Macrobrachium sundaicum ( Heller, 1862 )
|
publication ID |
https://doi.org/ 10.11646/zootaxa.2372.1.22 |
|
persistent identifier |
https://treatment.plazi.org/id/03843C5E-FFB9-FFF2-0490-7D3E7AB7FE61 |
|
treatment provided by |
Felipe |
|
scientific name |
Macrobrachium sundaicum ( Heller, 1862 ) |
| status |
|
Macrobrachium sundaicum ( Heller, 1862) View in CoL
( Figs. 5–7 View FIGURE 5 View FIGURE 6 View FIGURE 7 )
Palaemon sundaicus Heller, 1862: 415 View in CoL (part); 1865: 115 (part). Palaemon javanicus Heller, 1862: 421 View in CoL , Pl. 2, Fig. 48; 1865: 116. — von Martens 1868: 45. — Ortmann 1891: 732. Palaemon (Parapalaemon) Trompii De Man, 1898: 144 , Fig. 2 View FIGURE 2 . Palaemon (Eupalaemon) sundaicus View in CoL . — Roux 1932: 569. Palaemon (Parapalaemon) thienemanni Roux, 1932: 570 , Figs. a–b. Palaemon (Parapalaemon) trompi armatus Roux, 1936: 30 . Macrobrachium trompii View in CoL . — Holthuis 1950: 211. — Chace & Bruce 1993: 38. — Johnson 1961: 57. — Johnson 1963: 9;
1967: 421–429, 432; 1968: 306; 1969: 111. — Ng 1990: 196; 1992b: 793; 1997a: 268. — Ng & Choy 1990a: 304,
309; 1990b: 13. — Ng & Chong, 1993: 120. — Ng & Lim 1992: 261. — Cai et al. 2004: 605. — Wowor et al. 2004:
350, Fig. 10T. — Wowor et al. 2009: 342–344, 346–347.
Material examined. Type material. Palaemon sundaicus Heller, 1862 , female (cl 12.6, NHMW 1525, lectotype designated herein), Java, Indonesia, leg. I. Pfeiffer. Palaemon javanicus Heller, 1862 , male (cl 18.2, NHMW 7689a, lectotype designated herein), Java, Indonesia, leg. I. Pfeiffer; 2 paralectotypes ( NHMW 7689b), same data as lectotype. Palaemon (Parapalaemon) trompii De Man, 1898 , male (cl 17.4, RMNH D 1779a, lectotype designated herein), Ketungau River, Sintang Regency, W. Kalimantan, leg. M. Moret, 1894; paralectotype ov. female (cl 11.9, RMNH D 1779b), same data as lectotype. Palaemon (Parapalaemon) trompii De Man, 1898 , paralectotypes: male (cl 10.0, MNHN Na. 1435), 2 ov. females (cl 10.5–10.9, RMNH D 1520), ov. female (cl 11.3, ZMA De 102.816), Mandai River near Nanga Raun, W. Kalimantan, leg. Borneo Expedition, 1894. Palaemon (Parapalaemon) trompii De Man, 1898 , paralectotypes: 2 juveniles ( RMNH D 1783), Sintang, W. Kalimantan, leg. Borneo Expedition, 1894. Palaemon (Parapalaemon) thienemanni J. Roux, 1932 , male (cl 9.5, RMNH D 6434a, lectotype designated herein), Sg. Musi near Muara Klingi, S. Sumatra, leg. Prof. A. Thienemann – Deutsche Limnology Sunda Expedition, 10.V.1929; paralectotypes: 2 males (cl 6.1–6.3) ( RMNH D 6434b), same data as lectotype.
Other material examined. BORNEO: West Kalimantan: 3 males, 1 ov. female ( MZB), Sg. Pengkaran, Kapuas basin, Kapuas Hulu Regency, leg. Haryono et al., 26.VI.1996 ; 6 juveniles, 19 males, 13 females, 4 ov. females ( MZB), blackwater stream at ca. Km 373.5 Pontianak on Sekadau – Sintang road, leg. H. H. Tan et al., 22.IV.1998 ; 5 juveniles, 18 males, 9 females, 1 ov. female ( MZB), tea-coloured stream at ca. Km 377 Pontianak on Sekadau – Sintang road, near junction to Nanga Pinoh, leg. H. H. Tan et al., 22.IV.1998 ; 1 female, 1 ov. female ( MZB), Sg. Lumbai Dara, Sanggau, Kapuas basin, leg. T. R. Roberts & S. Wirjoatmodjo, 17.VII.1976 ; 43 juveniles, 22 males, 38 females, 1 ov. female ( MZB), Sg. Kepayang at Anjungan, leg. D. Wowor, 5.VI.1998 ; 9 juveniles, 10 males, 16 females, 5 ov. females ( ZRC), Sg. Kepayang, blackwater brook at Km 58 Pontianak on Pontianak – Anjungan road, leg. H. H. Tan et al., 29.IV.1998 ; 5 juveniles, 3 males, 1 female, 1 ov. female ( ZRC), unnamed blackwater tributary at ca. 2 km into Sg. Ketungau, Sintang Regency, leg. H. H. Tan et al., 23.IV.1998 ; 10 juveniles, 11 males, 14 females, 1 ov. female ( ZRC), peat swamps at Anjungan ‘D’, at Km 66 Pontianak on Pontianak – Anjungan road, leg. H. H. Tan et al., 28.IV.1998 ; 1 juvenile, 4 males, 2 females ( ZRC), blackwater stream at Km 325 Pontianak on Sekadau – Sintang road, Sanggau Regency, leg. H. H. Tan et al., 25.IV.1998 ; 1 juvenile, 3 males, 4 females ( MZB), Sg. Mujan 86.8 km from Putussibau, leg. Y. Y. Goh, 8.V.1998 ; Sarawak: 1 male ( ZRC), blackwater stream in forest at Km 7 on road from Kuching to Batu Kawa, leg. M. Kottelat et al., 3.VII.1992 ; 3 males ( ZRC), 11.8 km before junction to Sg. Cina Matang, leg. H. H. Tan et al., 4.IX.1995 ; 4 females ( ZRC), Sg. Stuum Toman, leg. O. Chia, 24.VI.1998 ; 1 male, 3 females ( ZRC 1995.254 View Materials ), middle stretches of Sg. Serait, Bako N. P., leg. N. Sivasothi et al., 1.VII.1994 ; 2 males, 1 female ( ZRC), Sg. Bejit, between Balai Ringin and Simunjan, 10 km after turnoff from Balai Ringin – Bandar Sri Aman road, leg. M. Kottelat et al., 2.VII.1992 ; NATUNA: 2 juveniles, 9 males, 4 females, 1 ov. female ( MZB), Sg. Datuk Kaya, a tributary of Sg. Segeram, leg. D. Wowor, 18.III.2002 ; KUNDUR: 1 juvenile, 11 males, 1 female, 1 ov. female ( MZB), Parit Gantung Sebesi, Tanjung Batu, leg. D. Wowor & H. K. Lua, 12.X.1998 ; 2 males, 4 females, 5 ov. females ( MZB), Parit Gantung Sei Ungar, Tanjung Batu, leg. D. Wowor & H. K. Lua, 13.X.1998 ; 2 males, 1 female ( MZB), Parit Pacitan, Urung District , leg. D. Wowor & H. K. Lua, 13.X.1998 ; 8 juveniles, 10 males, 9 females, 3 ov. females ( MZB), Sg. Nibong B, Sanglang district , nearby Kampung Baru, leg. D. Wowor & H. K. Lua, 13.X.1998 ; 10 males, 2 females, 6 ov. females ( ZRC), Tanjung Batu, leg. P. Yap, 10.IV.1998 ; SUMATRA: 5 males ( MZB), Sg. Mesjid, tributary of Sg. Alas, Gn. Leuser N.P., Aceh, leg. D. Wowor et al., 15.X.1982 ; 1 male, 1 ov. female ( MZB), Desa Pinang Sebatang, Bengkalis, Riau, leg. Haryono, 12.I.1996 ; 1 male ( MZB), drainage at Pinang Sebatang Industrial Forest , Siak Indrapura, Bengkalis, Riau, leg. Haryono, 14.I.1996 ; 1 major second pereiopod ( ZMA De 240510), Taluk, Riau, leg. J. P. Kleiweg de Zwaan ; 5 males ( MZB), Sg. Rokan, Riau, leg. A. Suyanto, 18.V.1997 ; 3 females ( ZRC), Sg. Keruh at Km 23 on road Jambi – Tembesi, leg. M. Kottelat, 28.V.1994 ; 9 males, 6 females ( ZRC), Sg. Bakung, stream joining Danau Arang-Arang, Jambi, leg. M. Kottelat, 29.V.1994 ; 1 male ( RMNH D 6433 About RMNH ), Taluk, Riau, leg. J. P. Kleiweg de Zwaan. SINGAPORE : 5 females, 1 ov. female ( ZRC), Nee Soon, leg. Y. Cai, 7.IV.1999 ; 1 male, 1 ov. female ( MZB), Nee Soon, leg. P. K. L. Ng, 8.IX.1998 ; 1 male ( ZRC 1989.2698 View Materials ), Nee Soon stream near Seletar reservoir, leg. P. K. L. Ng, II.1988 ; PENINSULAR MALAYSIA: 1 male, 5 females, 6 ov. females ( ZRC), a tributary of Sg. Titi Teras, Kongsi, P. Pinang, leg. H. H. Tan, 6.VIII.2001 ; 1 male, 2 females, 1 ov. female ( ZRC), N. Selangor, IX.1992 ; 3 males, 1 ov. female ( ZRC), a small tributary, between 4-5 km from Kuala Berang to Kuala Trengganu, Trengganu, leg. P. K. L. Ng & D. Wowor, 8.X.1997 ; 5 males, 3 females ( ZRC), first bridge to Panching, Kuantan, Pahang, leg. D. Wowor & N. K. Ng, 6.IX.1998 ; 6 males, 3 females ( ZRC 1985.3723 View Materials - 3730 View Materials ), stream 9 km after Jemaluang towards Kluang, Johor, leg. S. S. C. Chong & P. K. L. Ng, 12.VII.1985 ; 3 males ( ZRC), Clearwater flowing stream at Km 53 to Mersing, Km 106 to Batu Pahat, Johor, leg. M. Kottelat et al., 12.V.2000 ; 3 males, 2 females ( ZRC), clear water stream at Km 55 to Mersing and Km 106 to Batu Pahat, Johor, leg. M. M. Bahir et al. 23.IX.2001 ; 38 males, 18 females, 2 ov. females ( MZB), blackwater irrigation canal at Batu 30, Pekan Nanas, Pontian District , Johor, leg. D. Wowor, 14.III.1999 ; 1 male ( ZRC), Gn. Panti foothill, Kota Tinggi, Johor, leg. M. Kottelat et al. 22.I.1991 ; 1 male ( ZRC 1995.479 View Materials ), Sg. Mupor, Johor, leg. P. K. L. Ng, 14.I.1993 ; 2 juveniles, 14 males, 15 females, 3 ov. females ( ZRC), Sg. Bunyi, 4 km towards Pontian Kechil, Johor, leg. J. C. Y. Lai et al., 1.III.2003 ; for Thailand record see Cai et al. 2004.
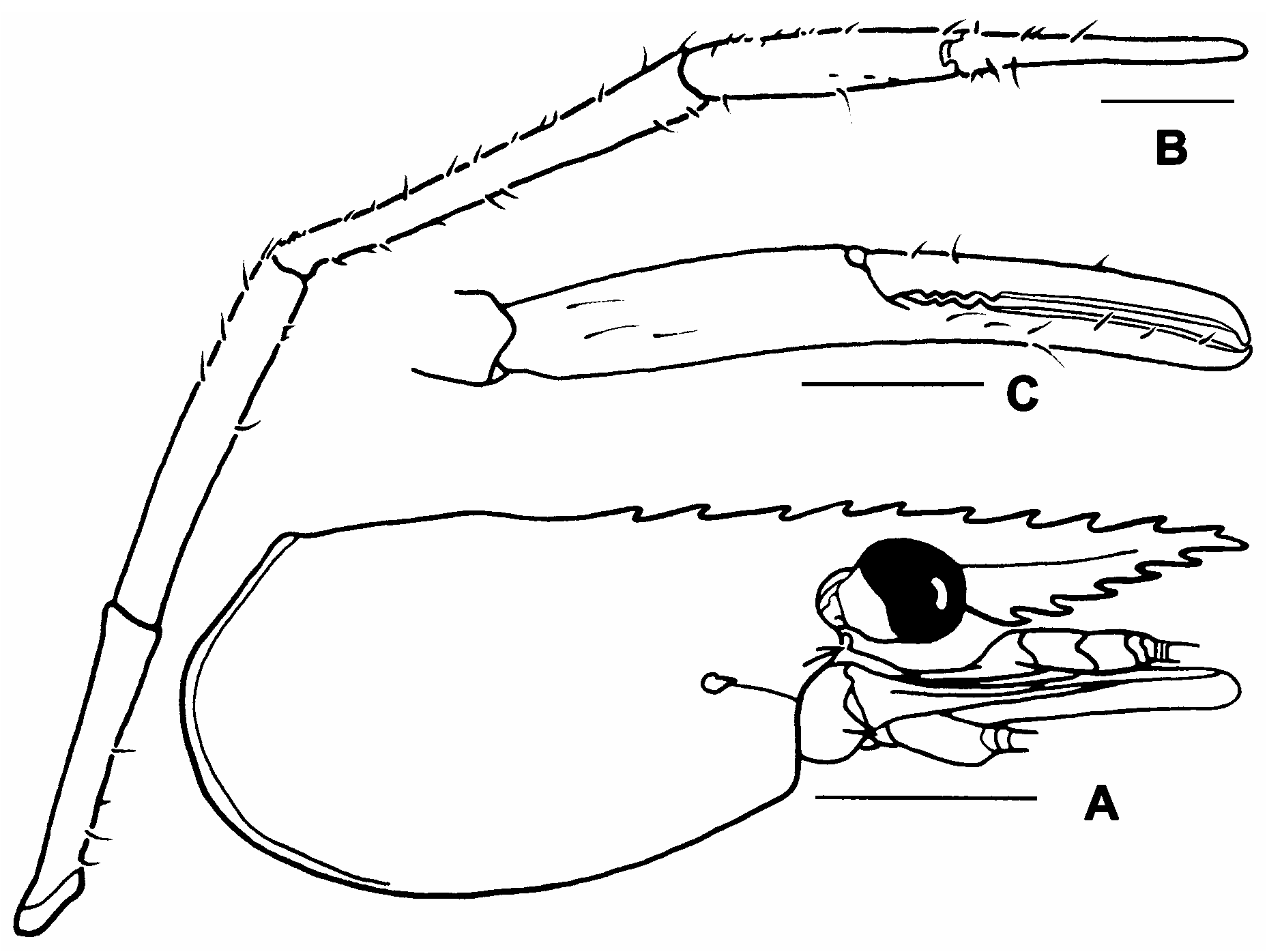
Description. The description is mainly based on the lectotype of Palaemon javanicus (cl 18.2, male, NHMW 7689). The designated lectotype of P. sundaicus is unfortunately a young female and the diagnostic second pereiopod is not fully developed ( Figs. 7A–C View FIGURE 7 ). Rostrum short, 0.66 of cl (0.63–0.74 of cl in other specimens) with tip not extending beyond distal end of scaphocerite but extending beyond distal end of third segment of antennular peduncle or tip slightly extending beyond distal end of scaphocerite in young specimens; moderate, maximum depth equal to maximum dorsoventral diameter of cornea; lateral carina well developed, continuing almost to tip; dorsal carina slightly convex, straight with tip directed anteriorly or curved upwards especially in young specimens, teeth subequally spaced, armed with 11 teeth (9–12 in other specimens, mode 11), 4 teeth completely postorbital (3 or 4 in other specimens, mode 4), postorbital teeth on anterior 0.41 of carapace (0.39–0.44 in other specimens); ventral carina with 3 teeth (4–6 in other specimens, mode 5), first tooth located at about proximal one-third ( Fig. 5A View FIGURE 5 ).
Carapace spinulate on branchiostegal region or glabrous in non-fully adult males. Ocular cornea well developed, 0.17 of cl (0.13–0.18 of cl in other specimens). Inferior orbital margin moderately produced, obtuse, postantennular carapace margin evenly rounded ( Fig. 5B View FIGURE 5 ). Antennal spine sharp, slender, continuing posteriorly as ridge, situated below lower orbital angle; hepatic spine smaller, situated behind and below antennal spine; branchiostegal suture running from hepatic spine to carapace margin. Ocular beak well developed but without expanded lateral tip. Epistome partly bilobed, lobes with blunt rounded margins ( Fig. 5C View FIGURE 5 ). Scaphocerite stout, 0.65 of cl (0.60–0.67 of cl in other specimens), length 2.89 times maximum breath (2.85–3.01 times in other specimens), lateral margin straight, distolateral tooth not reaching end of lamella. Third maxilliped with ultimate segment extending beyond antennal peduncle; ultimate shorter than penultimate segment, 0.76 times as long as penultimate (0.79–0.81 times in other specimens); exopod as long as ischiomerus.
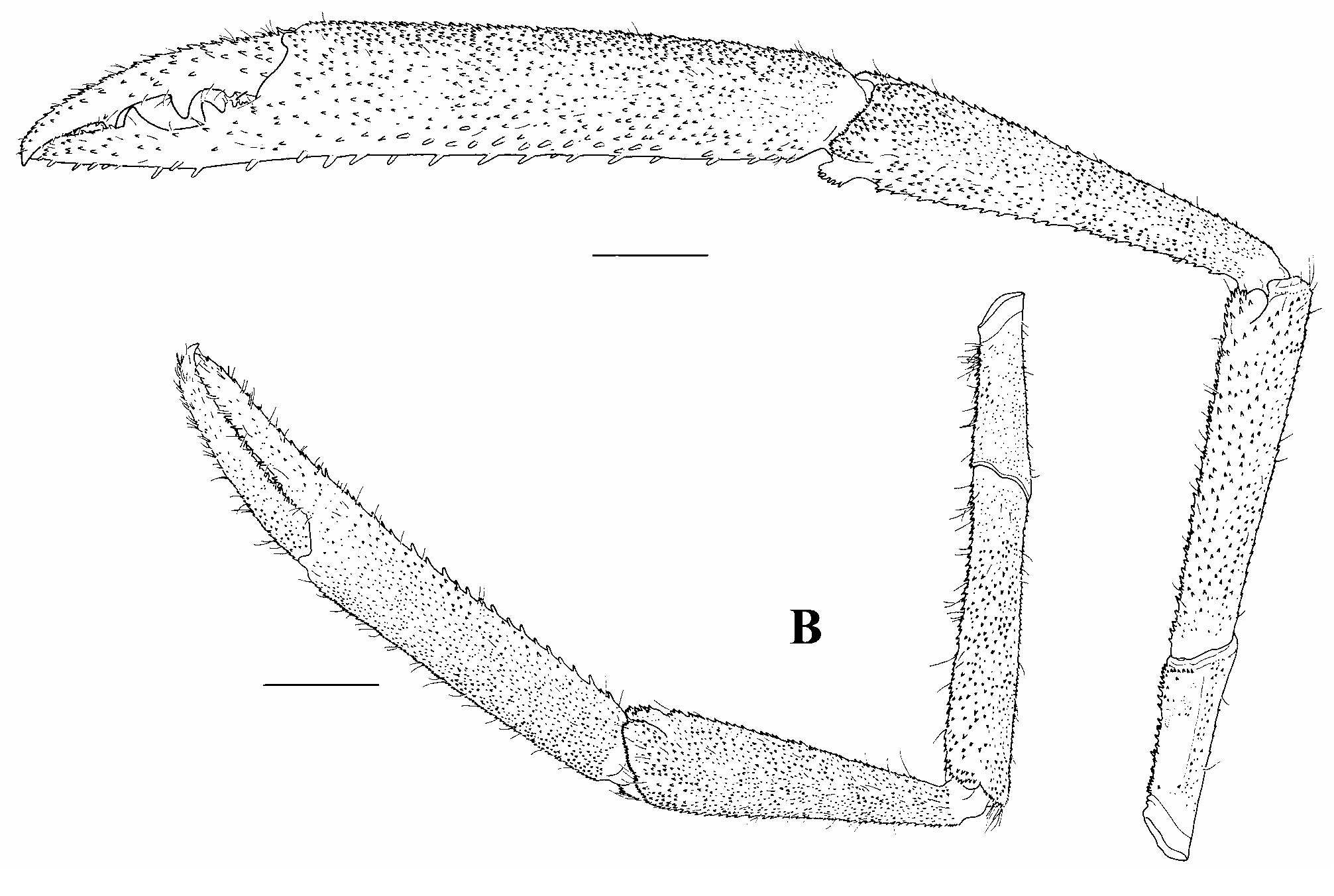
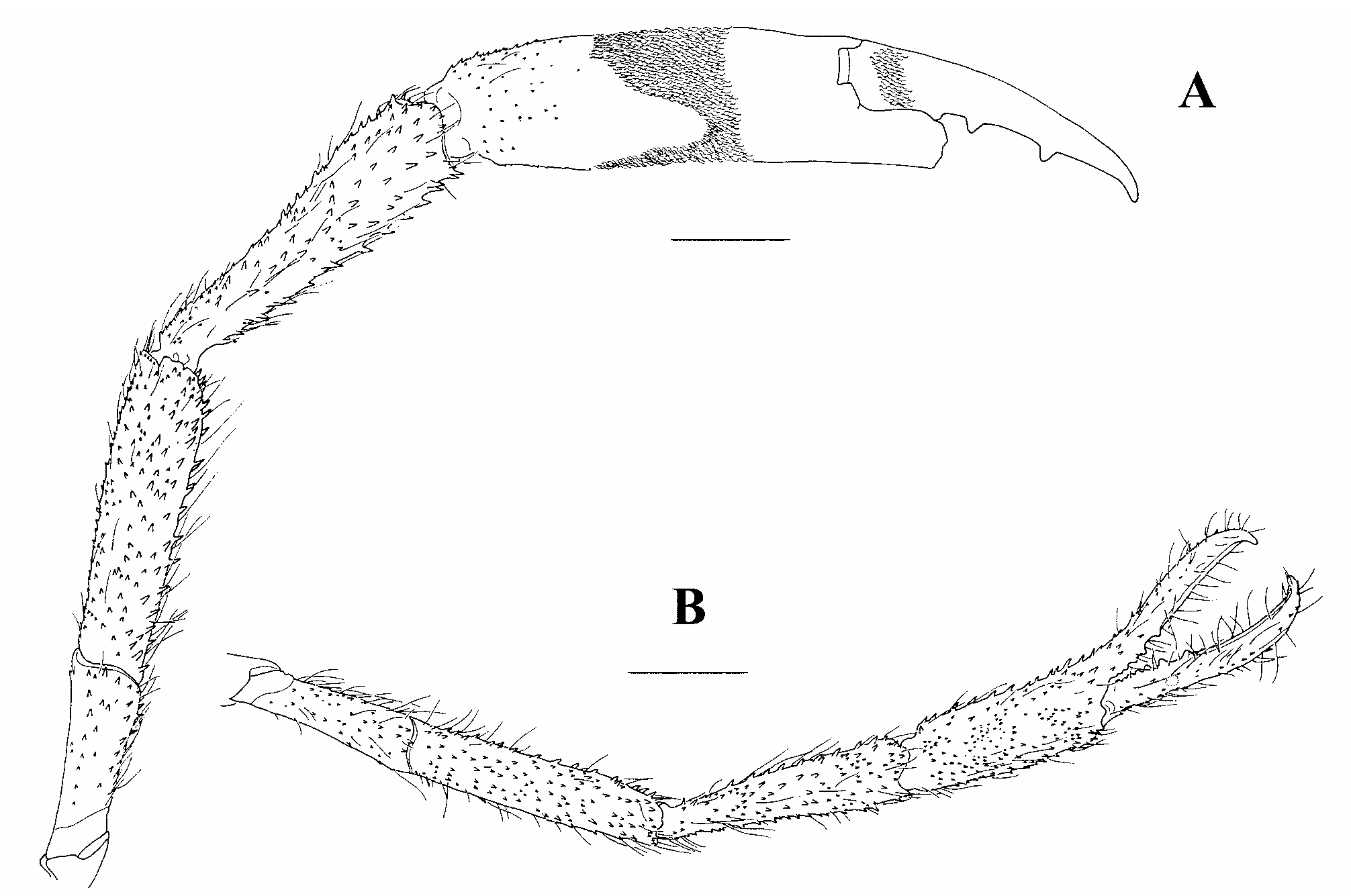
First pereiopods slender, exceeding scaphocerite by distal half of carpus; fingers about as long as palm; carpus 2.19 times chela length (1.94–2.25 times in other specimens), 1.32 times merus length (1.30–1.39 times in other specimens); few scattered long stiff setae present on all segments, otherwise glabrous ( Fig. 5I View FIGURE 5 ). Second pereiopods dissimilar in shape, unequal in size, robust; distal half of minor cheliped carpus extend beyond end of scaphocerite. Spines on inner margin of palm, carpus, merus and ischium larger but less dense than those on other margins. Major cheliped ( Fig. 6A View FIGURE 6 ) with chela densely covered with short velvety setae except part of proximal basal palm with spinules otherwise all segments covered with few scattered long stiff setae, chela 1.65 of cl (1.33–1.70 of cl in other specimens), length 5.02 times width (4.81–6.34 times in other specimens), outer and inner margins slightly convex and almost straight respectively, upper and lower margins rounded; palm subcylindrical, greater than maximum merus width, slightly compressed, width 1.24 times depth (1.09–1.23 times in other specimens); fingers thick, 0.82 times palm length (0.77–0.83 times in other specimens), about proximal 0.66 moderately gaping, distal rest overlapping when closed; dactylus with 2 large teeth, first tooth at proximal 0.62, second tooth at proximal 0.35 and 3 smaller teeth towards articulation of fingers (proximal 0.64 and 0.36 respectively with 2 smaller teeth towards articulation of fingers in another specimen), distal part of pollex broken and only with 3 smaller teeth present towards articulation of fingers (first tooth on proximal 0.54 and second tooth on proximal 0.32 with 4 smaller teeth towards articulation of fingers in another specimen), teeth unequally distributed along cutting edges; fingers uncinate at tip; carpus 0.96 palm length (0.94–1.02 in other specimens), conically long, length 4.08 times distal width (3.76–4.16 times in other specimens), 0.53 times chela length (0.51–0.57 times in other specimens), 1.17 times merus length (1.18–1.21 times in other specimens); merus not inflated, 1.46 times ischium length (1.32– 1.42 times in other specimens); ischium tapered. Minor cheliped ( Fig. 6B View FIGURE 6 ) resembles major cheliped; spines and few scattered long stiff setae present on all segments; cheliped length 0.72 times major cheliped (0.75– 0.82 times in other specimens); fingers 1.13 times palm length (0.99–1.11 times in other specimens), thin, distinctly gaping; dactylus with 4 small teeth, pollex with 5 small teeth, teeth subequaly distributed along proximal 0.39 of cutting edges; carpus shorter than chela, conically long, 1.36 times palm length (1.21–1.35 times in other specimens), 1.13 times merus length (1.12–1.16 times in other specimens); merus subcylindrical, 1.32 times ischium length (1.20–1.28 times in other specimens); ischium tapered.
Third pereiopods with entire dactylus extend beyond scaphocerite; few scattered long stiff setae present on all segments, otherwise glabrous; dactylus stout, curved, fringed with dorsolateral setae, ventral carina poorly developed; propodus length 14.17 times longer than width (12.0–13.5 times in other specimens); 11 ventral spines distributed along length of propodus, 2 distal most spines paired; carpus 0.58 times propodus length (0.56 times in other specimens); merus 1.06 times propodus length (1.0–1.07 times in other specimens), 1.84 times ischium length (1.67–1.93 times in other specimens) ( Figs. 5J–K View FIGURE 5 ).
Fourth pereiopods with entire dactylus extending beyond distal end of scaphocerite; few scattered long stiff setae present on all segments, otherwise glabrous; 16 ventral spines distributed along length of propodus, 2 distal most spines paired; merus 1.82 times ischium length (1.66–1.93 times in other specimens).
Fifth pereiopods with tip of dactylus reaching distal end of scaphocerite; few scattered long stiff setae present on all segments, otherwise glabrous; 15 ventral spines distributed along length of propodus; merus 0.94 times as long as propodus (0.88–0.96 times in other specimens), 1.82 times longer than ischium (1.67– 2.0 times in other specimens).
T4 without median process, without or with low posterior submedian plate ( Fig. 5D View FIGURE 5 ). T8 with moderately separated anterolateral lobes, without median process ( Fig. 5E View FIGURE 5 ). Abdomen smooth, glabrous. First 2 male abdominal sternites with large triangular median process of similar size and form; third abdominal sternite with small median process ( Fig. 5F View FIGURE 5 ). Inter-uropodal sclerite well developed, elevated as longitudinal preanal carina, carina small, smaller than posterolateral teeth of sixth abdominal somite. Telson moderate, stout, glabrous, 4.18 times median width (3.68–3.96 times in other specimens), lateral margin straight, convergent, 2 pairs of dorsal spines present, posterior subventral margin slightly convex with pointed median point, median projection overreached by inner pair of posterior spines ( Fig. 5H View FIGURE 5 ). Uropods with acute distolateral tooth, mobile mesial spine as large as distolateral tooth ( Fig. 5G View FIGURE 5 ); exopod 1.96 times longer than wide (1.94–2.08 times in other specimens). Developed eggs large, maximum size 1.9 × 1.5 mm, ovoid, few.
Remarks. As has been discussed earlier, what has been called “ M. javanicum ” since 1879 is actually not that species but an undescribed taxon (see Remarks under M. duri spec. nov.), and the real M. javanicum is actually conspecific with another well known species, M. trompii (De Man, 1898) .
Palaemon javanicus was described by Heller (1862) as a small-sized species with subcylindrical body form on the basis of three fully adult males from Java collected by Ida Pfeiffer; her trip to Java being between 1852 and 1853 (Holthuis, pers. comm.). The description of this species is concise and includes the important characters especially the morphology of the second pereiopods. Von Martens (1868) subsequently identified two males from Danau Sriang of Kapuas river basin in Borneo as Palaemon javanicus. Although the specimens of von Martens were lost during the war (Coleman, pers. comm.), the description of the rostrum, the second pereiopods, and the origin of the specimens are clear indications that they belong to P. javanicus Heller, 1862 , sensu stricto. The specimens have the carpus of the major second pereiopod almost as long as the palm and the fingers of the minor second pereiopod are as long as the palm. These are typical characters of the species in the strict sense.
Re-examination of the 5 type specimens of Palaemon sundaicus Heller, 1862 , shows that this material is actually a mix of two species: M. equidens ( Dana, 1852) , and M. javanicum (as presently defined). Although the largest type specimen (female, cl 12.6, NHMW 1525) is still relatively small, the absence of a median process on the T4 and the presence of a preanal carina clearly identify this specimen as been conspecific with M. javanicum sensu stricto. The four other, smaller, specimens are clearly M. equidens sensu stricto. The larger female specimen female (cl 12.6, NHMW 1525) from Java, Indonesia, is here designated as the lectotype of Palaemon sundaicus Heller, 1862 . The present action therefore makes Palaemon sundaicus Heller, 1862 , a subjective synonym of Palaemon javanicus Heller, 1862 . Since both names were simultaneously published in the same work by Heller (1862), we here invoke Article 24.2.1 of the Code, and as first revisors, select P. sundaicus Heller, 1862 , to have priority over P. javanicus Heller, 1862 , when the two names are regarded as synonyms, as is the case here.
In 1898, De Man described Palaemon (Parapalaemon) trompii on the basis of 1 young adult male, 1 young male, 4 ovigerous females and 2 juveniles from the Kapuas basin, western Borneo. The specimens described by De Man (1898) are not fully adult, especially the males, as the largest male has the fingers of the major second pereiopod closing along the cutting edges, with each of them armed with 3 minute basal teeth and the second legs are smooth (De Man 1898: 146–147). In addition, the second pereiopod of the herein designated lectotype also has only a few scattered stiff setae and both the major and minor pereiopods are of similar form and almost equal in size. One of the ovigerous female paralectotypes (cl 10.5, RMNH D 1520) has the major second pereiopod with velvety setae lightly covering the proximal half of the fingers. The above second pereiopods indicate that they are not yet fully developed. Palaemon trompii has a relatively long rostrum in which the tip slightly extends beyond the distal end of the scaphocerite and is slightly curved upwards, a rounded postantennular carapace margin, a partly bilobed epistome, an unarmed T4 without a posterior submedian plate, moderately separated anterior lobes of the T8 without a median process, presence of a preanal carina and a large mobile mesial spine which is larger than the distolateral tooth. These are all characters shared with P. sundaicus Heller and P. javanicus Heller , sensu stricto. The differences between these taxa are only in the size, form and ornamentation of the second pereiopods. As mentioned before, the second pereiopods of P. trompii are not fully developed, while those of P. javanicus are adult. Regardless, the carpus of the second pereiopods of all three taxa is longer than the merus. Also important is that the eggs of M. sundaicum , M. javanicum and M. trompii are relatively large and of the abbreviated type. The lectotypes of P. sundaicus Heller, 1862 and P. javanicus Heller, 1862 , are morphologically very close to the types of Palaemon (Parapalaemon) trompii De Man, 1898 , and we have no doubts that they are conspecific. Macrobrachium sundaicum ( Heller, 1862) has priority and M. trompii (De Man, 1898) becomes its subjective synonym.
Roux (1936) described a subspecies, Palaemon trompii armatus , from Gunong Pulai, Johor, Peninsular Malaysia. Although the specimens are mostly young, his subspecies has small spinules on the merus and carpus and a pubescence on the fingers of the second chelipeds, characters which appear when the specimens are almost mature. The merus is always somewhat shorter than carpus. Holthuis (1950) and Ng & Chong (1993) synonymised this subspecies with M. trompii sensu stricto. Although the type of P. trompii armatus was not re-examined during this study (they had, however, been studied by Ng & Chong 1993), a reasonable number of specimens of various sizes from Pontian District around Gunong Pulai were examined and they all agree very well with M. trompii as presently understood. The fully adult male specimens have the major second pereiopod covered with prominent spines except the chela, the base of the palm with few, smaller spines and the rest of the palm and fingers are densely covered with short velvety setae; the fingers always with 2 large teeth and 3 or 4 smaller teeth on each of them. The minor second pereiopod has a few scattered long stiff setae. The same features also exist in fully adult specimens, especially males from Kapuas basin, Riau Islands, east coast of Sumatra, Singapore and other parts of Peninsular Malaysia. All are all now referred to M. sundaicum (= M. trompii ) in the present study. Holthuis (1950) also synonymised Palaemon (Parapalaemon) thienemanni Roux, 1932 ; the types of which consist of small specimens from Sumatra, with M. trompii (now M. sundaicum ); a decision we concur with (see also Ng & Chong 1993).
A note on the supposed type locality of M. javanicum ( Heller, 1862) is needed. That it was purportedly collected from Java may have also misled De Man (1879, 1892) in thinking his Javanese specimens were this species (here referred to M. duri spec. nov.). Up to the present, no specimens of M. javanicum sensu stricto have ever been found in Java, with the exception of the original type specimens obtained by Ida Pfeiffer. Over the last 30 years, we and our colleagues have failed to find M. javanicum on Java, despite many surveys there. More telling is that even during the time of De Man, when the natural environment of Java was relatively less impacted by human, subsequent specimens were also not found (see Holthuis 1950: 211). The circumstantial evidence on hand suggests M. javanicum as presently defined may never have been found in Java. It is important to note that Ida Pfeiffer, the collector of the types of M. javanicum , also went to Borneo during her trip to Southeast Asia. During her trip to Borneo, she collected a male Macrobrachium specimen, which was described as Palaemon idae (presently Macrobrachium idae ) by Heller (1862). It is possible that the type material of P. javanicus Heller, 1862 , was actually from Borneo and there had been a mistake in labeling. We also need to keep in mind that the type locality of M. sundaicum ( Heller, 1862) is also supposedly Java, and this taxon is now regarded as synonymous with M. javanicum ( Heller, 1862) . As such, mistakes in labeling probably occurred there as well. It is well known that some of Heller’s material has been mislabeled (sometimes very badly) and this has caused innumerable problems before (e.g. see Rathbun 1905; Ng 1989; McLaughlin & Dworschak 2001). That a mistake in labeling took place makes biogeographical sense, especially since M. sundaicum (= M. javanicum ) as presently defined is common in Borneo (as M. trompii ; see Holthuis 1950; Johnson 1963; Ng & Chong 1993); and there are a number of other freshwater decapod species which are not shared by the two islands (e.g. see Ng 1997b; Ng & Tan 1998, 1999). However, without clear evidence that the original type localities of M. sundaicum and M. javanicum are wrong, we are not prepared to exclude the possibility that it used to be present in Java, and may yet be found in the future. As discussed earlier, we have selected the name Palaemon sundaicus Heller, 1862 , as having priority over P. javanicus Heller, 1862 , since both names are subjective synonyms and were published simultaneously by Heller (1862). As such, having the name M. sundaicum ( Heller, 1862) as the subjective senior synonym of M. trompii (De Man, 1898) is therefore also less confusing.
Macrobrachium sundaicum (Heller,) morphologically resembles M. malayanum ( Roux, 1934) , especially larger specimens. The similarities and differences between these two species and their first zoeas have been described in detail by Chong & Khoo (1987) and Ng & Chong (1993). Additional similar characters shared by these two species are: a rounded postantennular carapace margin, partly bilobed-epistome, unarmed T4 without a posterior submedian plate, moderately separated anterior lobes of the T8 and a large mobile mesial spine which is larger than the distolateral tooth (see Wowor et al. 2004). However, M. sundaicum can be distinguished from M. malayanum by the presence of a pre-anal carina (typical for the species group), a character absent in M. malayanum . This character is quite reliable and can be applied to young and adult specimens of both sexes.
Macrobrachium sundaicum as presently redefined, has only one other recognised species in its species- group, M. oxyphilus Ng, 1992a , from peat swamps in Peninsular Malaysia (see Ng 1992a, for details comparing it with M. trompii ).
Ecology. Macrobrachium sundaicum ( Heller, 1862) inhabits various water bodies, such as forest streams, streams at edge of reservoirs and plantations, freshwater and peat swamps. It prefers moderate to slowflowing streams and it is most abundant in the well shaded stretches of streams with abundant leaf litter. The waters are always acidic with pH 4.5–6.0. This species can also tolerate very high temperatures between 24.5– 35.5°C and is often found in low-oxygen habitats ( Johnson 1967; Ng & Chong 1993).
Distribution. West Kalimantan and southern Sarawak in Borneo, east coast of Sumatra and Riau Archipelago, Singapore, Peninsular Malaysia and southern Thailand.
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |
Macrobrachium sundaicum ( Heller, 1862 )
| Wowor, Daisy & Ng, Peter K. L. 2010 |
Palaemon sundaicus
| Chace, F. A. Jr. & Bruce, A. J. 1993: 38 |
| Johnson, D. S. 1963: 9 |
| Johnson, D. S. 1961: 57 |
| Holthuis, L. B. 1950: 211 |
| Roux, J. 1936: 30 |
| Roux, J. 1932: 569 |
| Roux, J. 1932: 570 |
| Man, J. G. de 1898: 144 |
| Ortmann, A. 1891: 732 |
| Martens, E. von 1868: 45 |
| Heller, C. 1862: 415 |
| Heller, C. 1862: 421 |