SFA coll., V.1938, 1 ♂ G ( CMN, slide 2033 T) same data, 2 ♀, 1 ♂ ( CMN, no number), SFA coll., IX.1938; 1 ♂, 1 ♂ G ( CMN, nº 21495), Nova Iguaçu, Tinguá (-22.759199º S, -43.451099º W), C. Ciardelli, J. Mata and Quimiciano coll., VI.1940; 2 ♂ G ( CMN, no number), Teresópolis (-22.412200º S, -42.965599º W), R.C. Shannon and SFA coll., IV.1938; 2 ♀ ( CCULI, nº 4601–4602), Rondônia: Ariquemes, mata (-9.913330º S, -63.040798º W), M.A. Motta coll., 25.V. II.1987, M.A. Motta det., 11.I.2018; 5 ♀ ( CMN, no number), São Paulo, Juquiá (- 24.319999º S, -47.630001º W), FLN coll., XI.1938, J. Lane det., 1941; 6 ♀, 1 ♀ G ( CEIOC, 188/5602, 190/5627, 190/5668, 190/5693, 190/5705, 190/5715, 190/5733), Ubatuba, Parque Estadual Serra do Mar, Núcleo Picinguaba (- 23.345004º S, -44.851123º W), B.E. Rocha and R. Machado coll., 13.IX.1989, 2 ♀ ( CEIOC, 208/6128–6129), same data except C. Spata and R. Machado coll., 18.X.1989, 2 ♀ ( CEIOC, 240/6688, 240/6701), same data except M. Garcia coll., 13.XII.1989, 1 ♀ ( CEIOC, 277/7726), same data except A. Guimarães coll., 07.II.1990, 1 ♀ ( CEIOC, 469/15754), same data except 17.IV.1991, 2 ♀ ( CEIOC, 348/10215, 349/10338), same data except B.E. Rocha coll., 18.VII.1990, 2 ♀ ( CEIOC, 356/10412, 363/10543), same data except B. Neto & V. Moraes coll., 14–15.VIII.1990, 3 ♀, 1♀ G ( CEIOC, 380/11287, 380/11308, 380/11314, 380/11317),? coll., same data except 20.IX.1990, 1 ♀ ( CEIOC, 393/11793),? coll., same data except 25.X.1990, 3 ♀ ( CEIOC, 410/12466, 410/12489, 410/12582),? coll., same data, except 22.XI.1990, 4 ♀ ( CEIOC, 422/13077, 422/13083, 422/13124, 425/13181), same data except R. Marinelli coll., 05.XII.1990, 2 ♀ ( CEIOC, 437/13528, 437/13535), same data except 05.I.1991, 22 ♀, 1 ♀ G ( CEIOC, 437/13536, 437/13550, 437/13552, 437/13565, 437/13569, 437/13611–13612, 437/13619, 437/13629, 437/13649, 437/13656–13657, 437/13659–13660, 437/13664, 437/13670, 437/13694, 437/13698, 437/13704, 437/13710, 437/13715, 439/13820, 439/13822), same data except R. Marinelli, R. Machado and M. Garcia coll., 16.I.1991, 2 ♀ ( CEIOC, 449/14355, 449/14383),? coll., same data except 07.III.1991, 6 ♀ ( CEIOC, 450/14452, 450/14468, 450/14503, 450/14523, 450/14544, 452/14701), same data except V. Moraes and R. Marinelli coll., 07.III.1991, 2 ♀ ( CEIOC, 456/14927, 456/14933),? coll., same data except 19.III.1991, 19 ♀, 1 ♀ G ( CEIOC, 456/14969, 456/14982, 456/14997–14998, 456/15008, 456/15054, 456/15056, 458/15195, 458/15214, 458/15228, 458/15271, 458/15273–15274, 458/15277, 458/15288, 458/15292–15294, 458/15299, 458/15301),? coll., same data except 19.III.1991, 2 ♀ ( CEIOC, 481/16160, 481/16162), R. Machado coll., 10.VII.1991.
Abreu, F. V. S. de, Ribeiro, I. P., Ferreira-de-Brito, A., Santos, A. A. C. dos, Miranda, R. M. de, Bonelly, I. de S., Neves, M. S. A. S., Bersot, M. I., Santos, T. P. dos, Gomes, M. Q., Silva, J. L. da, Romano, A. P. M., Carvalho, R. G., Said, R. F. do C., Ribeiro, M. S., Laperriere, R. da C., Fonseca, E. O. L., Falqueto, A., Paupy, C., Failloux, A. - B., Moutailler, S., Castro, M. G. de, Gomez, M. M., Motta, M. de A., Bonaldo, M. C. & Lourenco-de-Oliveira, R. (2019) Haemagogus leucocelaenus and Haemagogus janthinomys are the primary vectors in the major yellow fever outbreak in Brazil, 2016 - 2018. Emerging Microbes & Infections, 8 (1), 218 - 231. https: // doi. org / 10.1080 / 22221751.2019.1568180
Belkin, J. N., Schick, R. X. & Heinemann, S. J. (1971) Mosquito Studies (Diptera, Culicidae) XXV. Mosquitoes originally described from Brazil. Contributions of the American Entomological Institute, 7 (5), 1 - 64.
Brilhante, A. F., Paula, M. B. de, Nagaki, S. S., Avila, M. M. de, Rocha, R. da C. & Souza, J. L. de. (2020) Uma revisao da diversidade e distribuicao das especies de culicideos (Diptera: Culicidae) registradas no Estado do Acre, Brasil. In: Silveira, M., Silva, E. da & Lima, R. A. (Eds.), Biodiversidade e Biotecnologia no Brasil. Vol. 1. Stricto Sensu, Rio Branco, Acre, pp. 91 - 106. [https: // sseditora. com. br / ebooks / biodiversidade-e-biotecnologia-no-brasil- 1 /] https: // doi. org / 10.35170 / ss. ed. 9786586283280.05
Cerqueira, N. L. (1961 b) Distribuicao geografica dos mosquitos da Amazonia (Diptera, Culicidae, Culicinae) Revista Brasileira de Entomologia, 10, 111 - 168.
Colecao de Culicidae (2021) CCULI-Fundacao Oswaldo Cruz. Available from: http: // cculi. fiocruz. br / catalogue (accessed 14 September 2021)
Coquillett, D. W. (1906) New Culicidae from the West Indies and Central America. Proceedings of the Entomological Society of Washington, 7 (4), 182 - 186. [for 1905, https: // repository. si. edu / handle / 10088 / 68401]
Dutra, A. P., Natal, D., Tubaki, R. M., Barata, J. M. S., Menezes, R. M. T., Urbinatti, P. R. & Costa, A. I. P. (1996) Mosquitos (Diptera, Culicidae) da Reserva Estadual Pedro de Toledo (Juquitiba, SP, Brasil). Revista Brasileira de Entomologia, 40 (3 - 4), 375 - 378.
Dyar, H. G. & Shannon, R. C. (1924) The subfamilies, tribes, and genera of American Culicidae. Journal of the Washington Academy of Sciences, 14 (20), 472 - 486. https: // www. jstor. org / stable / 24527294
Forattini, O. P., Rabello, E. X. & Cotrim, M. das D. (1970) Catalogo das Colecoes Entomologicas da Faculdade de Saude Publica da Universidade de Sao Paulo (1. ª Serie) Culicidae [sic]. Revista de Saude Publica, 4 (N. º Especial), 1 - 100.
Forattini, O. P., Gomes, A. de C., Natal, D. & Santos, J. L. F. (1986 a) Observacoes sobre atividade de mosquitos Culicidae em mata primitiva da encosta no Vale do Ribeira, Sao Paulo, Brasil. Revista de Saude Publica, 20 (1), 1 - 20 [Errata. (1986) Revista de Saude Publica, 20 (2), 1 - 3]
Forattini, O. P., Kakitani, I., Massad, E. & Marucci, D. (1993 b) Studies on mosquitoes (Diptera: Culicidae) and anthropic environment. 4 - Survey of resting adults and synanthropic behaviour in South-Eastern, Brazil. Revista de Saude Publica, 27 (6), 398 - 411. https: // doi. org / 10.1590 / S 0034 - 89101993000600002
Guimaraes, A. E., Motta, M. A., Arle, M., Machado, R. M. & Goncalves, L. D. (1989) Bionomia de mosquitos (Diptera: Culicidae) em areas da Mata Atlantica no municipio de Itaguai, estado do Rio de Janeiro, Brasil: I. Frequencia intra, peri e extradomi- ciliar. Memorias do Instituto Oswaldo Cruz, 84 (Supplement IV), 243 - 254. https: // doi. org / 10.1590 / S 0074 - 02761989000800044
Guimaraes, J. H. (1997) Systematic database of Diptera of the Americas South of the United States (family Culicidae). Editora Pleiade, Sao Paulo, Sao Paulo, ix + 286 pp.
Guimaraes, A. E., Gentile, C., Lopes, C. M. & Mello, R. P. de (2000 a) Ecology of mosquitoes (Diptera: Culicidae) in areas of Serra do Mar State Park, State of Sao Paulo, Brazil. II - Habitat distribution. Memorias do Instituto Oswaldo Cruz, 95 (1), 17 - 28. https: // doi. org / 10.1590 / S 0074 - 02762000000100002
Guimaraes, A. E., Gentile, C., Lopes, C. M. & Mello, R. P. de. (2000 b) Ecology of mosquitoes (Diptera: Culicidae) in areas of Serra do Mar State Park, State of Sao Paulo, Brazil. III - daily biting rhythms and lunar cycle influence. Memorias do Instituto Oswaldo Cruz, 95 (6), 753 - 760. https: // doi. org / 10.1590 / S 0074 - 02762000000600002
Guimaraes, A. E., Mello, R. P. de, Lopes, C. M. & Gentile, C. (2000 d) Ecology of mosquitoes (Diptera: Culicidae) in areas of Serra do Mar State Park, State of Sao Paulo, Brazil. I - Monthly frequency and climatic factors. Memorias do Instituto Oswaldo Cruz, 95 (1), 1 - 16. https: // doi. org / 10.1590 / S 0074 - 02762000000100001
Hall, C. R., Howard, T. M. & Harbach, R. E. (1999) Sabethes (Peytonulus) luxodens, a new species of Sabethini (Diptera: Culicidae) from Ecuador. Memorias do Instituto Oswaldo Cruz, 94 (3), 329 - 338. https: // doi. org / 10.1590 / S 0074 - 02761999000300009
Harbach, R. E. (1995 a) A new Sabethes of the subgenus Peytonulus (Diptera: Culicidae) with an unusual fourth-instar larva. Entomologica Scandinavica, 26 (1), 87 - 96.
Harbach, R. E. & Howard, T. M. (2002) Sabethes (Peytonulus) paradoxus, a new species of Sabethini (Diptera: Culicidae) from Panama. Proceedings of the Entomological Society of Washington, 104 (2), 363 - 372. [https: // www. biodiversitylibrary. org / part / 54892 # / summary]
Harbach, R. E. (2018) Culicipedia: species-group, genus-group and family-group names in Culicidae (Diptera). CABI, Wallingford, Oxfordshire and Boston, Massachusetts, xviii + 378 pp. https: // doi. org / 10.1079 / 9781786399052.0000
Harbach, R. E. (2021) Mosquito Taxonomic Inventory. Available from: http: // mosquito-taxonomic-inventory. info (accessed 6 July 2021)
Heinemann, S. J. & Belkin, J. N. (1978) Collection records of the Project Mosquitoes of Middle America 10. Panama, including Canal Zone (PA, GG). Mosquito Systematics, 10 (2), 119 - 196. https: // www. biodiversitylibrary. org / part / 132524
Horsfall, W. R. (1955) Mosquitoes: their bionomics and relation to disease. The Ronald Press Company, New York, New York, viii + 723 pp.
Howard, L. O., Dyar, H. G. & Knab, F. (1913) The mosquitoes of North and Central America and the West Indies. Vol. 2. Plates. Carnegie Institution of Washington, No. 159, Washington, D. C., x + 150 pp. [for 1912,
Howard, L. O., Dyar, H. G. & Knab, F. (1915) The mosquitoes of North and Central America and the West Indies. Vol. 3. Systematic description (in two parts). Part I. Carnegie Institution of Washington No. 159. Carnegie Institution of Washington, Washington, D. C., vi + 523 pp. [https: // www. biodiversitylibrary. org / item / 116022 # page / 7 / mode / 1 up]
Hutchings, R. S. G., Sallum, M. A. M., Ferreira, R. L. M. & Hutchings, R. W. (2005 a) O acervo de mosquitos (Diptera, Culicidae) de Nelson L. Cerqueira na Colecao de Invertebrados do Instituto Nacional de Pesquisas da Amazonia, Manaus, Brasil. Revista Brasileira de Entomologia, 49 (1), 15 - 28. https: // doi. org / 10.1590 / S 0085 - 56262005000100004
Knight, K. L. & Stone, A. (1977) A catalog of the mosquitoes of the world (Diptera, Culicidae). The Thomas Say Foundation. Vol. VI. 2 nd Edition. Entomological Society of America, College Park, Maryland, xi + 611 pp.
Lane, J. & Cerqueira, N. L. (1942) Os sabetineos da America (Diptera, Culicidae). Arquivos de Zoologia do Estado de Estado de Sao Paulo, 3 (9), 473 - 849.
Lane, J. (1953) Neotropical Culicidae. Vol. II. University of Sao Paulo, Sao Paulo, Sao Paulo, 564 pp.
Lutz, A. (1905) Novas especies de mosquitos do Brasil. Imprensa Medica de Sao Paulo, 13 (2, 3, 4, 5, 6, 7, 9, 11, 14, 15, 16, 18), 26 - 29, 48 - 52, 65 - 70, 81 - 84, 101 - 104, 125 - 128, 169 - 172, 202 - 204, 269 - 271, 287 - 290, 311 - 314, 347 - 350.
Marchon-Silva, V., Lourenco-de-Oliveira, R., Almeida, M. D. de, Silva-Vasconcelos, A. da & Costa, J. (1996) The type specimens of mosquitoes (Diptera, Culicidae) deposited in the Entomological Collection of the Instituto Oswaldo Cruz, Rio de Janeiro, Brazil. Memorias do Instituto Oswaldo Cruz, 91 (4), 471 - 478. https: // doi. org / 10.1590 / S 0074 - 02761996000400014
Motta, M. A. & Lourenco-de-Oliveira, R. (1995) Wyeomyia luteoventralis Theobald, the type species of the subgenus Dendromyia Theobald (Diptera: Culicidae). Memorias do Instituto Oswaldo Cruz, 90 (3), 375 - 385. https: // doi. org / 10.1590 / S 0074 - 02761995000300012
Santos, E. B., Favretto, M. A. & Navarro-Silva, M. A. (2019) Community structure of mosquitoes (Diptera: Culicidae) in the coast of Southern Brazil. Austral Entomology, 58 (4), 826 - 835. https: // doi. org / 10.1111 / aen. 12412
Silva, A. M. da, Santos, D. R. dos, Cristovao, E. C., Ferreira, A. C., Postai, C., Westphal-Ferreira, B. & Silva, M. A. N. da. (2019) First records of the occurrence of twelve species of Sabethini (Diptera, Culicidae) in the state of Parana, southern Brazil. Check List, 15 (1), 193 - 201. https: // doi. org / 10.15560 / 15.1.193
Stone, A., Knight, K. L. & Starcke, H. (1959) A synoptic catalog of the mosquitoes of the world (Diptera, Culicidae) The Thomas Say Foundation. Vol. VI. The Thomas Say Foundation, Entomological Society of America, College Park, Maryland, vi + 358 pp.
Talaga, S., Dejean, A., Carinci, R., Gabirot, P., Dusfour, I. & Girod, R. (2015) Update checklist of the mosquitoes (Diptera: Culicidae) of French Guiana. Journal of Medical Entomology, 52 (5), 770 - 783. https: // doi. org / 10.1093 / jme / tjv 109
Wilkerson, R. C., Linton, Y. - M. & Strickman, D. (2021) Mosquitoes of the world. Vols. 1 & 2. Johns Hopkins University Press, Baltimore, Maryland, 1332 pp.
Xavier, S. M., Mattos, S. da S. & Correa, I. dos R. (1989) Lista de especies e generos de culicideos encontrados nos estados do Brasil. X. Estado do Acre (Diptera, Culicidae). Acta Amazonica, 19, 307 - 317. https: // doi. org / 10.1590 / 1809 - 43921989191317
FIGURE 1. Sabethes (Peytonulus) shannoni. A, Female: habitus (lateral). B–D, Holotype male: B, thoracic pleura (prealar setae absent); C, D, gonostylus.
FIGURE 2. Sabethes (Peytonulus) shannoni. A, Sternum IX of male genitalia. B–I, Female genitalia: B, cercus (dorsal); C, spermathecal capsules; D, postgenital lobe (dorsal); E, postgenital lobe (ventral); F, tergum IX; G, insula; H, tergum VIII; I, sternum VIII.
FIGURE 3. A, B, Sabethes (Peytonulus) shannoni, holotype male: A, proboscis (dorsal); B, proboscis (ventral). C, D, Sabethes (Peytonulus) harbachi, adult male: C, proboscis (dorsal); D, proboscis (ventral).
FIGURE 4. Sabethes (Peytonulus) shannoni. A, Pupa: abdominal segment VIII and paddle. B–F, Larval mouthparts: B, dorsomentum; C,D, maxilla (C, ventral; D, dorsal); E,F, mandible (E, ventral; F, dorsal). LR1, laciniarastrum 1; MPlp, maxillary palpus; MnB, mandibular brush; MRB, mandibular rake blade; MSA, mandibular spiculose area; MxB, maxillary brush; MnS1, MnS2 mandibular sweeper 1 and 2; VT 0, VT 1, VT 2, VT 3, VT 4, ventral teeth 0–4; 1–6, maxillary setae 1–6-Mx; 2-Mn, mandibular seta.
| CMN |
Canadian Museum of Nature |
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |
|
|
SubGenus |
Peytonulus |
Sabethes (Peytonulus) shannoni ( Lane & Cerqueira, 1942 )
| Nascimento-Pereira, Agostinho C., Neves, Maycon Sebastião Alberto Santos, Guimarães, Anthony Érico, Motta, Monique De Albuquerque & De-Oliveira, Ricardo Lourenço- 2021 |
Wyeomyia shannoni
| Brilhante, A. F. & Paula, M. B. de & Nagaki, S. S. & Avila, M. M. de & Rocha, R. da & Souza, J. L. de 2020: 100 |
| Silva, A. M. da & Santos, D. R. dos & Cristovao, E. C. & Ferreira, A. C. & Postai, C. & Westphal-Ferreira, B. & Silva, M. A. N. da 2019: 195 |
| Santos, E. B. & Favretto, M. A. & Navarro-Silva, M. A. 2019: 829 |
| Harbach, R. E. 2018: 119 |
| Guimaraes, J. H. 1997: 131 |
| Motta, M. A. & Lourenco-de-Oliveira, R. 1995: 384 |
Sabethes (Sabethinus)
| Heinemann, S. J. & Belkin, J. N. 1978: 193 |
Wyeomyia shannoni
| Abreu, F. V. S. de & Ribeiro, I. P. & Ferreira-de-Brito, A. & Santos, A. A. C. dos & Miranda, R. M. de & Bonelly, I. de & Neves, M. S. A. S. & Bersot, M. I. & Santos, T. P. dos & Gomes, M. Q. & Silva, J. L. da & Romano, A. P. M. & Carvalho, R. G. & Said, R. F. do & Ribeiro, M. S. & Laperriere, R. da & Fonseca, E. O. L. & Falqueto, A. & Paupy, C. & Failloux, A. - B. & Moutailler, S. & Castro, M. G. de & Gomez, M. M. & Motta, M. de & Bonaldo, M. C. & Lourenco-de-Oliveira, R. 2019: 221 |
| Hutchings, R. S. G. & Sallum, M. A. M. & Ferreira, R. L. M. & Hutchings, R. W. 2005: 26 |
| Marchon-Silva, V. & Lourenco-de-Oliveira, R. & Almeida, M. D. de & Silva-Vasconcelos, A. da & Costa, J. 1996: 447 |
| Dutra, A. P. & Natal, D. & Tubaki, R. M. & Barata, J. M. S. & Menezes, R. M. T. & Urbinatti, P. R. & Costa, A. I. P. 1996: 376 |
| Forattini, O. P. & Rabello, E. X. & Cotrim, M. das 1970: 91 |
Wyeomyia (Dendromyia) shannoni
| Xavier, S. M. & Mattos, S. da & Correa, I. dos 1989: 312 |
| Guimaraes, A. E. & Motta, M. A. & Arle, M. & Machado, R. M. & Goncalves, L. D. 1989: 248 |
| Forattini, O. P. & Gomes, A. de & Natal, D. & Santos, J. L. F. 1986: 7 |
| Knight, K. L. & Stone, A. 1977: 331 |
| Belkin, J. N. & Schick, R. X. & Heinemann, S. J. 1971: 12 |
| Cerqueira, N. L. 1961: 160 |
| Stone, A. & Knight, K. L. & Starcke, H. 1959: 87 |
| Horsfall, W. R. 1955: 329 |
| Lane, J. 1953: 868 |
1 (by plazi, 2021-12-17 08:53:29)
2 (by ExternalLinkService, 2021-12-17 16:44:23)
3 (by ExternalLinkService, 2021-12-17 17:14:58)
4 (by diego, 2022-01-04 11:58:39)
5 (by ExternalLinkService, 2022-01-04 12:23:50)
6 (by diego, 2022-01-04 13:53:33)
7 (by diego, 2022-01-04 14:06:24)
8 (by diego, 2022-01-04 14:07:24)
9 (by plazi, 2023-11-08 11:31:07)