Oligobregma mucronata, Blake, James A., 2015
|
publication ID |
https://doi.org/ 10.11646/zootaxa.4033.1.3 |
|
publication LSID |
lsid:zoobank.org:pub:9C0A63B6-5532-484D-BBD7-EDD5250D4ABA |
|
DOI |
https://doi.org/10.5281/zenodo.6102463 |
|
persistent identifier |
https://treatment.plazi.org/id/038D87CD-FFA1-BC0E-FF16-FC23FBB6FD54 |
|
treatment provided by |
Plazi |
|
scientific name |
Oligobregma mucronata |
| status |
sp. nov. |
Oligobregma mucronata View in CoL new species
Figures 8‒9 View FIGURE 8 View FIGURE 9
Material examined. East Antarctic Peninsula, RVIB Nathaniel B. Palmer Cruise 2000-03, Collector, J.A. Blake.— Larsen-A Ice Shelf Area, Greenpeace Trough: Sta. NBP-06, 733 m, 2 specimens poorly preserved (JAB); Sta. NBP-07A, 839 m, 3 paratypes (2 complete, LACM-AHF Poly 7011); Sta. NBP-07B, 839 m, 1 specimen (JAB); Sta. NBP- 16, 713 m, 5 specimens (1 adult, 4 juveniles) (JAB); Sta. NBP- 17, 719 m, 1 juvenile (JAB); Sta. NBP- 18, 665 m, 3 paratypes, 1 adult, 2 juveniles (AHF-Poly 7012); Sta. NBP- 19, 879 m, 1 specimen (JAB); Sta. NBP- 20, 899 m, 7 paratypes ( USNM 1281914); Sta. NBP- 21, 912 m, holotype and 4 paratypes (LACM-AHF Poly 7013, 7014); Sta. NBP- 22, 868 m, 4 specimens (JAB).— LIS-A Area, transect along border with Larsen Ice Shelf B: Sta. NBP- 13, 323 m, 1 specimen (JAB).
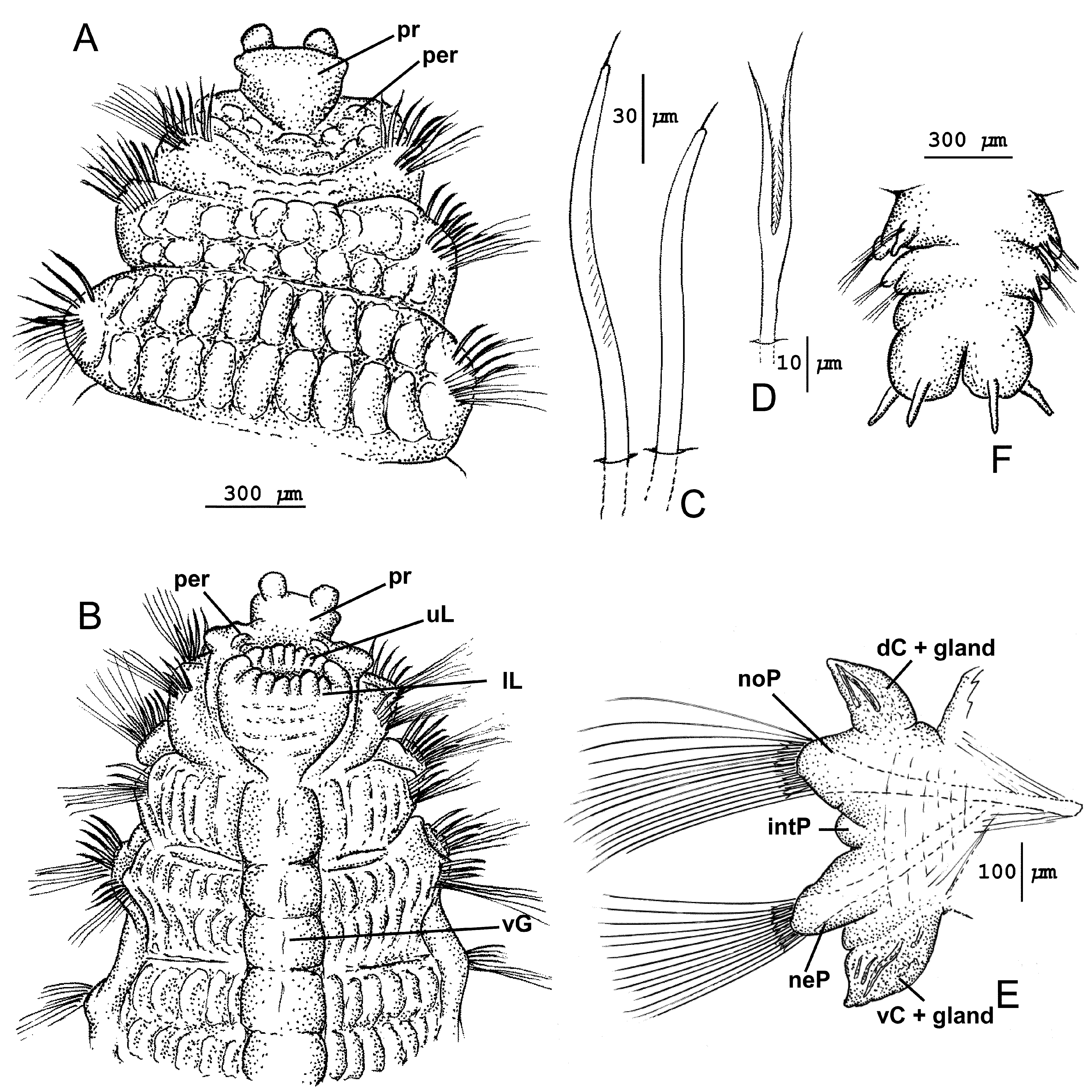
Description. Holotype incomplete, 16 mm long, 3.5 mm wide for 30 setigerous segments; two complete paratypes from Sta. NBP-21, one 11 mm long, 1.4 mm wide with 27 setigerous segments; second 9.5 mm long, 1.5 mm wide with 28 setigerous segments. Body expanded through first eight setigers, thereafter narrowing to posterior end. Color in alcohol light pink, without body pigment. Body segments with transverse rows of weakly raised pads; setigers 1‒3 uniannulate to biannulate ( Fig. 8 View FIGURE 8 A‒B); setigers 4 and subsequent segments becoming quadriannulate with low inconspicuous pads throughout ( Fig. 9 View FIGURE 9 A). Venter with prominent ventral midline from setiger 2 composed of a row of large pads within a groove ( Fig. 8 View FIGURE 8 B); each segment with at least two pads, each subdivided at middle by transverse groove, forming four structures per segment, corresponding to quadriannulate rows of segmental pads ( Fig. 9 View FIGURE 9 A). Branchiae absent. Pygidium of holotype with indistinct lobes, with two cirri present; paratypes with up to four anal cirri ( Fig. 8 View FIGURE 8 F).
Prostomium broadly curved across anterior margin, weakly expanded laterally, narrowing posteriorly; with two short, rounded lobes emerging subapically from anterior margin and extending forward forming short frontal horns ( Figs. 8 View FIGURE 8 A‒B, 9A); eyes absent; nuchal organs not observed; proboscis not everted. Peristomium a doublelobed ring around prostomium dorsally ( Fig. 8 View FIGURE 8 A), ventrally forming upper and lower lips of mouth; upper lip with 4‒5 narrow lobes; lower lip with about seven large, elongate inflated lobes, together forming a ring of elongate lobes around oral opening ( Figs. 8 View FIGURE 8 B, 9A).
Parapodia with short, conical-shaped podial lobes in anterior third of body, becoming longer posteriorly; dorsal and ventral cirri from setiger 14 on holotype, these podial lobes inconspicuous anteriorly, becoming longer and more prominent posteriorly, both cirri asymmetrical; dorsal cirri triangular, broad, basally tapering to narrow nipple-like tip ( Figs. 8 View FIGURE 8 E, 9B‒C); ventral cirri strongly asymmetrical with broad basal attachment narrowing to elongated, nipple-like tip ( Figs. 8 View FIGURE 8 E, 9B‒D); both dorsal and ventral cirri with darkly pigmented internal tubularshaped glands extending toward nipple-like tips ( Fig. 9 View FIGURE 9 C‒D). Interramal papilla present, best developed in posterior parapodia ( Fig. 8 View FIGURE 8 E).
Heavy curved acicular spines present in both noto- and neuropodia of setigers 1‒3 ( Fig. 8 View FIGURE 8 A‒B); notopodia with up to 12 spines arranged in two rows in setigers 1‒2 and single row with up to seven spines in setiger 3, spines accompanied posteriorly by single row of capillaries; neuropodia with 3‒5 spines in single row in setigers 1‒3, accompanied by posterior row of capillaries; spines curved, narrowing to blunt tip bearing thin terminal arista ( Fig. 8 View FIGURE 8 C); internal fibrils in spines apparent; notopodial spines more robust than those of neuropodia. Short spinous setae anterior to heavy spines absent. Setiger 4 with lyrate setae anterior to a row of short curved pointed setae and a third row of long, thin capillaries; setiger 4 therefore transitional between first three setigers bearing heavy curved spines and subsequent body segments having long thin capillaries. Lyrate setae continue on subsequent segments anterior to capillaries that generally occur in two rows from setiger 5. Lyrate setae from setiger 4, short, with unequal tynes bearing short bristles ( Figs. 8 View FIGURE 8 D, 9K), numbering 3‒4 per noto- and neuropodium in anterior segments and 8‒10 in posterior most segments ( Fig. 9 View FIGURE 9 K).
Reproduction. Sperm platelets were observed in one large paratype measuring 10 mm long and with 27 setigers of Oligobregma mucronata n. sp. from Sta. NBP-20 ( Fig. 9 View FIGURE 9 J). These are similar in size and appearance to those described earlier for Scalibregma australis n. sp. Individual sperm were observed to have a short rounded nucleus, middle piece consisting of two spherical mitochondria and a long tail or flagellum. As with S. australis n. sp., the sperm structure for O. mucronata n. sp. suggests that they are ect-aquasperm, which are discharged into the sea.
Morphology of juveniles. The smallest juveniles available for study had 20‒21 setigers and measured 2.3‒2.8 mm long. The noto- and neuropodial acicular spines of setigers 1‒3 develop early; these smallest juveniles had the full complement of notopodial spines on setigers 1‒3, but spines were present only on neuropodia of setigers 1‒2. A 24-setiger specimen that was 3.0 mm long had spines on setigers 1‒3 in both noto- and neuropodia. Lyrate setae were present from setiger 4 in the smallest 20-setiger specimen examined.
While the setae or hard structures develop early, the soft morphology develops later. Changes to the prostomial morphology is shown in Figure 9 View FIGURE 9 : E, 20 setigers (2.3 mm long); F, 21 setigers (2.8 mm long); G, 24 setigers (3.0 mm long), H, 28 setigers (4.0 mm long); and I, 30 setigers (9.5 mm long). The initial prostomial shape in the available specimens is blunt across the anterior margin with a nearly square overall shape. With growth, the anterior margin becomes rounded or curved, eventually developing lateral bulges and a medial notch. The characteristic subapical lobes that become elongate and directed frontally do not develop until later, but were observed in 28-setiger juveniles that were 5‒6 mm in length.
The development of segments in O. mucronata n. sp. appears to be limited to a maximal number of 28‒30 setigers; within this range, the body length ranges from 4–16 mm indicating that continued growth of the worm is within a defined number of final segments. Parapodia develop dorsal and ventral cirri relatively early, at about 24 setigers and a length of 4.0 mm. These cirri also develop the tubular internal glands.
Remarks. These specimens of Oligobregma mucronata n. sp. were originally identified as O. collare due to the prostomium having two short anteriorly subapical frontal horns. Further study, however, showed that the large anterior acicular spines occurred on both the noto- and neuropodia of setigers 1‒3 instead of only the notopodia and that most of these spines terminated in a thin arista. Further, the dorsal and ventral cirri were larger and of a different shape than in O. collare , with both cirri bearing internal glands similar to those described for Scalibregma australis n. sp. and Pseudoscalibregma palmeri n. sp. To date, O. mucronata n. sp. is the only species of the genus from Antarctic waters known to have large curved acicular spines in both noto- and neuropodia of anterior setigers. Examination of specimens of the type-species, O. aciculata (from deep-water off the Western North Atlantic collected as part of the Atlantic Continental Slope and Rise Program (ACSAR)) permitted elaboration of the original description which was based on a single incomplete specimen. O. aciculata , like O. mucronata n. sp. has acicular spines in noto- and neuropodia of setigers 1–3 and similar appearing frontal horns. However, a few short spinous setae occur anterior to the large acicular spines in O. aciculata , but are absent in O. mucronata n. sp. In addition, the form of the dorsal and ventral cirri differs significantly between the two taxa. In O. aciculata the dorsal cirrus is small oval and rounded on the tip and the ventral cirrus is larger, round and globular (not pointed as in Hartman (1965)); whereas in O. mucronata n. sp., the dorsal cirrus is triangular and pointed at the tip and the ventral cirrus has a broad asymmetrical base and tapers to a pointed tip. Further, the acicular spines of O. aciculata taper to a long capillary-like tip and are not mucronate; blunt-tipped acicular spines reported by Hartman (1965) were not observed in the new material.
Oligobregma mucronata n. sp. bears some resemblance to O. quadrispinosa from deep water in the Weddell Sea. However, the prostomium and nature of the dorsal and ventral cirri is different in O. quadrispinosa and this species is reported to have heavy spines in the notopodia of setigers 1‒4 but no neuropodial spines ( Schüller & Hilbig 2007).
The development of juvenile morphology indicates that the noto- and neuropodial acicular spines are present at least by the 20-setiger stage and with a length of 2.3 mm. In contrast, the characteristic form of the prostomium is not developed until at least 28 setigers with a length of 5‒6 mm have developed. These results, like those for Scalibregma australis n. sp. suggest that small specimens of scalibregmatids cannot be specifically identified without sufficient numbers of specimens available to identify a growth sequence and transition of characters.
Before reaching the final configuration, the prostomium passes through growth phases that have been described for other species.
Etymology. The epithet is derived from the Latin mucro, referring to a sharply pointed thin aristate-like tip on some of the large acicular spines of setigers 1‒3.
Ecology. Specimens of Oligobregma mucronata n. sp. collected as part of the LIS-A survey were mostly limited to the nearshore Greenpeace Trough, newly open to the sea and discovered by multibeam bathymetry during the survey in May 2000. These nearshore sediments were described from 20‒25 cm cores as coarse-grained in the upper 5 cm near the surface, overlying fine-grained silt and clay size sediments with depth ( Gilbert & Domack 2003). While the nature of coarse-grained surficial sediments and deeper fine-grained sediments provide a variable sedimentary habitat, the seafloor at this site also appeared to be in a constant state of disturbance due to the presence of numerous deposit-feeding elasapoid holothurians, Elpidia glacialis Théel, 1876 , which were observed by video to be constantly moving over the surface. Several of the holothurians were observed on the surface of 10 X 10 X 50 cm megacore tubes collected in the Greenpeace Trough (Blake & Maciolek unpublished). As burrowing deposit feeders, scalibregmatids would be better adapted to such an environment than tube-building species. Scalibregma australis n. sp. was also abundant in these sediments.
Distribution. East Antarctic Peninsula, former Larsen Ice Shelf A area, 323‒ 912 m.
| USNM |
Smithsonian Institution, National Museum of Natural History |
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Family |
|
|
Genus |