Dipsadoboa montisilva Branch, Conradie & Tolley, 2019
|
publication ID |
https://doi.org/ 10.11646/zootaxa.4646.3.6 |
|
publication LSID |
lsid:zoobank.org:pub:AE4B0255-50CB-41E6-93B5-B42DC67C9E3F |
|
persistent identifier |
https://treatment.plazi.org/id/038C87E8-FF92-DF4A-FF7A-4094FD8CFE7C |
|
treatment provided by |
Plazi |
|
scientific name |
Dipsadoboa montisilva Branch, Conradie & Tolley |
| status |
sp. nov. |
Dipsadoboa montisilva Branch, Conradie & Tolley sp. nov.
Montane Forest Tree Snake
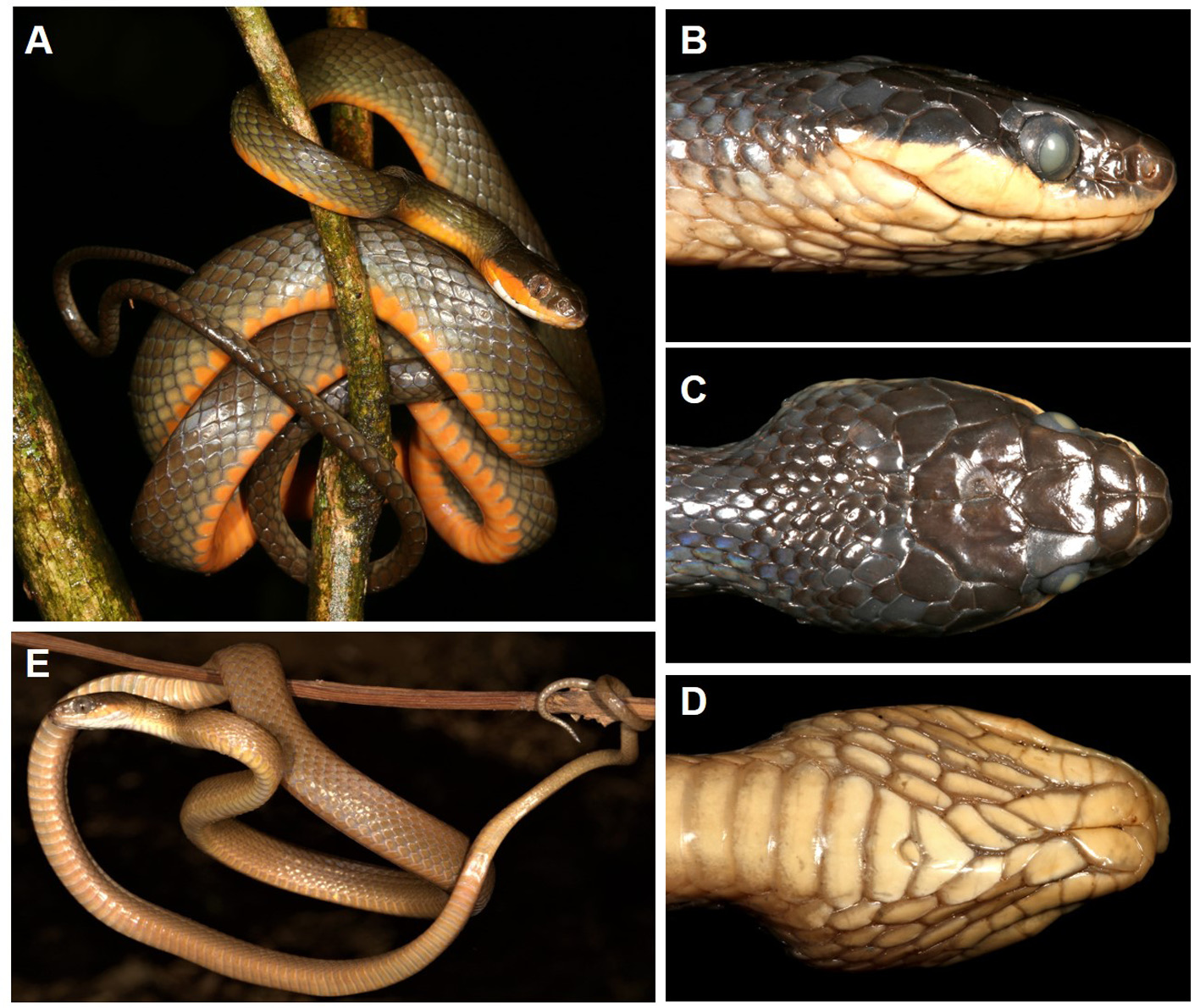
( Fig. 3 View FIGURE 3 , 4 View FIGURE 4 , 5 View FIGURE 5 )
http://zoobank.org/ urn:lsid:zoobank.org:act:ACAF8954-A17F-4830-9B1A-84F8E08BE5AC
Synonymy:
Dipsadoboa sp. ( Timberlake et al. 2012; Bayliss et al. 2014)
Dipsadoboa cf. shrevei ( Conradie et al. 2016) .
Type Material: Holotype: PEM R21122, adult male with everted hemipenes, preserved in formalin and transferred to 70% ethanol. Body coiled and with a small incision ventrally. Collected 21 November 2014 by K.A. Tolley, S.P. Loader, W. Conradie, G.B. Bittencourt-Silva, M. Menegon, H.M. Engelbrecht, and C. Nanvonamuquitxo.
Type locality: Mt Mabu Forest Base Camp (16°17’10.4”S, 36°24’00.2”E, 919 m above sea level ~a.s.l.), Zambezia Province, Mozambique.
Paratypes: Five specimens: PEM R21115 , male, Mt Mabu Forest Summit Camp (16°17’48.5”S, 36°23’32.7”E, 1644 m a.s.l.), Zambezia Province, Mozambique, collected 20 November 2014 by KAT, SPL, WC, GBBS, MM, HME, CN; PEM R21123 GoogleMaps , male, Mt Mabu Forest Base Camp (16°17’10.4”S, 36°24’00.2”E, 919 m a.s.l.), Zambezia Province, Mozambique, collected 20 November 2014, by KAT, SPL, WC, GBBS, MM, HME, CN; NHMUK 2018.02398 About NHMUK (previously PEM R21116) GoogleMaps , male, Mt Mabu Forest Summit Camp (16°17’48.5”S, 36°23’32.7”E, 1644 m a.s.l), Zambezia Province, Mozambique, collected 20 November 2014 by KAT, SPL, WC, GBBS, MM, HME, CN; MHNM GoogleMaps . Rep. 2014.0011, male, Mt Mabu Forest Base Camp (16°17’10.4”S, 36°24’00.2”E, 919 m a.s.l.), Zambezia Province, Mozambique, collected 20 November 2014 by KAT, SPL, WC, GBBS, MM, HME, CN; PEM R21937 GoogleMaps , female, Mt Mabu Forest Base Camp (16°17’10”S, 36°24’04”E, ~ 950 m a.s.l.), Zambezia Province, Mozambique, collected on 15 October 2012 by JB GoogleMaps .
Additional Material: One specimen, PEM R21195, juvenile female, M’pàluwé Ridge Forest , Ribáuè Massif (14°54’10.0”S, 38°18’04”E, 1416 m a.s.l.) Nampula Province, Mozambique, collected 1 December 2014 by KAT, WC, GBBS, MM and HME GoogleMaps .
Etymology. The name is derived from the Latin words ‘ montem ’ = mountain and ‘ silva ’= forest, which is in reference to the isolated mountain forest habitat in which it is found on Mt Mabu, Zambezia Province, Mozambique. The name is in the masculine form.
Diagnosis. Dipsadoboa montisilva sp. nov. is distinguished from all other members of the genus by having divided subcaudals (single in the D. unicolor group), an entire anal (divided in the D. aulica group), and 19 MSR (17 MSR in D. duchesnii group). It differs from other members of the D. werneri group in having low ventral and subcaudal counts (higher in D. werneri , see Table 4 View TABLE 4 ), and usually only two supralabials (4–5) entering the eye (three supralabials (3–5) in others)), 1+1 or 1+2 temporals (1+ 1 in D. s. shrevei and D. s. kageleri ), and an entire anal and 19 MSR (divided anal and 17 MSR in D. s. kageleri ).
Description. (Paratype and additional material variation in parentheses). Body elongated, slightly laterally compressed, tapering gradually to a relatively long tail. Dorsal scales smooth with single or double apical pits and in 19-19-15 scale rows; 203 ventrals (195– 210 males, 194– 201 females); anal entire; 98 subcaudals (95–100 in males, 89–90 in females). Head depressed and broad and distinct from the neck; snout bluntly rounded and about half as long again as horizontal diameter of orbit (1.53x OD, 1.31–1.87x in paratypes); rostral just visible from above, much broader than deep; internasals rectangular and broader than long, but shorter than rectangular prefrontals, which are in broad contact laterally with loreal; frontal pentangular, slightly longer than broad, in broad contact with large supraocular, and posteriorly inserting slightly between the very large parietals; very large nasal shield divided, region posterior to nostril deeply excavated with the characteristic Dipsadoboa nasal depression, below contacting 1 st and 2 nd supralabials; loreal deeper than long, in contact with nasal, 2 nd supralabial and prefrontal, but excluded from orbit by the single preocular, which is well-separated from the frontal. Eye large, vertical diameter half as deep again as distance from lip, and with a vertical pupil; two postoculars, the lower slight larger than upper and in contact with 5 th and 6 th subralabials; temporals 1+1 on right, 1+2 on left (four with 1+2, three with 1+1); supralabials eight, 4 th and 5 th entering orbit (3–5 th entering orbit in PEM R21115, and nine supralabials, 4–6 th entering orbit in NHMUK 2018.02398), and 6–7 th supralabials largest; infralabials 10, first 5 in contact with anterior chin shields (first 5 on right, and first 6 on left in NHMUK 2018.02398); anterior chin shields slight longer than posterior chin shields. Further details and meristics for the type series are summarized in Table 3 View TABLE 3 .
Colouration. Typical with minor differences for all adults (> 600 mm total length, Fig. 3A View FIGURE 3 ). Ground colour on dorsum olive-brown, lighter on two lateral scale rows, and becoming bright brown on dorsum of tail; ventrum bright orange throughout, becoming paler beneath the tail to become light olive at the tip. Ventral orange colour extends onto all supralabials (only proximal onto supralabials in two adults, where supralabials 1–4 are cream to light orange). Throat white on preventrals and adjacent gulars and extending onto most proximal infralabials, but becoming infused with orange on chin shields and adjacent infralabials. Eye white both heavily reticulated in red-brown giving a reddish tinge. Extended tongue tri-coloured, with white tines merging to a dark grey distal half and pink base.
In juveniles (<400 mm total length) the whole body and tail dorsally is uniform light orange-brown with a paler ventral surface, with the only different coloration being a slight flush of orange on the proximal supralabials in juveniles approaching 500 mm total length (e.g. PEM R21195, Fig. 3E View FIGURE 3 ).
After preservation the orange ventral colouration was lost becoming dirty ivory in colour, and the dorsum became grey-green ( Fig. 3 View FIGURE 3 B–D).
Hemipenis. Both hemipenes of the holotype are fully everted as the retractor muscle was cut through a small lateral incision in the subcaudals. Due to leakage of fixative, the organ became slightly deflated but all details of ornamentation are visible ( Fig. 4 View FIGURE 4 ). The hemipenis is typical for the Boigini, being a simple organ with an undivided sulcus, basal spines and calyculate distal ornamentation ( Bogert 1940; Rasmussen 1986). The base is unadorned and the simple sulcus has marginally raised lips and turns initially to the posterior surface of the organ (left in Fig. 4 View FIGURE 4 ) and then along the length of the organ to drain into the ornamented cap. The middle of the organ includes rows of basal spines with slightly hooked, ossified tips that reduce in size distally. As with other Dipsadoboa hemipenes ( Rasmussen 1993) the rows of spines are asymmetrical, with two rows of 4–5 spines lying medial to the sulcus and only one row of smaller spines lateral to the sulcus. In addition, the two largest spines lie at the bottom of the outer medial row and border a zone of small scattered spines on the asulcal surface. The final distal third of the organ is an ornamented cap with numerous calyces with raised, papillate edges.
Size. Largest female ( PEM R21137) 597 + 185 = 782 mm; largest male (holotype, PEM R21122) 825 + 259 = 1084 mm. The small series includes only one adult female and thus precludes determination of possible sexual size dimorphism. In both D. werneri and D. s. shrevei , where larger series are available males are longer than females ( Rasmussen 1986; Haagner et al. 2000).
Distribution. Known only from the evergreen Afrotemperate forest patches on Mt Mabu and Mt Ribáuè (M’pàluwé Forest), Zambezia Province, Mozambique from altitudes between 919 and 1644 m a.s.l. ( Fig. 5A View FIGURE 5 ). There is comparatively lower genetic divergence between individuals from these montane isolates than between other species in the genus. This suggests that the species may be more widely distributed in association with evergreen forest patches in northern Mozambique. For example, it could also occur in the Afromontane forest on neighbouring Mt Namuli, as a single specimen of Atheris mabuensis ( Branch & Bayliss 2009) was also found at this site following its discovery at Mt Mabu. The lack of specimens (including any Atheris sp.) from the well-surveyed Mt Mulanje in adjacent southern Malawi ( Stevens 1974; Broadley 2001; Branch & Cunningham 2006) suggests that it is absent from that massif. Spawls et al. (2018) plot a record of D. s. shrevei from the Rondo Plateau in southeastern Tanzania, but the affinities of this specimen with other members of the D. werneri group should be reassessed (see below).
Habitat. All Mt Mabu specimens collected were either active on the forest floor or in low branches ( Fig. 5B View FIGURE 5 ), while the Mt Ribáuè specimen was collected from low branches (ca. 1.5 m high) of a small tree in the understory. Dipsadoboa montisilva sp. nov. inhabits evergreen montane forest in contrast to the related D. shrevei , which inhabits miombo woodland and gallery forest ( Broadley et al. 2003). All specimens were collected between 919–1644 m a.s.l. on Mt Mabu and at 1416 m a.s.l. at Mt Ribáuè (M’pàluwé Forest). It is possible that the species occurs higher in the canopy but was not visible, so the collections in the lower branches should not be considered as the limits of this species habitat. The habitat on Mt Mabu comprises closed-canopy forest, except for small gaps caused by tree-falls and along stream gullies. Tall trees (up to 40–50 m height) emerge from the canopy, with Strombosia scheffleri dominant and others including Newtonia sp., Chrysophyllum gorungosanum , Maranthes goetzeniana , Ficus thonningii , Blighia unijugata and Funtumia africana . Smaller trees in the understorey include Allophylus sp., Canthium sp., Oxyanthus speciosus , Rawsonia lucida , Tabernaemontana ventricosa , Vepris stolzii and small saplings of Cola greenwayi , Drypetes sp. and Maranthes sp. Canopy lianas are dominated by Millettia lasiantha (Dowsett-Lemaire & Dowsett, unpubl. report 2008; Timberlake et al. 2012).
Biology. There is no direct knowledge about the species’ biology however, all Dipsadoboa species are currently considered nocturnal and arboreal. Chameleons have been recorded in the diet of all other members of the D. werneri group ( Rasmussen 1986; Haagner et al. 2000), and are plentiful in the forests of Mt Mabu ( Branch & Tolley 2010; Branch et al. 2014) and Mt Ribáuè ( Conradie et al. 2016). In contrast, frogs form the dominant diet of the D. aulica group (Stevens 1964; Broadley & Stevens 1971; Rasmussen 1989a).
Conservation. The species is currently known from two small mid-altitude evergreen forests on Mt Mabu (79 km 2) and Mt Ribáuè (M’pàluwé Forest ~ 1.9 km 2). It has not yet been recorded from the larger forest patch (~ 4.3 km 2) on the western Mt Ribáuè massif. None of the forest on either mountain is currently formally protected, although Mt Mabu is under the stewardship of the Mt Mabu Conservation Project, aiming to manage nature-based tourism activities in the future ( Bayliss et al. 2014). While the forest at Mt Mabu is essentially intact, the two endemic chameleons ( Nadzikambia baylissi and Rhampholeon maspictus ) are considered Near Threatened because human encroachment and fires for agriculture are threatening the ecological integrity of the forest ( Tolley & Bayliss 2014; Tolley et al. 2014). The situation might be similar for D. montisilva sp. nov. as well as other endemic species such as Atheris mabuensis ( Branch & Bayliss 2009) . In stark contrast, Mt Ribáuè is heavily threatened by ongoing habitat destruction in association with subsistence agriculture, particularly at the M’pàluwé Forest section on the eastern massif, which has already been reduced to less than 2 km 2 and is heavily fragmented and degraded. Dipsadoboa montisilva sp. nov. was found in the small degraded Mt M’pàluwé section, but it is very likely to also occur in the larger forest patch of Mt Ribáuè.
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |
Dipsadoboa montisilva Branch, Conradie & Tolley
| Branch, William R., Bayliss, Julian, Bittencourt-Silva, Gabriela B., Conradie, Werner, Engelbrecht, Hanlie M., Loader, Simon P., Menegon, Michele, Nanvonamuquitxo, Cristóvão & Tolley, Krystal A. 2019 |
Dipsadoboa cf. shrevei (
| Conradie 2016 |
Dipsadoboa
| Gunther 1858 |