Cyanocharax itaimbe Malabarba & Weitzman, 2003
|
publication ID |
https://doi.org/ 10.5281/zenodo.10813265 |
|
DOI |
https://doi.org/10.5281/zenodo.10810791 |
|
persistent identifier |
https://treatment.plazi.org/id/03808793-801E-FFDA-CC50-FAFBFB02F95D |
|
treatment provided by |
Juliana |
|
scientific name |
Cyanocharax itaimbe Malabarba & Weitzman |
| status |
sp. nov. |
Cyanocharax itaimbe Malabarba & Weitzman View in CoL , new species
( Figs. 1 View Figure 1 , 13 View Figure 13 , 17-23 View Figure 17 View Figure 18 View Figure 19 View Figure 20 View Figure 21 View Figure 22 View Figure 23 , Tables 1 View Table 1 and 2 View Table 2 )
Diagnosis. The strongly pigmented adipose fin (dark in alcohol preserved specimens) distinguishes C. itaimbe from C. alburnus , C. lepiclastus , C. macropinna , and C. alegretensis . Cyanocharax itaimbe differs from C. dicropotamicus by the larger eye diameter (43.0-50.0% versus 37.8-46.8% of HL), and higher number of longitudinal series of scale rows (11 -13 scale rows between dorsal-fin origin and pelvic-fin origin versus 10- 11, respectively). The completely perforated lateral line separate C. itaimbe from C. tipiaia that has 7- 11 perforated lateral-line scales. The unpigmented tip of the anterior lobe of the anal fin, and the concave distal border of the anal fin of mature males further distinguishes C. itaimbe from C. lepiclastus , C. macropinna and C. alegretensis , which have a pigmented anal fin and convex or nearly straight distal border of the anal fin.
Description. Based on specimens from rio Mampituba drainage only - see discussion under Distribution and Geographical Variation. Morphometric data given in Table 1 View Table 1 . Body moderately elongate and compressed. Dorsal and ventral profiles nearly equally convex from head to caudal peduncle. Greatest depth at dorsal-fin origin, or somewhat anterior of that point if belly expanded. Body profile along anal-fin base nearly straight. Caudal peduncle slightly longer than deep. Dorsal and ventral profiles of caudal peduncle slightly concave.
Head small (21.8-24.8% of SL). Eyes large (43.0-50.0% of HL). Maxilla positioned at approximately 45 degrees angle relative to long body axis. Posterior tip of maxilla reaches or passes vertical through anterior border of pupil.
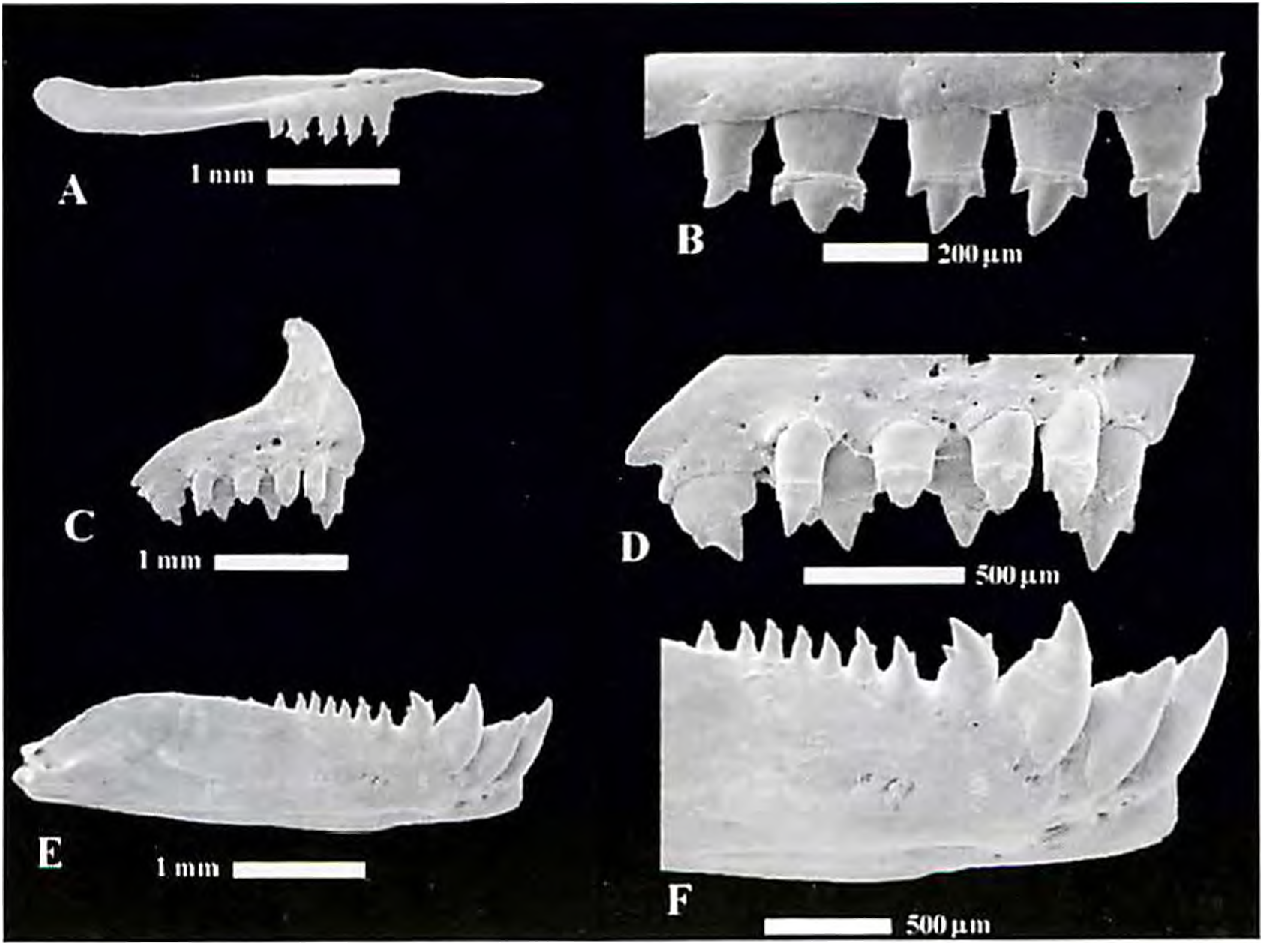
Premaxilla with two series of teeth. Teeth of outer series 3 to 5, usually tricuspid and smaller than inner series. Inner series with 4 or 5 teeth with 3 to 5 cusps. Teeth with 4 or 5 cusps with fourth and fifth cusps very small, almost imperceptible. Maxilla with 5 to 7 teeth usually ranging from 3 cusps anteriorly to conical posteriorly, rarely with 5 cusps anteriorly. Dentary with 4 large anterior dentary teeth followed posteriorly by series of 9 to 13 much smaller ones teeth. Dentary teeth ranging from 3 cusps anteriorly to conical posteriorly. Anterior large dentary teeth with 3 to 5 cusps. Those teeth with 4 or 5 cusps with fourth and fifth cusps usually very small, almost imperceptible. Second dentary tooth relatively short and inserted at more ventral position on bone such that tip of tooth reaches only as high as tip of second largest cusp of first and third dentary teeth ( Fig. 23 View Figure 23 ).
Dorsal-fin rays ii,8 (n = 29). First unbranched ray about one-half length of second ray. Dorsal-fin origin at, or slightly posterior to, midlength of body. Adipose-fin origin just dorsal to vertical through insertion of posteriormost anal-fin ray.
Anal-fin rays iii-v (usually iv or v), 22-27 (x = 23.9, n = 88). Anal-fin origin clearly posterior to vertical through dorsal-fin origin. Distal border of anal fin concave in both sexes, with anterior 3-4 branched rays longer, forming prominent anterior lobe. One mature male ( Fig. 17 View Figure 17 - holotype) with long unbranched and first and second branched rays longer than usual, with anterior lobe of anal fin reaching further posteriorly than longest dorsal-fin rays, possibly a secondary sexual feature. Anal-fin rays of males with small retrorse bony hooks present on longest unbranched ray and usually 8 anteriormost branched rays. Hooks present on distal 2/3 of longest unbranched ray, first 4 branched rays, and usually distal one-half length of branched rays 5 to 8. Minute hooks distributed along third distal portion of some remaining rays. Hooks mostly present on posterior branches of rays. Usually one pair of bony hooks per ray segment; two pair rarely occur on anterior branched rays.
Pectoral-fin rays i,9- l 1 (x̄ = 10, n = 29). Tips of longest rays extend to or close to pelvic-fin origin. Pelvic-fins i,6 (n = 29; i, 7 in 2 specimens). Pelvic-fin origin anterior to vertical through dorsal-fin origin. In females longest ray reaches or falls short of anal-fin origin. In males longest pelvic-fin ray reaches anal-fin origin. Pelvic fins with ventromedial, usually unpaired retrorse bony hooks on branched rays only. Principal caudal-fin rays 10/9.
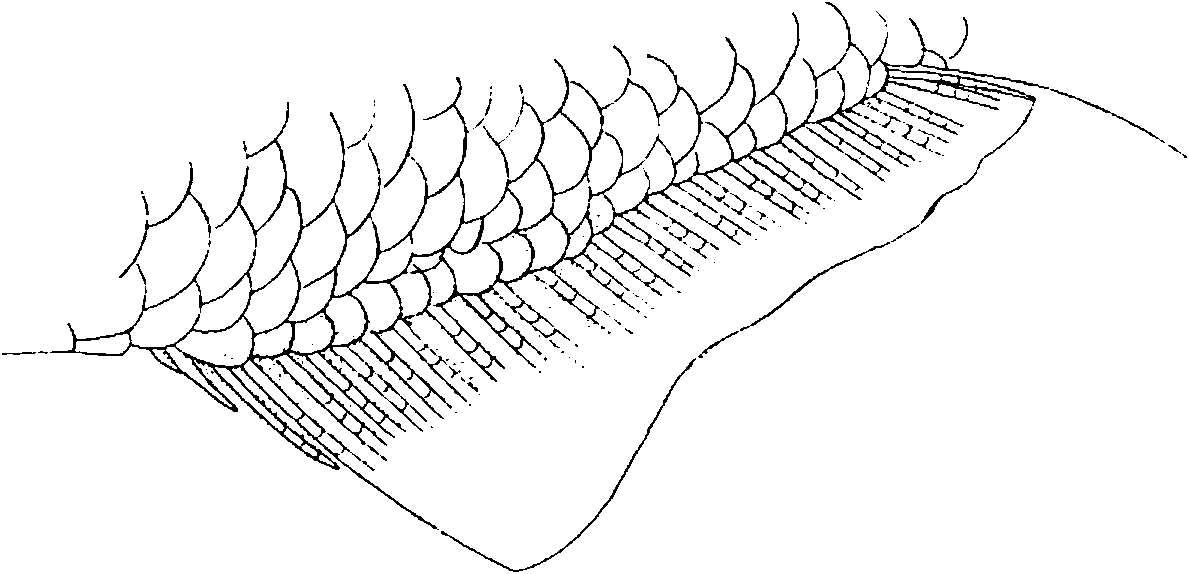
Scales cycloid. Lateral line usually complete. Of 22 specimens counted, one with an alternate series of 25 perforated, 11 non-perforated and 4 perforated scales and one specimen with incomplete lateral line having 29 perforated scales. Total number of scales in lateral-line row, including perforated and non-perforated scales, 37-40 (x̄= 38.2, n = 22). Scale rows between dorsal-fin origin and lateral line 6 to 7 (x ̄ = 6.7, n = 27). Scale rows between lateral line and pelvic-fin origin 4-5 (x̄= 4.1, n = 29). Predorsal scales 11 - 15 (x̄ = 13.0, n = 16), usually irregularly ar ranged. Males and females with scale sheath on anal-fin base consisting of one row with 7 to 13, usually 9 to 10, small scales covering bases of unbranched rays and first 7 to 11 branched rays.
Vertebrae 35-38 (x̄= 36.8, n = 61), including vertebrae of Weberian apparatus and posterior half centrum (counts taken from radiographs of USNM 323414 and USNM 323416).
Color in alcohol. Figs. 17- 19 View Figure 17 View Figure 18 View Figure 19 and 22 View Figure 22 . Body pale brownish yellow in specimens preserved in formalin long enough to destroy guanine pigment. Lateral body stripe broad and dark posteriorly, becoming pale and narrow anterior to dorsal-fin origin. Dark humeral spot vertically elongate, centered on fourth and fifth scales of scale row just dorsal to lateral line. Caudal fin remarkably darkly pigmented in mature males, primarily along middle caudal-fin rays and extending to termination of those rays. Branched rays of ventral caudal-fin lobe also darkly pigmented. Dorsal caudal-fin lobe not pigmented basally, and weakly pigmented distally. Dorsal and anal fins darkly pigmented, except for a distinct unpigmented area at tip of dorsal fin and tip of anterior lobe of anal fin. Adipose fin very darky pigmented. Head black to gray dorsally, especially dark near nape. Sides of head and opercle silvery where guanine pigment not destroyed by formalin; otherwise whitish yellow. Exposed borders of scales of dorsal part of body delineated by dark chromatophores. Specimens collected during late spring and summer, presumably corresponding to reproductive period ( Figs. 17 View Figure 17 , 18 View Figure 18 , 20 View Figure 20 , 22 View Figure 22 ), more strongly pigmented than those collected during fall and winter months ( Figs. 19 View Figure 19 , 21 View Figure 21 ).
Color in life. Field observations show that specimens collected during late spring and summer, presumably corresponding to the reproductive period, have intense blue pigmentation, mostly along midbody, as shown in Fig. 20A View Figure 20 . Specimens collected during fall and winter months and presumably not in reproductive mode, are whitish, with more intense yellow pigmentation on caudal fin ( Fig. 21 View Figure 21 ).
Sexual dimorphism. Males with hooks on anal and pelvic fins. Also, mature males with slightly longer pelvic fins than females ( Table 1 View Table 1 ).
Etymology. Itaimbe, meaning precipice, comes from the Tupi-Guarani language of Brazilian Amerindians. In reference, to the most popular of the deep canyons of the Parque Nacional de Aparados da Serra, Canion do Itaimbezinho, at the border of the states of Rio Grande do Sul and Santa Catarina. All deep canyon rivers flowing from the Parque Nacional de Aparados da Serra are tributaries to the rio Mampituba drainage, the type locality of C. itaimbe .
Ecological notes. Cyanocharax itaimbe , although until recently appar ently absent in fish collections, seems to be a relatively common fish spe cies in mainstream clear and cold waters of the small rivers draining from Serra Geral formation. Twenty of the 22 MCP lots originated in clear waters, with the bottom covered with rocks and stones in all 22 samples, and with some areas of sand and mud in 7 of these 22 samples. All sited had a low to medium speed water currents.
Distribution and geographical variation. The description of C. itaimbe is based exclusively on population samples collected from the rio Mampituba drainage; however, two other geographically isolated population samples were tentatively identified as C. itaimbe . One occurs in the Atlantic drainage immediately to the North, the rio Araranguá and its tributar ies. The second in the immediately southern drainage of the rio Tramandaí. In this latter drainage, C. itaimbe was found in rio Maquiné and rio Três Forquilhas valleys of the Serra Geral, that are partially connected in the coastal plain through lagoa dos Quadros and lagoa Itapeva, but was absent in fish samples taken on the coastal plain itself ( Fig. 1 View Figure 1 ).
We found no clear differences among these populations (see Tables 1 View Table 1 and 2 View Table 2 ), except for different ranges and means in anal-fin ray counts. Cyanocharax specimens from the rio Araranguá drainage have a relatively low number of anal-fin rays (21 -25, median = 23, x ̄= 22.9, n = 104) compared to the Cyanocharax populations from the coastal drainages of the rio Mampituba (22-27, median = 24, x̄= 23.9, n = 88), and rio Tramandaí (rio Tres Forquilhas, 22-27, median = 25, x̄= 24.6, n = 76; rio Maquiné, 22-27, median = 25, x̄ = 24.7, n = 121). The parametric t-test used for comparing these four samples, showed the differences in the mean values between the rio Araranguá population and each of the three remaining populations are greater than would be ex pected by chance (P = 0.001). Because these population samples did not have a normal distribution, we subjected them to non-parametric Mann-Whitney Rank Sum Tests, and found a statistically significant difference in the median values between the rio Araranguá population and each of the three remaining populations (P = 0.001). The type series from rio Mampituba also was statistically significantly different from the rio Maquiné and rio Três Forquilhas means (P = 0.01), and medians (P = 0.001 and P = 0.002, respectively), but a comparison between the rio Tres Forquilhas and rio Maquiné populations (both from rio Tramandaí drainage) showed no statistically significant differences in both tests. Ac cording to these statistical results for anal-fin ray counts, we could rec ognize two (rio Araranguá and rio Mampituba + Três Forquilhas + Maquiné) or three (rio Araranguá, and rio Mampituba, and rio Três Forquilhas + Maquiné) different species among these C. itaimbe populations. However, we found no additional characters supporting the recognition of different species and consequently prefer not to describe more than one species for these populations.
Additionally we tested statistical differences among population samples from the same drainage taken at different tributaries on the same date (tributaries of the rio Mampituba drainage, 15 Jan 1991: USNM 323414, rio dos Mengue; USNM 323410, arroio Facão) and among samples taken in the same tributary at different years (rio Jordão, rio Araranguá tributary: USNM 319746, 2 Dec 1977; MCP 15000, 7 Jun 1985). We found that the statistical differences in the mean and median values between samples taken at the same tributary in different years, MCP 15000 (range 22-25, median = 24, x ̄= 23.4, n = 21) and USNM 319746 (range 21-25, median = 23, x̄= 22.7, n = 83), are also greater than would be expected by chance (P = 0.003 for means and P = 0.003 for medians), as well as differences between samples taken at different tributaries of the same drainage on the same date USNM 323414 (range 22-27, median = 24, x̄= 24.4, n = 31) and USNM 323410 (range 22-26, median - 24, x = 23.6, n = 48), (P = 0.001 for means and P = 0.005 for medians). This seems to show that, at least for C. itaimbe , slight changes in the range, mean and median of anal-fin rays counts observed within drainages are influenced by unknown factors affecting sample composition, and may not be appropriate for species recognition.
Although the three isolated population samples of C. itaimbe may constitute more than one species, in view of the small differences discussed so far, these populations samples are considered as constituting a natural assemblage comparable to the remaining Cyanocharax species. This is additional support for the recognition of an area of endemism in some of the small Atlantic coastal drainages of Southern Brazil, first proposed to include the rio Maquiné, rio Três Forquilhas, and rio Mampituba by Malabarba & Isaia (1992), and later extended to the rio Araranguá by Reis & Schaefer (1999).
Taxa with a geographical distribution identical to Cyanocharax itaimbe are the characids Mimagoniates rheocharis Menezes & Weitzman (1990) , and Deuterodon stigmaturus ( Lucena & Lucena, 1992; Reis & Schaefer, 1999); the loricariid Epactionotus , with three species, one in the rio Maquiné plus rio Três Forquilhas, one from the rio Mampituba, and one from rio Araranguá drainage ( Reis & Schaefer, 1999); an undescribed species of Rineloricaria ( Reis & Schaefer, 1999) , and two monophyletic groups of Hemipsilichthys species (Edson H. L. Pereira, pers. comm., and Reis & Schaefer, 1999). The characid Odontostoechus lethostigmus Gomez is also endemic to the rio Tramandaí and rio Mampituba drainages, and is putatively closely related to an undescribed species from the rio Araranguá drainage ( Malabarba, 1998). The pimelodid Microglanis cibelae Malabarba & Mahler (1998) has been found only in the rio Maquiné, rio Três Forquilhas and rio Mampituba drainages, but no other species of this genus have been collected as yet in the rio Araranguá drainage.
Holotype: MCP 25972 , male, 49.6 mm SL, arroio Facão at Mae dos Homens , tributary rio Canoas - rio Mampituba , Praia Grande, Santa Catarina, Brazil; 15 Jan 1991.
Paratypes: Rio Mampituba drainage: Santa Catarina, Brazil: MCP 14788 (46: 10 c&s, 28.0- 45.8 mm SL), USNM 323410 (51), MZUSP 43549 (50, 29.3 -47.0 mm SL), MNRJ 23841 (15, 31.4-45.8 mm SL), collected with holotype . MCP 14707 (9, 31.7-42.9 mm SL), rio Canoas , between Praia Grande and Mae dos Homens , 8 km from Praia Grande, Praia Grande; 16 Jan 1991 . Rio Grande do Sul, Brazil: MCP 14838 (13, 12.5-35.1 mm SL), USNM 323416 (10), MZUSP 43544 (10, 25.2-30.1 mm SL), rio das Pacas , near Morro Azul , tributary oflagoa Jacaré , Três Cachoeiras; 15 Jan 1991 . MCP 14710 (47, 14.3-37.3 mm SL), USNM 323414 (41), MZUSP 43548 (40, 14.1 -33.0 mm SL), rio dos Mengue , between Morro Azul and Rua Nova, tributary oflagoa Jacaré , Torres; 15 Jan 1991.
Non-type specimens). Rio Araranguá drainage, Santa Catarina, Brazil: MCP 15000 (21: 4 c&s), rio Jordão , Siderópolis; 2 Dec 1977 . MCP 15089 (85: 5 c&s), MZUSP 44136 (79), USNM 319746 (85), rio Jordão , Jordão Alto , Nova Veneza; 7 Jun 1985 . USNM 323413 (15), USNM 326248 (6), USNM 326249 (20), rio Pique , Morro Cortado, road off Meleiro, near Limeira , turn off at Km 407, BR 101; 22 Sep 1977 . MCP 25437 (13), rio Itoupava , nearly 3 Km W of Ermo; 5 Jan 2000 . Rio Mampituba drainage, Santa Catarina, Brazil: MCP 15091 (70), MZUSP 44137 (68), USNM 319747 (68), rio Faxinalzinho at Mae dos Homens, Praia Grande; 9 Jun 1985 . MCP 14798 (18), USNM 323411 (10), creek tributary of rio Canoas , between Praia Grande and Mae dos Homens, about 2 km from Praia Grande, Praia Grande; 16 Jan 1991 . Rio Três Forquilhas drainage, Rio Grande do Sul, Brazil: MCP 14819 (260), USNM 323408 (100), rio Tres Pinheiros , 8 km from BR 101 on road to Itati, Terra de Areia; 15 Jan 1991 . MCP 14796 (130), USNM 323412 (80), rio Mitmann , Vila Nova, 10 km from BR 101 on road to Itati, Terra de Areia; 15 Jan 1991 . MCP 14791 (8), USNM 323415 (8), rio do Padre , Itati, Osorio; 15 Jan 1991 . MCP 14569 (6), rio Tres Forquilhas , Porto Alagio, Torres; 25 May 1986 . MCP 14290 (21: 6 c&s), rio Tres Forquilhas , on road Tres Forquilhas-Itati, Torres; 12 Dec 1989 . MCP 14237 (6), rio Tres Forquilhas , on road Tres Forquilhas-Itati, 100 m from bridge, Tones; 12 Dec 1989 . MCP 15404 (121), USNM 319749 (120), MZUSP 44138 (71), Chapéu, Torres; 25 May 1986 . MCP 14263 (13: 4 c&s;), rio Tres Forquilhas , at second dam after bridge, Torres; 12 Dec 1989 . MCP 25308 (105), creek on road Terra de Areia to Itati, nearly 8 Km from BR 101 highroad, Vila Nova; 29 Dec 1999 . Rio Maquiné drainage, Rio Grande do Sul, Brazil: MCP 14856 (3), arroio Água Parada , Maquiné; 14 Jan 1991 . MCP 14549 (9), arroio Água Parada , Maquiné; 25 May 1986 . MCP 13697 (7), arroio Água Parada , Maquiné, Osório; 1 Oct 1989 . MCP 14795 (8), USNM 323409 (10: 5 c&s), rio Pinheiro , Maquiné; 14 Jan 1991 . MCP 14562 (27), rio Maquiné , Maquiné; 25 May 1986 . MCP 15088 (99), USNM 319748 (95), MZUSP 44139 (95), rio Maquiné , 2 km above Maquiné; 25 May 1986 . MCP 13604 (9), rio Maquiné , near Maquiné; 1 Oct 1989 . UFRGS 4998 (4), Balneário Maquiné , rio Maquiné , Maquiné; 25 Jan 2001 .
Table 1. Morphometries of Cyanocharax itaimbe, new species. Standard length is expressed in mm; other measurements through head length arc percentages of standard length; the last four entries arc percentages of head length. Described ranges include measurements of the holotype MCP 25972, male, and paratypes from rio Mampituba drainage (MCP 14710, 8; MCP 14838,6; MCP 14707, 9; MCP 14788, 12); and comparative material from rio Tramandaí drainage (MCP 13604, 9; MCP 14856, 3; MCP 14795, 8; MCP 13697, 7; MCP 14791, 8; MCP 14263, 13) and rio Araranguá drainage (MCP 15000, 20; USNM 326248, 5; USNM 326249, 3; USNM 323413,2).
| holotype + paratypes | rio Tramandaí | rio Araranguá | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| rio Mampituba | |||||||||||||
| Character | holotype | n | low | high | n | low | high | x | n | low | high | x | |
| Standard length (mm) | 49.6 | 35 | 28.4 | 49.6 | 38.8 | 48 | 29.4 | 52.6 | 40.3 | 30 | 29.0 | 51.4 | 38.2 |
| Snout to anal-fin origin | 60.1 | 35 | 54.2 | 61.2 | 57.2 | 48 | 55.7 | 59.9 | 57.7 | 30 | 55.4 | 60.5 | 58.6 |
| Snout to dorsal-fin origin | 53.0 | 35 | 50.3 | 55.5 | 53.2 | 48 | 51.1 | 55.5 | 53.3 | 30 | 50.8 | 55.5 | 53.3 |
| Snout to pelvic-fin origin | 42.3 | 34 | 40.4 | 45.6 | 43.0 | 48 | 41.0 | 46.6 | 43.3 | 30 | 41.2 | 46.1 | 43.4 |
| Dorsal-fin base length | 12.1 | 35 | 9.7 | 12.8 | 11.4 | 48 | 10.1 | 13.1 | 11.7 | 30 | 9.1 | 13.2 | 11.4 |
| Anal-fin base length | 31.9 | 35 | 30.4 | 36.0 | 33.2 | 48 | 29.6 | 37.1 | 33.0 | 30 | 28.2 | 34.2 | 31.4 |
| Caudal peduncle length | 13.3 | 35 | 11.4 | 15.2 | 13.1 | 48 | 11.0 | 16.2 | 13.1 | 30 | 9.6 | 16.2 | 13.1 |
| Caudal peduncle depth | 11.1 | 35 | 9.2 | 11.4 | 10.3 | 48 | 9.9 | 12.2 | 11.0 | 30 | 8.6 | 11.0 | 10.2 |
| Depth at dorsal-fin origin | 32.1 | 34 | 27.0 | 35.0 | 30.0 | 48 | 26.1 | 36.9 | 31.6 | 29 | 24.6 | 33.2 | 28.6 |
| Dorsal-fin height | 28.2 | 35 | 21.8 | 28.6 | 24.4 | 46 | 21.9 | 27.2 | 23.9 | 29 | 21.2 | 27.4 | 23.8 |
| Pelvic-fin length males | 17.9 | 21 | 13.4 | 17.9 | 15.0 | 18 | 12.8 | 17.1 | 15.6 | 11 | 14.8 | 17.4 | 16.1 |
| Pelvic-fin length females | 13 | 12.5 | 15.1 | 13.9 | 29 | 12.2 | 15.8 | 14.0 | 13 | 13.5 | 15.7 | 14.4 | |
| Pectoral-fin length | 22.0 | 34 | 18.8 | 23.6 | 20.7 | 46 | 18.6 | 23.1 | 21.0 | 29 | 18.2 | 23.8 | 21.1 |
| Bony head length | 23.2 | 35 | 21.8 | 24.8 | 23.3 | 48 | 21.8 | 24.2 | 23.0 | 30 | 21.0 | 24.4 | 23.4 |
| Snout length | 21.7 | 35 | 20.7 | 23.1 | 21.9 | 48 | 19.3 | 25.0 | 22.0 | 29 | 18.8 | 23.3 | 21.1 |
| Upper jaw length | 36.5 | 35 | 36.2 | 43.9 | 39.8 | 48 | 33.8 | 42.0 | 38.5 | 30 | 32.5 | 44.7 | 39.4 |
| Horizontal eye diameter | 43.5 | 35 | 43.0 | 50.0 | 46.0 | 48 | 38.5 | 48.1 | 44.1 | 30 | 41.6 | 48.3 | 45.3 |
| Least interorbital width | 33.9 | 35 | 31.5 | 36.6 | 33.7 | 48 | 31.0 | 37.3 | 34.4 | 30 | 30.2 | 37.4 | 33.9 |
Table 2. Counts of Cyanocharax itaimbe, new species. Described ranges include counts of the holotype MCP 25972, male, and paratypes from rio Mampituba drainage (MCP 14710, 8; MCP 14838, 6; MCP 14707, 5; MCP 14798, 5; MCP 14788, 5), and comparative material from rio Tramandaí drainage (MCP 14791, 8; MCP 14263, 13; MCP 14290, 7; MCP 13604, 9; MCP 14856, 3; MCP 14795, 8) and rio Araranguá drainage (MCP 15000, 14; MCP 15089, 14). Branched anal-fin rays counts taken from x-rays of USNM 323410, USNM 323414 and USNM 323416 (rio Mampituba), MCP 13604, USNM 323409 and USNM 323412 (rio Tramandaí), and USNM 319746 and MCP 15000 (rio Araranguá).
| holotype + paratypes | rio Tramandaí | rio Araranguá | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| rio Mampituba | |||||||||||||
| Character | Holotype | n | low | high | x | n | low | high | x | n | low | high | x |
| Unbranched anal-fin rays | 3 | 29 | 3 | 5 | 4.4 | 48 | 3 | 5 | 4.4 | 27 | 4 | 5 | 4.6 |
| Branched anal-fin rays | 23 | 88 | 22 | 27 | 23.9 | 197 | 22 | 27 | 24.7 | 104 | 21 | 25 | 22.9 |
| Branched dorsal-fin rays | 8 | 29 | 8 | 8 | 8.0 | 48 | 7 | 9 | 8.0 | 26 | 8 | 8 | 8.0 |
| Branched pelvic-fin rays | 6 | 29 | 6 | 7 | 6.1 | 48 | 5 | 7 | 5.9 | 26 | 6 | 6 | 6.0 |
| Branched pectoral-fin rays | 10 | 29 | 9 | 11 | 10.0 | 48 | 9 | 1 1 | 9.7 | 26 | 9 | 11 | 10.0 |
| Principal caudal-fin rays | 19 | 29 | 19 | 19 | 19.0 | 48 | 17 | 20 | 19.0 | 25 | 19 | 19 | 19.0 |
| Lateral line perforated scales | 38 | 22 | 25 | 40 | 37.1 | 40 | 4 | 41 | 37.1 | 19 | 15 | 40 | 36.1 |
| Longitudinal scales at lateral line series | 38 | 22 | 37 | 40 | 38.2 | 39 | 37 | 41 | 38.5 | 19 | 38 | 40 | 38.4 |
| Scales between lateral line and dorsal-fin origin | 6 | 27 | 6 | 7 | 6.7 | 43 | 6 | 7 | 6.7 | 24 | 6 | 7 | 6.3 |
| Scales between lateral line and pelvic-fin origin | 4 | 29 | 4 | 5 | 4.1 | 42 | 4 | 6 | 4.6 | 25 | 4 | 5 | 4.4 |
| Number of predorsal scales | 15 | 16 | 11 | 15 | 13.0 | 27 | 11 | 16 | 13.1 | 9 | 11 | 15 | 13.0 |
| MCP |
MCP |
| USNM |
USA, Washington D.C., National Museum of Natural History, [formerly, United States National Museum] |
| MZUSP |
MZUSP |
| MNRJ |
Brazil, Rio de Janeiro, Sao Cristovao, Universidade do Rio Janeiro, Museu Nacional |
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Order |
|
|
Family |
|
|
Genus |