Scyliorhinus retifer ( Garman, 1881 )
|
publication ID |
https://doi.org/ 10.11646/zootaxa.4601.1.1 |
|
publication LSID |
lsid:zoobank.org:pub:8A695352-8382-458F-A86A-17A198F780CA |
|
persistent identifier |
https://treatment.plazi.org/id/03B94378-D035-0667-FF7D-F9AFFE53ABD5 |
|
treatment provided by |
Plazi |
|
scientific name |
Scyliorhinus retifer ( Garman, 1881 ) |
| status |
|
Scyliorhinus retifer ( Garman, 1881) View in CoL
( Figs. 19C View FIGURE 19 , 63–69 View FIGURE 63 View FIGURE 64 View FIGURE 65 View FIGURE 66 View FIGURE 67 View FIGURE 68 View FIGURE 69 , Tabs. 2 View TABLE 2 , 3 View TABLE 3 , 15)
Common names: chain dogfish ( United States).
Scyllium retiferum Garman, 1881: 233 View in CoL (original description, type locality: Delaware, United States).
Scylliorhinus retifer : Jordan & Gilbert, 1882: 869 –870 (catalogue, North America); Goode & Bean, 1896: 16 –17, pl. IV, figs. 14, 15 (brief account, Northwestern Atlantic).
Catulus retifer: Smith, 1907: 31 (catalogue, North Carolina); Garman, 1913: 76 –77 (brief account); White, 1937: 107, 117 (listed, systematics).
Catulus retifer : Jordan & Evermann, 1896: 25 (brief account, North America).
Scyliorhinus retifer: Springer & Sadowsky, 1970: 88 View in CoL –89 (taxonomic review, Western Central Atlantic); Cadenat & Blache, 1981: 182 –184, fig. 124a (catalogue, western Africa).
Scyliorhinus retifer: Regan, 1908: 457 View in CoL (listed, classification); Nichols, 1931: 38–39 (egg capsules); Bigelow & Schroeder, 1948: 207 –211, fig. 33 (brief account, North America); Bigelow & Schroeder, 1953: 34, fig. 9a (catalogue, Gulf of Maine); Bigelow, Schroeder & Springer, 1953: 214 (catalogue, Northwestern Atlantic and Gulf of Mexico); Springer, 1966: 602 –603, figs. 2, 5a, 6, 7g, 8 (taxonomic review, Northwestern Atlantic); Springer, 1979: 141 –142, fig. 93 (taxonomic review); Compagno, 1984: 364 –365 (FAO catalogue); Castro et al., 1988: 740 –746, figs. 1–15 (reproductive biology); Sminkey & Tabit, 1992: 251 –253 (reproductive biology); Compagno, 1999: 480 (listed); Kiraly et al., 2003: 16 (catalogue, Atlantic coast of United Stated); Moore et al., 2003: 167 –168 (catalogue, New England); Compagno et al., 2005: 251 –252, pl. 42 (compilation); Castro, 2011: 342 –345, figs. 8a–e (catalogue, North America); Kyne et al., 2012: 59 (catalogue, North America and Caribbean Sea); Ebert et al., 2013a: 374, 382, pl. 52 (compilation); Ebert & Stehmann, 2013: 209 –2010, figs. 240, 241 (FAO catalogue, North Atlantic); Weigman, 2016: 44 (listed).
Holotype. MCZ 825 View Materials , male, 311 mm TL (Delaware, U.S.A., 38° N 073° W). Additional material examined. 263 specimens (see Appendix ).
Diagnosis. Scyliorhinus retifer differs from all congeners by presenting a color pattern composed of dark lines forming geometric figures and bordering the saddles (vs. lines absent in all other species); light spots absent (vs. present in all other species, except S. cervigoni , S. garmani and S. meadi ); anterior nasal flaps not reaching the upper lip (vs. flaps reaching the upper lip, and sometimes covering it in S. canicula , S. cervigoni , S. comoroensis , S. duhamelii , S. garmani , and S. stellaris ); clasper with envelope medially expanded (vs. poorly developed or absent in other species, except S. boa and S. hesperius ); cover rhipidion with no dermal denticles (vs. denticles present in other, except in S. boa , S. cervigoni and S. hesperius ). The following combination of characters, although less conspicuous, also helps distinguish this species: nasoral grooves absent and anterior nasal flaps situated on the posterior border of excurrent apertures (vs. grooves present and flaps situated laterally in S. canicula and S. duhamelii ); mesonarial ridge not exceeding the posterior border of anterior nasal flaps (vs. exceeding in S. stellaris ); interdorsal distance greater than anal base (vs. smaller or similar to anal base in S. canicula , S. capensis , S. cervigoni , S. comoroensis , S. duhamelii , S. garmani , S. stellaris , and S. torazame ); oral canal of lateral line system with 8–10 pores (vs. 5–6 in S. hesperius ; 10–12 in S. duhamelii ; 9–13 in S. torrei ); commissural teeth with two cusplets (vs. one in S. cervigoni , S. torazame and S. torrei ; three or more in S. boa , S. canicula and S. hesperius ); pelvic apron extending to 2/3 of length of pelvic inner margins (vs. extending through almost entire length in S. canicula , S. capensis , S. duhamelii , S. torazame , and S. torrei ); clasper terminal dermal cover smooth (vs. rough in S. canicula and S. capensis ); terminal 3 cartilage present (vs. absent in other species, except in S. boa , S. canicula , S. capensis , and S. torazame ); dorsal terminal 2 cartilage elongated and rodlike, corresponding to 1/4 of dorsal terminal cartilage (vs. reduced and subtriangular in S. cabofriensis , S. capensis , S. cervigoni , S. haeckelii , and S. ugoi ; 1/3 of dorsal terminal cartilage in S. boa and S. comoroensis ; cartilages the same length in S. torazame ); neurocranium width across nasal capsules smaller than nasobasal length (vs. greater in S. meadi ); nasal capsule width 0.9–1.2 times its length (vs. 1.4 times in S. meadi ); counts of monospondylous vertebrae 37–43 (vs. 44–46 in S. capensis ; 35–37 in S. duhamelii ; 48 in S. garmani ; 46–48 in S. meadi ; 43–47 in S. stellaris ; 30–35 in S. torrei ); adult males between 395–580 mm TL and adult females 380–590 mm TL (vs. adult sizes greater than 600 mm TL in S. capensis , S. cervigoni , S. meadi , and S. stellaris ; 269 mm TL and 294 mm TL, respectively, in S. torrei ).
Description. Morphometric and meristic data are given in Table 15, and neurocranial measurements in Table 2 View TABLE 2 .
Body slender and cylindrical, tapering considerably posterior to cloaca ( Fig. 63 View FIGURE 63 ). Prepectoral length 0.4 times the prepelvic length. Trunk shorter than tail; snout-vent length 0.8–0.9 (0.8) times vent-caudal length. Pectoralpelvic space 1.3–1.5 (1.5) times the pelvic-anal space. Interdorsal space 1.7–2.8 (2.7) times the dorsal-caudal space ( Tab. 15). No interdorsal, postdorsal or postanal ridges; lateral crest on caudal peduncle absent.
Head moderately broad and depressed; head length 1.7 times head width ( Figs. 63 View FIGURE 63 , 64 View FIGURE 64 ). Snout relatively short, preoral length 0.6–0.7 (0.7) times mouth width and 0.7–0.8 (0.8) times smaller than preorbital length. Prenasal length 0.3–0.5 (0.6) times internarial space; preorbital length 0.6–1 (1.0) times interorbital space.
Eye large and slitlike, eye length 1.6–1.9 (2.3) times its height and 0.2 times smaller than head length ( Figs. 63 View FIGURE 63 , 64 View FIGURE 64 ). Eye dorsolateral on head, with lower edge medial to horizontal head rim in dorsal view; subocular ridge strong. Nictitating lower eyelid of rudimentary type, with shallow subocular pouch and secondary lower eyelid free from upper eyelid. Spiracle close behind but well separated from eyes, dorsolaterally on head and somewhat lower than level of eye notch. Spiracle diameter goes 4.1–5.8 (4.2) times in eye length and 8.1–10.6 (7.9) times in interorbital width.
First two gill openings about equally wide; first one twice as long as fifth. All gill openings slightly concave and not elevated on dorsolateral surface of head; gill filaments not visible externally.
Nostril with broad incurrent aperture, without nasoral groove or nasal barbel, and small and oval excurrent aperture. Anterior nasal flap large, triangular, covering posterior nasal flap and excurrent aperture, and extending just anterior to mouth, close to the upper lip but not touching it ( Figs. 65 View FIGURE 65 A–B). Mesonarial ridge distinct but not exceeding the posterior border of the anterior nasal flap. Posterior nasal flap rectangular, situated on the posterior border of the excurrent aperture. Mesonarial superior and inferior flaps triangular and corresponding to 1/3 of anterior nasal flap. Internarial space 0.8–0.9 (0.8) times smaller than interorbital space.
Mouth arched, moderately wide and short, its length goes1.5–1.9 (1.6) times in mouth width ( Figs. 65 View FIGURE 65 A–B). Lower labial furrow short and narrow, 2.9–3.1 (3.6) times smaller than mouth width. Dorsal labial cartilage 1.3 times the ventral cartilage; anterior tip of dorsal labial cartilage reaching the orbital process of the palatoquadrate. Tongue flat and rounded, light-colored, with oral papillae hardly detectable.
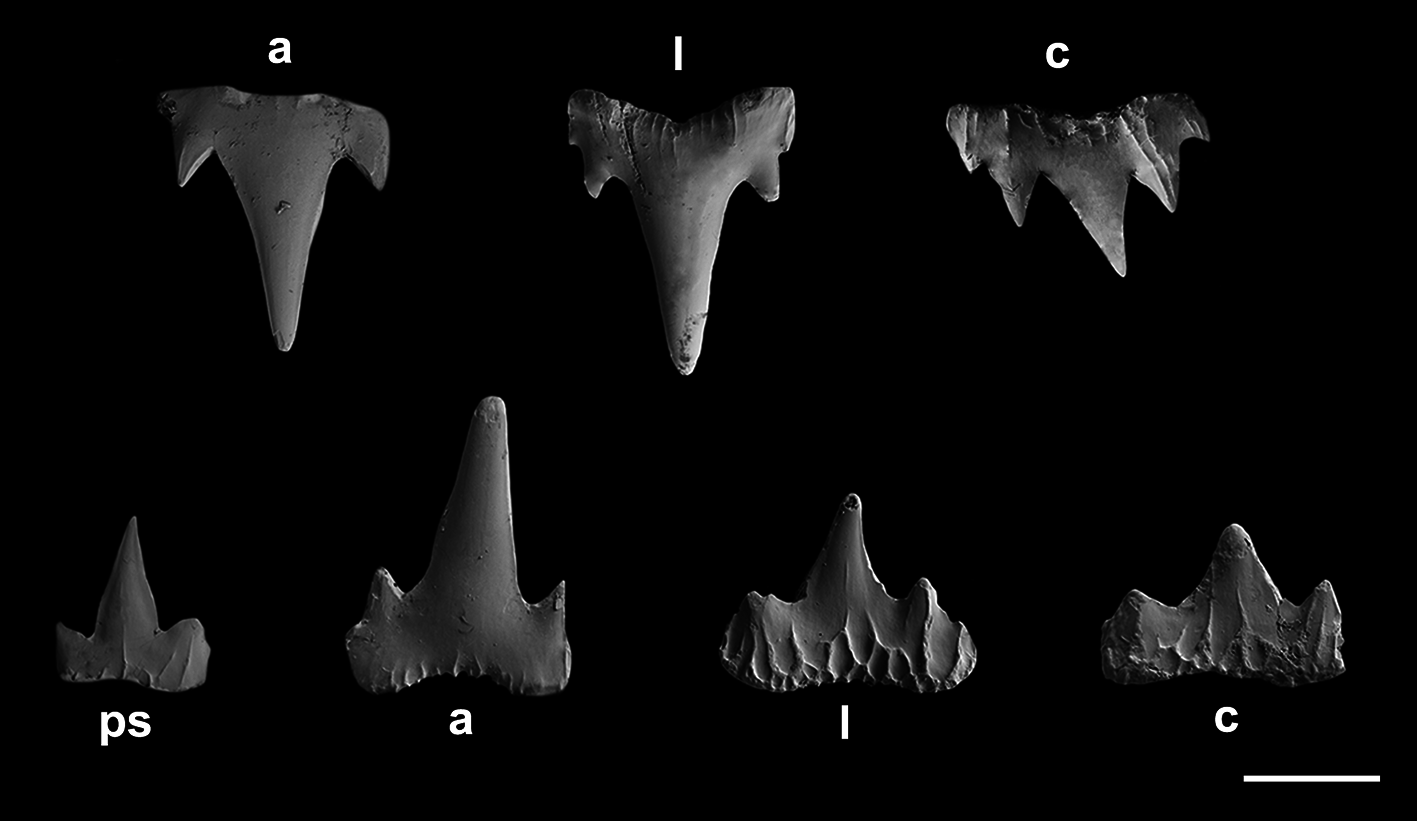
Monognathic heterodonty gradual well developed; anterior teeth abruptly larger than the parasymphysial ones and lateral teeth smaller distally, with smaller and thicker principal cusps ( Fig. 66 View FIGURE 66 ). Sexual heterodonty well pronounced, with females presenting anterior teeth with lower principal cusp and well-developed cusplets in relation to males. Tooth counts 18–26 18–28/ 17–25 1 16 –24 (20–21/ 19–1–20). Parasymphysial teeth with a principal cusp flanked by one cusplet on each side; cusplets half the height and the width of the principal cusp. Protuberances on medial portion of the crown base and striae restricted to the crown base. Anterior teeth larger than the parasymphysial and principal cusp less stout. Anterior teeth with four cusplets; marginal cusplets corresponding to half the height of proximal cusplets in males and 2/ 3 in females. Proximal cusplets corresponding to less than half the height and the width of the principal cusp in both sexes. Protuberances on the crown base; striae restricted to the crown base in males and extending through more than half the height of the principal cusp in females. Lateral teeth with four cusplets; marginal cusplets 2/3 the height of proximal cusplets. Protuberances on the crown base; striae more prominent and running from base toward the apex of principal cusp in females, and poorly developed and reduced in males. Commissural teeth with two cusplets; principal cusp stronger and laterally situated. Cusplets corresponding to 2/3 the width and the height of the principal cusp. Protuberances present and striae extending to half the height of the principal cusp. Ectodermal pits present in lateral and commissural teeth, restricted to the crown base.
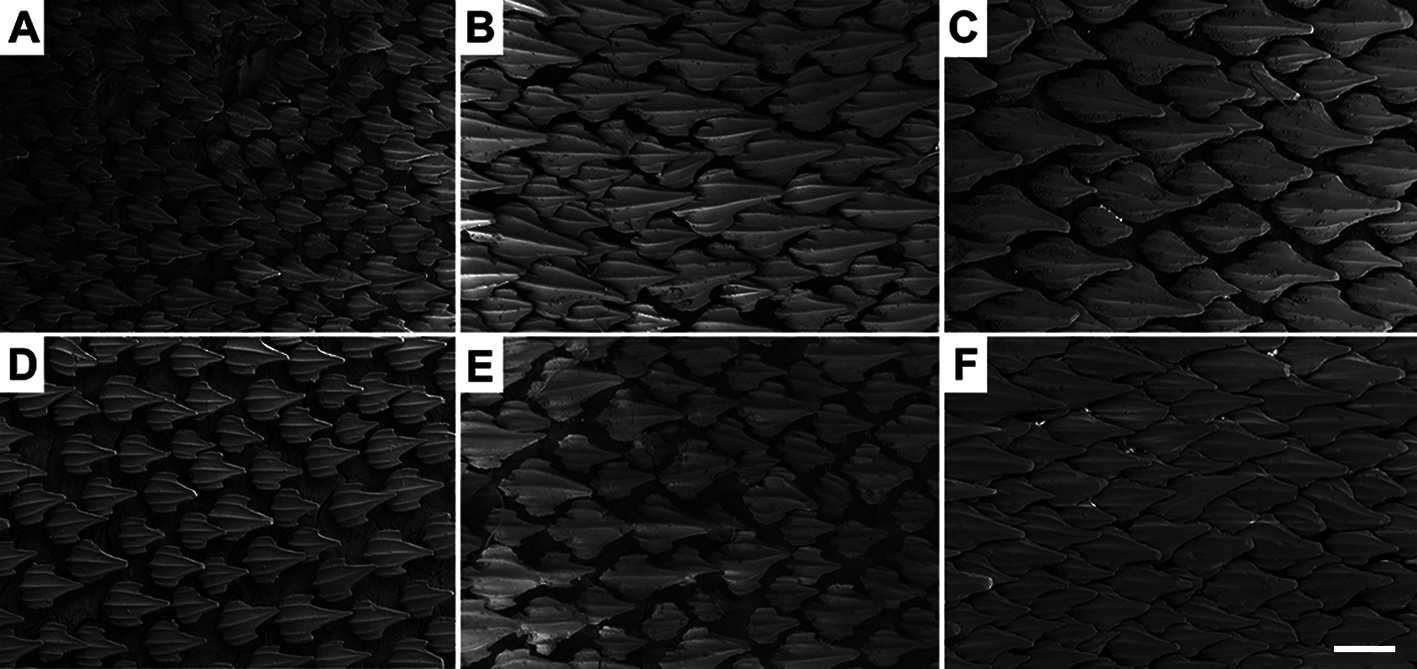
Lateral trunk denticles with flat, elongated teardrop-shaped crowns, 1.6–2.2 times as long as wide ( Tab. 3 View TABLE 3 ); anterior part covered with ectodermal pits. Dermal denticles above the pectoral fin presenting five ridges, median ridge less prominent than in denticles of other regions; lateral ridges not extending until the intersection between principal cusp and cusplets. Denticles below dorsal fins longer and presenting median and lateral ridges prominent, extending until the distal tip of cusplets; projected base with one or two ridges restricted to it and medial to lateral ridges. Marginal ridges present in denticles of all regions ( Fig. 67 View FIGURE 67 ).
Pectoral base 0.9–1.1 (0.9) times mouth width ( Fig. 65C View FIGURE 65 ). Pectoral anterior margin 1.5–2.2 (2.2) times its base and 1.2–1.6 (1.2) times the posterior margin. Pectoral fin skeleton aplesodic with radials mostly divided into three segments. Propterygium and mesopterygium trapezoids; former smaller than latter. Propterygium with one proximal segment; mesopterygium with 3–4 proximal segments fused proximally. Metapterygium with 8–9 segments. Metapterygial axis rectangular and corresponding to 1/4 of metapterygium.
Pelvic fin subtriangular ( Fig. 65F View FIGURE 65 ); pelvic anterior margin 1–1.4 (1.1) times the posterior margins and 1–1.4 (1.1) times the pelvic base. Pelvic inner margins of males fused for 2/3 of their extension; claspers of juveniles evident without lifting the pelvic apron.
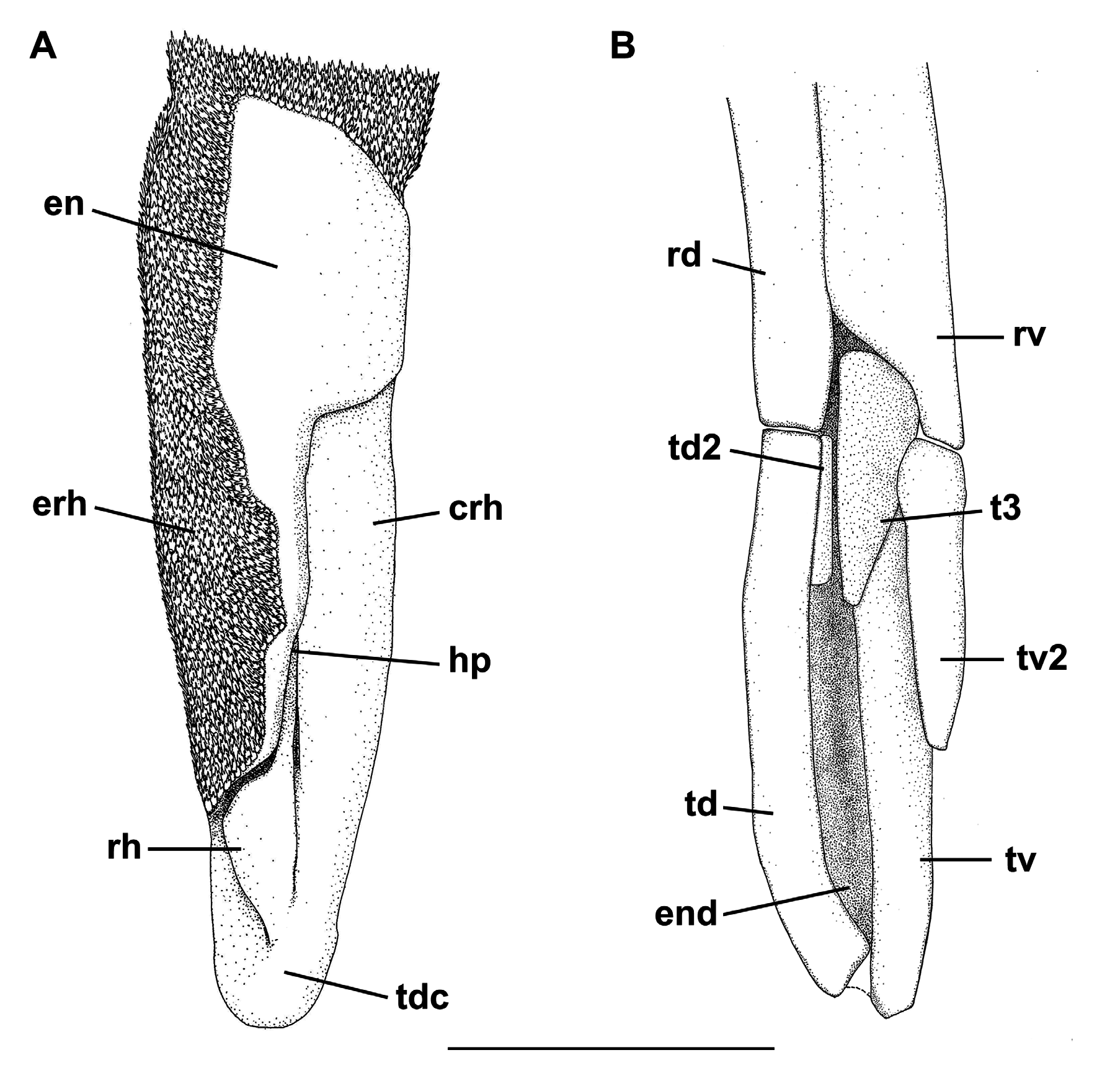
Clasper short and cylindrical ( Fig. 65F View FIGURE 65 ), sometimes extending beyond free rear tips of pelvic fins; clasper inner length 0.9–1.2 (1.1) times the pelvic anterior margin, 1.3–1.6 (2.2) times the clasper outer length and 4.7–5.1 (5.4) times the clasper base. Most of clasper surface except dorsomedial surface of glans, envelope, cover rhipidion, rhipidion, and terminal dermal cover, covered by dermal denticles with anteriorly directed crowns ( Fig. 68A View FIGURE 68 ). Clasper hooks absent. Rhipidion well-developed, partly covered medially by a prominent exorhipidion and anteriorly by the cover rhipidion; insertion of rhipidion at anterior portion of dorsal terminal 2 cartilage and extending to the end of glans. Cover rhipidion nearly straight, reaching medially a slightly posterior exorhipidion; both cover rhipidion and exorhipidion covering the clasper groove. Envelope expanded medially and covering the anterior border of the cover rhipidion; pseudosiphon distinct and strong. Terminal dermal cover extending for 1/3 of the clasper glans and covering the distal tips of cover rhipidion and exorhipidion.
Clasper skeleton relatively simple ( Fig. 68B View FIGURE 68 ). Ventral terminal cartilage beginning anteriorly, but ending together with the dorsal terminal. Terminal 3 cartilage rodlike and medially situated on the ventral terminal cartilage; corresponding to 3/4 of ventral terminal 2 cartilage. Dorsal terminal 2 cartilage elongated and rod-like, medially positioned on the dorsal terminal cartilage; this cartilage supports the rhipidion and corresponds to 1/4 of the length of dorsal terminal cartilage. Ventral 2 cartilage similar in shape to terminal 3 cartilage and corresponding to half-length of ventral terminal cartilage; ventral terminal 2 cartilage beginning slightly anterior to dorsal terminal 2.
First dorsal fin triangular or square-tipped, with nearly straight anterior margin, rounded apex and angular free rear tip ( Fig. 63 View FIGURE 63 ). First dorsal fin origin opposite or slightly posterior to pelvic fin insertion in females and opposite to one half length of pelvic inner margins in males. First dorsal fin insertion opposite to the anterior 1/3 of pelvicanal distance. Anterior margin 1.1–1.4 (1.5) times first dorsal fin base; first dorsal fin height 0.5–0.7 (0.7) times its base.
Second dorsal fin smaller than the first and triangular ( Fig. 63 View FIGURE 63 ). Second dorsal fin origin opposite to the posterior 2/5 of anal base or opposite to the anal fin insertion. Second dorsal fin insertion posterior to distal tip of anal fin. Anterior margin 1.2–1.3 (1.3) times base of second dorsal fin; second dorsal base 1.5–1.8 (1.9) times its height and 1.2–1.9 (1.9) times the dorsal-caudal distance. First dorsal fin 1.1–1.3 (1.3) times larger than the second dorsal fin.
Anal fin triangular, apically narrow and not falcate ( Fig. 63 View FIGURE 63 ); anal fin base 1.5–1.7 (1.4) times the second dorsal fin base. Anal fin anterior margin nearly straight, apex narrowly rounded, free rear tip acutely pointed, and inner margin straight. Anal fin base 1.1–1.2 (1.1) times the interdorsal distance and 1.7–3.2 (2.9) times the dorsalcaudal distance. Anal fin anterior margin 1.4–2.1 (2.2) times the posterior margin; anal fin height 0.2–0.4 (0.4) times its base.
Caudal fin narrow-lobed and asymmetrical ( Fig. 63 View FIGURE 63 ). Dorsal caudal lobe 1.2–1.3 (1.5) times larger than preventral lobe; subterminal caudal margin 1–1.2 (1.3) times the terminal margin. Caudal crest of enlarged denticles absent on caudal fin margins.
Neurocranium broad and somewhat flattened, corresponding to 8.8–10.6% TL. Rostrum length 1.5–1.7 times the distance between lateral rostral cartilages in females and 1.9–2.4 times in males. Nasal capsule wider than long, oval-shaped and expanded laterally; width 0.9–1.2 times its length. Width across nasal apertures proportionately greater in females (24–31.3%NL) than in males (22.8–23.6% NL); anterior fontanelle width proportionately greater in females (22.7–28.1% NL) than in males (17.5–18.6% NL). Orbital region 2.1–2.3 times smaller than nasobasal length. Otic capsule short, its length 4.3–5.8 times smaller than nasobasal length and width 2.3–3.2 times otic capsule length. Width across postorbital processes 1.1–1.2 times the preorbital processes width ( Tab. 2 View TABLE 2 ).
Coloration in alcohol. Holotype with body cream and light brown lines, faded and with little contrast in relation to the background color ( Fig. 63 View FIGURE 63 ). Other specimens presenting a body cream to beige with seven rectangular saddles, bordered by dark brown to black lines ( Fig. 64 View FIGURE 64 ); lines forming geometric figures inside the saddles (rectangles, diamonds and hexagons). Lines not very distinct in region anterior to spiracles; triangles situated anterior to eyes. Light brown lines, crossed by narrow black stripes, uniting with other lines and forming a web of polygons. Unique dark line between predorsal and first dorsal saddles and interdorsal and second dorsal saddles; one line between spiracles. Subsaddles between saddles anterior to the first dorsal fin. Individual variation in relation to geometric patterns present, mainly in the anteriormost saddles (branchial, pectoral and predorsal). Dark or light spots usually absent; when present, spots dark and greater than spiracles, continuous to lines. Belly and ventral surface of paired and anal fins without lines, cream in color.
Distribution. This species is widely distributed, known to occur from the Gulf of Mexico, from off Campeche, Mexico (95°W) to Florida (83°W), to the northeast coast of United States, from Georgia (30°N) to Connecticut (41°N) ( Fig. 69 View FIGURE 69 ).
Biological data. Adult males examined 395–580 mm TL; largest male examined 513 mm TL. Adult females between 380–590 mm TL; largest female examined 520 mm TL. Specimens from northern regions present greater sizes at maturity than those from southern regions ( Castro et al. 1988; Sminkey & Tabit 1992). Maximum size recorded 590 mm TL. Newly born specimens between 100–110 mm TL.
Females can store sperm through extended periods, producing viable eggs without males for two years or more. Egg capsules deposited in pairs in 8 to 15 days in captivity, during spring and summer, about 44 to 52 eggs per year ( Castro et al. 1988; Compagno et al. 2005). Castro et al. (1988) presents descriptions of the mating process and embryonic development. Egg capsules amber to dark brown with golden edges ( Fig. 19C View FIGURE 19 ). Anterior edge nearly straight and wider with strong tendrils on lateral sides; tendrils crossed at posterior edge. Longitudinal lines along the egg capsule present; lateral walls reinforced and without striae. Mean values of egg capsule dimensions: 51.9 mm in length, 21.6 mm in width, and 10.2 mm in height (n = 11).
Stomach contents include squid beaks, bony fishes, polychaetas, annelid worms and crustaceans. A preponderance of squid remains suggests that, even presenting a sluggish habit, this species is probably an ambush predator ( Ford 1921).
This species is a benthic dweller in depths of 73– 550 m. It is apparently more abundant in deeper waters off Virgina and North Carolina; specimens have been captured in shallower waters (50–220 m) in the northern regions and in deeper waters (230–450 m) in Cape Hatteras. In comparison with males, females have been recorded in deeper waters in the north (91–457 m vs. 38–405 m for males) and in shallower waters at the south (73–512 m vs. 239–795 m for males). Adults prefer rocky bottoms and juveniles remain on sandy bottoms. This species presents no interest to fisheries. Its distribution seems to be irregularly interrupted in areas where adults are rare; its preference for rough bottom habitats is generally considered by fishermen as not suited for trawling. Egg laying may be concentrated in specifically limited nursery areas. This species occurs in temperatures 8.5° to 11.3° C ( Springer 1979; Ebert & Stehmann 2013). Species frequently exhibited in aquariums of the United States. Conservation status ‘Less concern’ with increasing population trend (Sherrill-Mix et al. 2006).
Etymology. The specific name ‘retifer’ refers to its unique reticulated color pattern.
Remarks. Scyliorhinus retifer presents a reticulated color pattern, similar to Cephaloscyllium fasciatum . It is distinguished from C. fasciatum by presenting a flap on upper lip margin that is laterally projected and covers the lower labial furrow (vs. absent in C. fasciatum ), postoral groove absent (vs. present), dorsal fins more posteriorly situated, and males with pelvic apron extending to 2/3 of length of pelvic inner margins (vs. pelvic apron absent).
The type locality given by Springer (1979), as situated in Virginia, consisted in an error as noted by Hartel & Dingerkus (1997); mapping of the geographical coordinates points to the type locality being Delaware as related by Garman (1913). Records for the San Andrés Archipelago, Providencia, and Catalina in the Western Colombian Caribbean, probably refer to S. boa ( Bolaños-Cubillos et al. 2015) . Material previously identified as a subspecies of S. retifer ( Springer & Sadowsky 1970) was examined and reidentified by us, updating data on the geographic distribution of this species (see Appendix). Thus, S. retifer is restricted to the eastern coast of United States and Gulf of Mexico, with its southernmost record the southwestern coast of Mexico (18°N), not overlapping with S. boa and S. hesperius .
TABLE 15. Morphometric and meristic data of Scyliorhinus retifer. SD, standard deviation; n, number of examined specimens. Total length (TL) in mm, other measurements as percentages of TL.
| Characters | Holotype | n | Range | Mean | SD |
|---|---|---|---|---|---|
| Total length (TL) | 310.9 | 96.0—537.0 | 305.2 | 84.4 | |
| Precaudal length | 74.0 | 256 | 71.3–89.0 | 75.3 | 2.1 |
| Eye-spiracle length | 1.2 | 254 | 0.4–1.5 | 1.1 | 0.2 |
| Prenasal length | 3.1 | 256 | 1.4–4.7 | 3.2 | 0.5 |
| Preoral length | 5.3 | 254 | 2.5–7.8 | 5.2 | 0.6 |
| Preorbital length | 6.9 | 253 | 3.2–11.0 | 7.2 | 0.6 |
| Prespiracular length | 11.1 | 254 | 4.9–16.0 | 11.1 | 0.8 |
| Prebranchial length | 16.0 | 256 | 13.3–20.9 | 16.1 | 1.1 |
| Head length | 20.7 | 256 | 19.2–28.4 | 20.8 | 1.3 |
| Prepectoral length | 18.3 | 253 | 17.4–23.6 | 18.8 | 2.0 |
| Prepelvic length | 42.2 | 256 | 35.4–45.5 | 42.1 | 3.1 |
| Snout-vent length | 43.1 | 256 | 42.5–48.6 | 44.2 | 3.9 |
| Vent-caudal length | 56.7 | 253 | 45.6–54.8 | 56.7 | 3.3 |
| Pre-first dorsal length | 47.3 | 256 | 40.5–56.2 | 48.9 | 4.1 |
| Interdorsal distance | 10.1 | 256 | 6.1–13.0 | 10.6 | 1.1 |
| Dorsal-caudal distance | 3.7 | 253 | 2.2–7.5 | 4.4 | 1.3 |
| Pectoral-pelvic distance | 16.7 | 254 | 9.0–12.0 | 16.8 | 2.0 |
| Pelvic-anal distance | 11.1 | 256 | 7.8–15.3 | 11.3 | 1.5 |
| Anal-caudal distance | 5.7 | 256 | 2.6–9.5 | 7.0 | 1.0 |
| Interorbital distance | 7.1 | 256 | 5.3–10.6 | 7.5 | 0.8 |
| Internarial distance | 5.6 | 256 | 4.1–9.4 | 5.6 | 0.6 |
| Mouth length | 5.0 | 256 | 2.2–6.7 | 5.2 | 0.5 |
| Mouth width | 7.5 | 256 | 4.1–10.4 | 8.3 | 0.8 |
| Lower labial furrow length | 2.1 | 256 | 1.4–3.4 | 2.2 | 0.3 |
| Eye length | 3.8 | 254 | 2.9–5.4 | 4.0 | 0.5 |
| Eye height | 1.6 | 256 | 1.5–3.4 | 1.6 | 0.3 |
| Spiracle length | 0.9 | 256 | 0.5–1.3 | 1.0 | 0.2 |
| First gill slit height | 2.8 | 256 | 1.8–4.1 | 2.9 | 0.4 |
| Fifth gill slit height | 1.5 | 256 | 0.7–2.1 | 1.5 | 0.2 |
| Pectoral length | 14.3 | 256 | 9.9–16.9 | 13.5 | 1.1 |
| Pectoral anterior margin | 15.2 | 256 | 6.9–18.4 | 14.5 | 1.4 |
| Pectoral base | 6.9 | 256 | 4.7–9.0 | 6.8 | 0.6 |
| Pectoral posterior margin | 7.4 | 256 | 5.5–11.4 | 8.2 | 1.2 |
| Pectoral inner margin | 7.1 | 254 | 3.5–8.2 | 6.5 | 0.8 |
| Pelvic length | 12.0 | 256 | 6.9–14.4 | 11.5 | 1.3 |
......continued on the next page
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |
Scyliorhinus retifer ( Garman, 1881 )
| Soares, Karla D. A. & De, Marcelo R. 2019 |
Scyliorhinus retifer:
| Cadenat, J. & Blache, J. 1981: 182 |
| Springer, S. & Sadowsky, V. 1970: 88 |
Scyliorhinus retifer: Regan, 1908 : 457
| Ebert, D. A. & Stehmann, M. 2013: 209 |
| Kyne, P. M. & Carlson, J. K. & Ebert, D. A. & Fordham, S. V. & Bizzarro, J. J. & Graham, R. T. & Kulka, D. W. & Tewes, E. E. & Harrison, L. R. & Dulvy, N. K. 2012: 59 |
| Castro, J. I. 2011: 342 |
| Compagno, L. J. V. & Dando, M. & Fowler, S. 2005: 251 |
| Kiraly, S. J. & Moore, J. A. & Jasinsky, P. H. 2003: 16 |
| Moore, J. A. & Hartel, K. E. & Craddock, J. E. & Galbraith, J. K. 2003: 167 |
| Compagno, L. J. V. 1999: 480 |
| Sminkey, T. R. & Tabit, C. R. 1992: 251 |
| Castro, J. I & Bubucis, P. M. & Overstrom, N. A. 1988: 740 |
| Compagno, L. J. V. 1984: 364 |
| Springer, S. 1979: 141 |
| Springer, S. 1966: 602 |
| Bigelow, H. B. & Schroeder, W. C. & Springer, S. 1953: 214 |
| Bigelow, H. B. & Schroeder W. C. 1948: 207 |
| Regan, C. T. 1908: 457 |
Catulus retifer: Smith, 1907 : 31
| White, E. G. 1937: 107 |
| Garman, S. 1913: 76 |
| Smith, H. M. 1907: 31 |
Catulus retifer
| Jordan, D. S. & Evermann, B. W. 1896: 25 |
Scylliorhinus retifer
| Goode, S. B. & Bean, T. H. 1896: 16 |
| Jordan, D. S. & Gilbert, C. H. 1882: 869 |
Scyllium retiferum
| Garman, S. 1881: 233 |