Perigona (Neoperigona) spelunca, Pellegrini & Ferreira & Vieira, 2022
|
publication ID |
https://doi.org/ 10.5852/ejt.2022.806.1707 |
|
publication LSID |
lsid:zoobank.org:pub:EDD27920-FFF5-40C8-8EA5-85B079CB5BBA |
|
DOI |
https://doi.org/10.5281/zenodo.6406211 |
|
persistent identifier |
https://treatment.plazi.org/id/7E95F064-CE59-4043-AA2F-EB6DEC2BED47 |
|
taxon LSID |
lsid:zoobank.org:act:7E95F064-CE59-4043-AA2F-EB6DEC2BED47 |
|
treatment provided by |
Felipe |
|
scientific name |
Perigona (Neoperigona) spelunca |
| status |
sp. nov. |
Perigona (Neoperigona) spelunca sp. nov.
urn:lsid:zoobank.org:act:7E95F064-CE59-4043-AA2F-EB6DEC2BED47
Figs 1–5 View Fig View Fig View Fig View Fig View Fig
Diagnosis
The new species is a Perigona (Neoperigona) ranges in size from 3.72 to 4.42 mm, is microphthalmous and brachypterous, with long antennae, also characterized by a smooth and glabrous surface. The pronotum is transverse; its margins sinuate before hind angles. The elytra are coarctate, curved laterally and slightly lengthened, stria almost completely obsolete. Elytra fixed setae represented by one basal seta at the beginning of the second stria, and one preapical discal setae at the end of the junction of the 4 th and 5 th striae of the elytra, umbilicate series of the 8 th stria with 15 setae arranged linearly and not divided in three groups.
Differential diagnosis
Perigona (Neoperigona) spelunca sp. nov. differs from all other Perigona in the following set of characters: dorsal surface smooth and glabrous; brachypterous; microphthalmous with depigmented eyes; elytra with only two discal setae, a basal one and a preapical one inserted on the ends of the 4 th and 5 th striae; median group of umbilicate series of the eighth stria arranged linearly, without an evident separation among the three groups of umbilicate series.
Etymology
The specific epithet refers to the Latin word ‘ spelunca ’ meaning ‘cave’.
Type material
Holotype BRAZIL • 1 ♂; Minas Gerais State, Pains Municipality, Gruta do Éden cave ; 20°23ʹ5ʺ S, 45°39ʹ59ʺ W; 4 Aug. 2012; P. Ratton et al. leg; ISLA 66143 . GoogleMaps
Paratypes BRAZIL • 1 ♀, entire abdomen was removed for genitalia dissection; same collection data as for holotype; 27 Aug. 2009; R.A. Zampaulo et al. leg.; ISLA 363 GoogleMaps • 1 ♂; same collection data as for holotype; 27 Aug. 2009; R.A. Zampaulo et al. leg.; ISLA 360 GoogleMaps • 1 ♂, left proleg is broken; same collection data as for holotype; 4 Aug. 2012; P. Ratton et al. leg.; ISLA 66142 GoogleMaps • 1 ♂; same collection data as for holotype; 27 Aug. 2009; R.A. Zampaulo et al. leg.; ISLA 73707 GoogleMaps • 1 ♂; same collection data as for holotype; 22 Apr. 2012; P. Ratton et al. leg.; ISLA 75679 GoogleMaps .
Additional material
BRAZIL • 1 ♀; Minas Gerais State, Pains Municipality , Gruta do Zezinho Beraldo cave; 20°21ʹ25.04ʺ S, 45°50ʹ4.68ʺ W; 21 Jan. 2009; R.A. Zampaulo et al. leg.; ISLA 359 GoogleMaps • 1 ♀; Minas Gerais State, Pains Municipality , Gruta Santuário cave; 20°25ʹ11.61ʺ S, 45°46ʹ27.25ʺ W; 20 May 2006; T.O. do Carmo et al. leg.; ISLA 65792 GoogleMaps • 1 ♂; Minas Gerais State, Pains Municipality , Gruta Cinderela cave; 20°26ʹ44ʺ S, 45°36ʹ22ʺ W; 18 Sep. 2009; R.A. Zampaulo et al. leg.; ISLA 354 GoogleMaps • 1 ♂; Minas Gerais State, Pains Municipality , Gruta da Fazenda Amargoso Cave 20°23ʹ53.63ʺ S, 45°35ʹ38.34ʺ W; 29 Jan. 2009; R.A. Zampaulo et al. leg.; ISLA 353 GoogleMaps • 1 ♂; Minas Gerais State, Pains Municipality , Gruta Serro Azul Cave; 20°22ʹ31.97ʺ S, 45°38ʹ48.35ʺ W; 30 Jun. 2009; R.A. Zampaulo et al. leg.; ISLA 361 GoogleMaps • 1 ♂; Minas Gerais State, Pains Municipality , Gruta do Brega cave; 20°25ʹ4.37ʺ S, 45°46ʹ20.16ʺ W; 5 Jul. 2012; R.L. Ferreira et al. leg.; ISLA 65795 GoogleMaps • 3 ♂♂, 1 ♀; Minas Gerais State, Pains Municipality , Gruta do Santuário cave; 26 Aug. 2015; R.L. Ferreira et al. leg.; ISLA 65792 • 2 ♂♂; Minas Gerais State, Pains Municipality , S1- GEC-035 Cave ; 20°23ʹ26.1ʺ S, 45°35ʹ54.6ʺ W; 18 Aug. 2017; L.G.S. Soares leg., ISLA 47701 GoogleMaps • 2 ♀♀; same locality data as for preceding; 23 Jan. 2018; L.G.S. Soares leg.; ISLA 47702 GoogleMaps .
Type locality
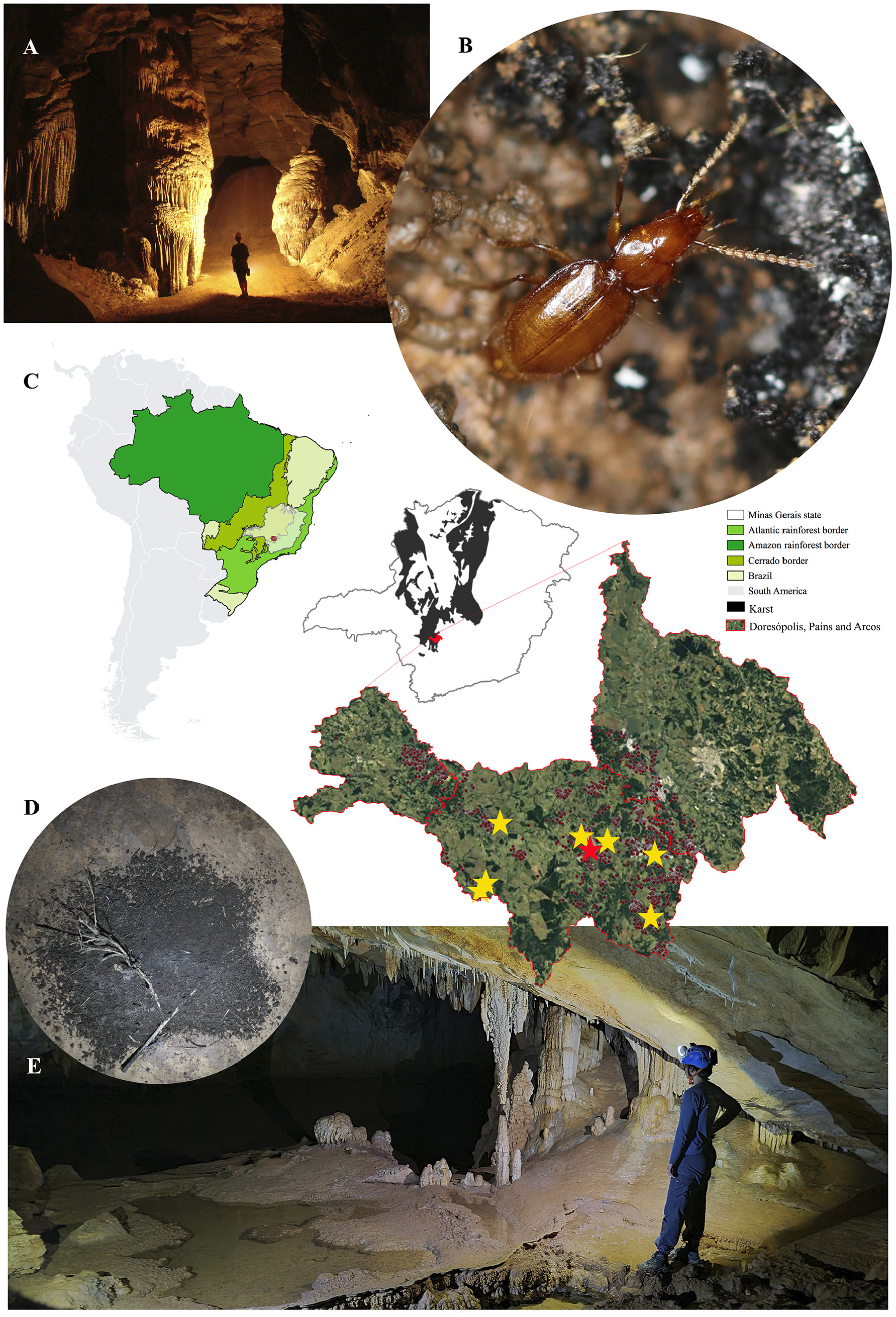
The holotype was collected in the Gruta do Éden cave (20°23ʹ5ʺ S, 45°39ʹ59ʺ W), located in Pains municipality in Minas Gerais state, Brazil ( Fig. 5 View Fig ).
Description
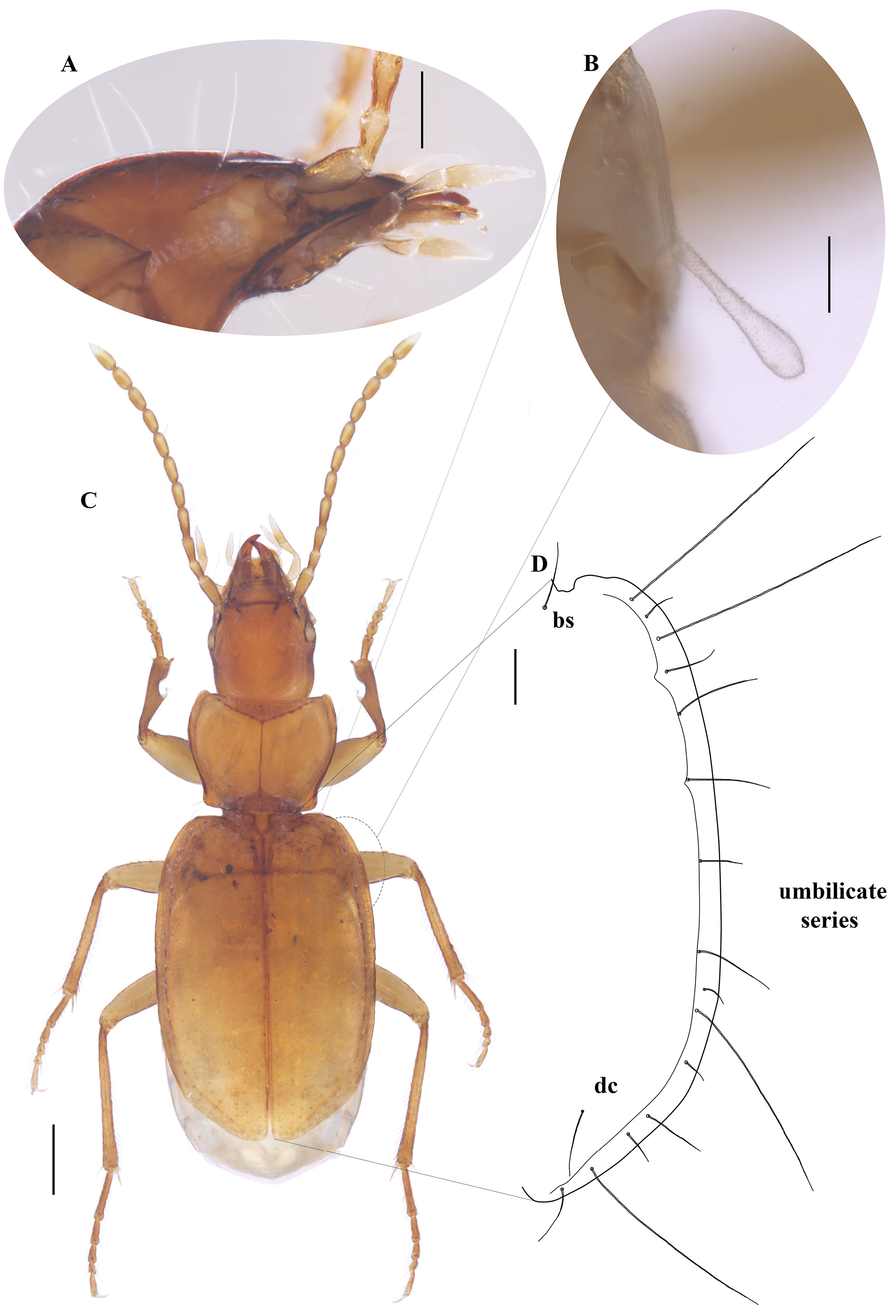
Holotype male ( Fig. 1 View Fig ). OBL: 4.20 mm; EW: 1.53 mm; HW/PW: 0.72. Body coloration orangish brown ( Fig. 1C View Fig ). Dorsal integument glabrous, with vanished microsculpture transversally on pronotum and vertically on the elytra.
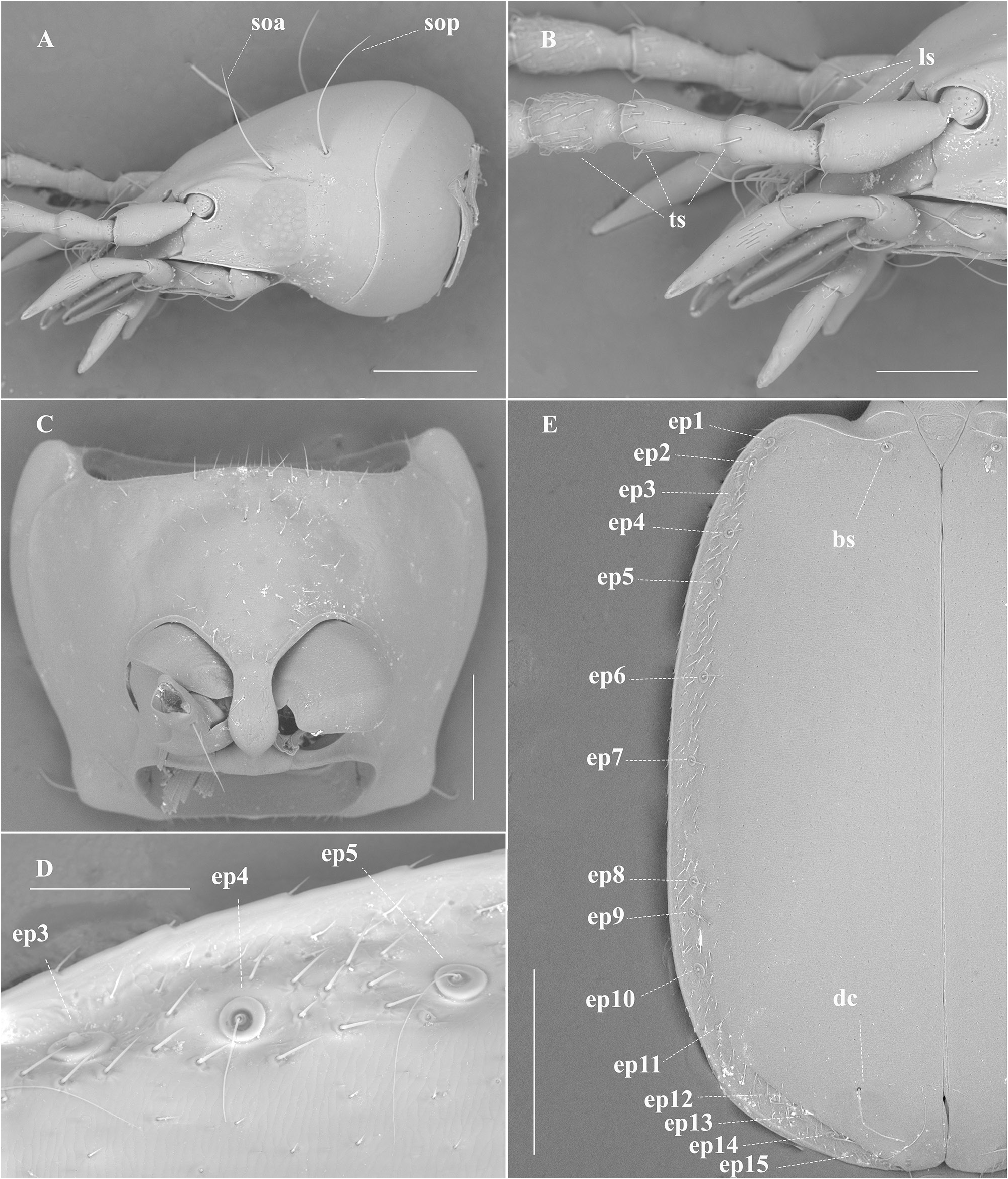
HEAD. Head relatively large ( Figs 1–2 View Fig View Fig ), HL/HW: 1.44; HL: 1.10 mm, and narrower than pronotum. Eyes reduced, flattened and depigmented, with vestigial eyes scars, situated laterally at the end of the genal sulcus ( Fig. 1A View Fig ), with two supraorbital setae on each side ( Fig. 2A View Fig ). Frons slightly depressed. Anterior margin of the clypeus subrectilinear, with two setae on the anterior angles. Labrum with a subrectilinear front margin and with 6 setae, with the larger ones on the side edges becoming smaller inward. Antennae stocky, relatively long and exceeding the base of the elytra ( Fig. 1C View Fig ) AL: 2.01 mm, AL/OBL: 0.48. First antennomere (scape) with a long seta distally close to the apical portion, and a row of several semi-erect trichoid setae; 2 nd short; 2 nd and 3 rd with semi-erect trichoid setae ( Fig. 2B View Fig ). Segments 4 th –11 th subequal, pubescent with semi-erect setae distally, and almost round in cross-section ( Fig. 2B View Fig ).
PRONOTUM. Shape trapezoidal and slightly transverse, it sinuates before hind angles, PW/PL: 1.31 (PL: 0.79) ( Fig. 1C View Fig ). Maximum width approximately in the anterior third and wider than the head. Anterior angle rounded; posterior angle also rounded, narrowed towards base, which is slightly narrowed than the anterior edge. Dorsal surface with two pairs of lateral marginal erect setae: one longer at the anterior third and the other shorter, close to the posterior-lateral angles. Ventral surface with a fine pubescence distributed in the middle portion of the prosternum ( Fig. 2C View Fig ).
ELYTRA ( Figs 1C–D View Fig , 2D–E View Fig ). Elytra coarctate, curved laterally and slightly lengthened EL/EW: 1.51 (EL: 2.31). Maximum width in the middle. Presence of an entire basal groove from the humeri to the scutellum. Humeri indicated but not rounded; post-humeral margin with a fine and delicate pubescence restricted to the 8 th stria interval; apex of elytra is slightly angular, presenting a small break that coincides with the height of the discal setae, giving a small closure to the angle towards the junction of the apex of the elytra. ( Fig. 1C–D View Fig ). Marginal groove wide and distinct until the apical border. Elytral chaetotaxy: one basal seta at the beginning of the second stria; and one preapical discal inserted at the end of the 5 th stria interval; Umbilicate series of the 8 th stria with 15 setae arranged linearly and not divided in three groups, of these 4 setae (1 st and 3 rd humeral, 10 th lateral and 14 th distal) are quite long (0.86 mm long), going beyond the end of the femur ( Figs 1D View Fig , 2D–E View Fig )
HIND WINGS. Brachypterous, HWL: 0.271, EL/HWL: 8.16 ( Fig. 1B View Fig ).
LEGS. Long and slender, protarsi with 2 nd, 3 rd and 4 th segments dilated in the male ( Fig. 1C View Fig ).
ABDOMEN. Abdominal tergites with a very short and fine pubescence; 3 nd, 4 th and 5 th tergites with one pair ventral of setae at its posterior margin; male sixth sternite with two pairs of ventral setae at its posterior margin and female sixth sternite with four pairs of ventral setae at its posterior margin.
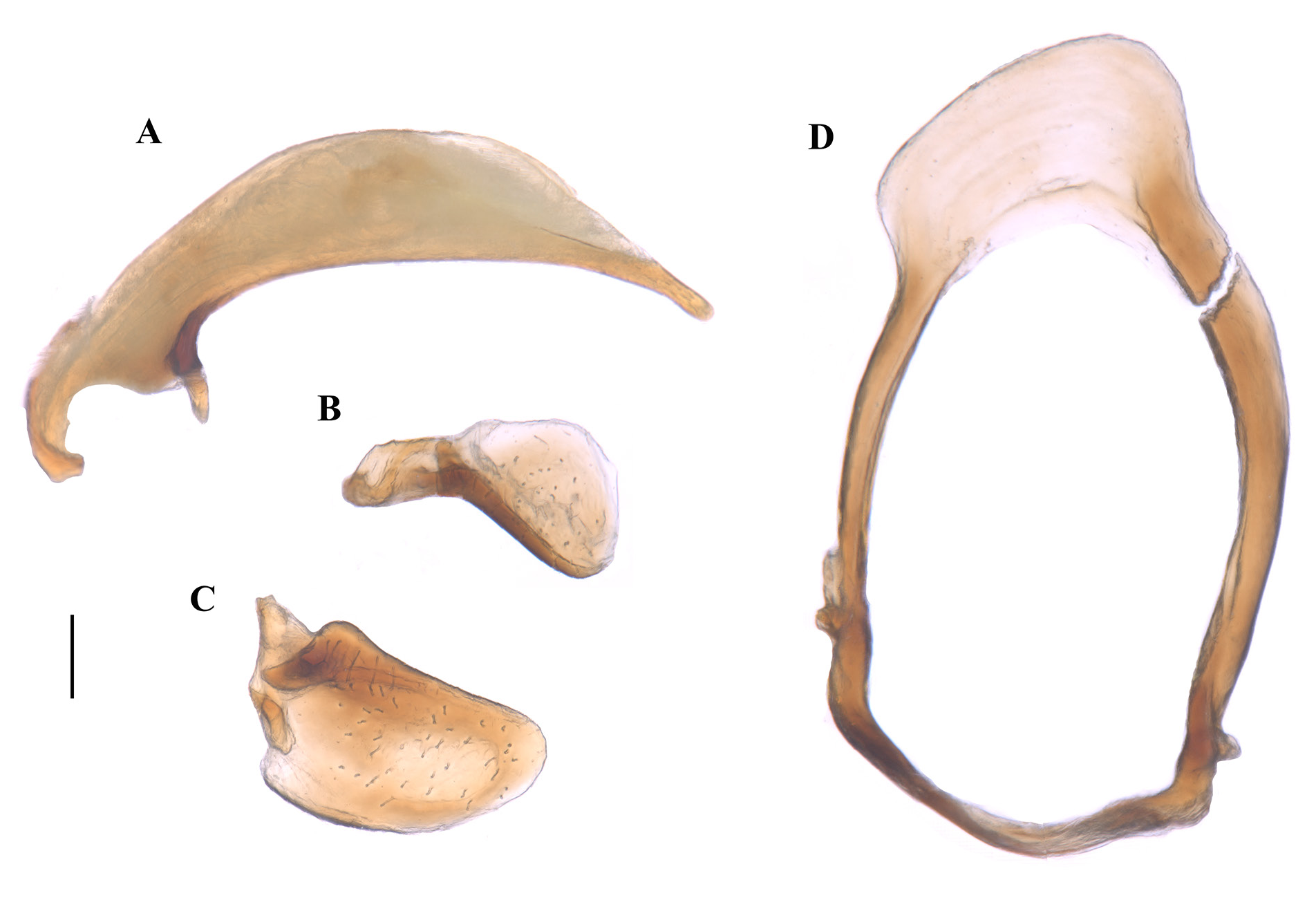
MALE GENITALIA ( Fig. 3 View Fig ). Phallus (i.e., median lobe of the aedeagus) elongate and narrow (PAL: 0.89; PAW/PAL: 0.22), slightly curved ventrally, tip narrowed and rounded ( Fig. 3A View Fig ). Parameres short and robust ( Fig. 3B–C View Fig ), the left is oval and widened in the basal third; the right is smaller, with a thinner base and a convex apex in the shape of a spoon. Male gonosomite ovoid and elongated, two apophyses in the distal part in the form of small hooks ( Fig. 3D View Fig ).
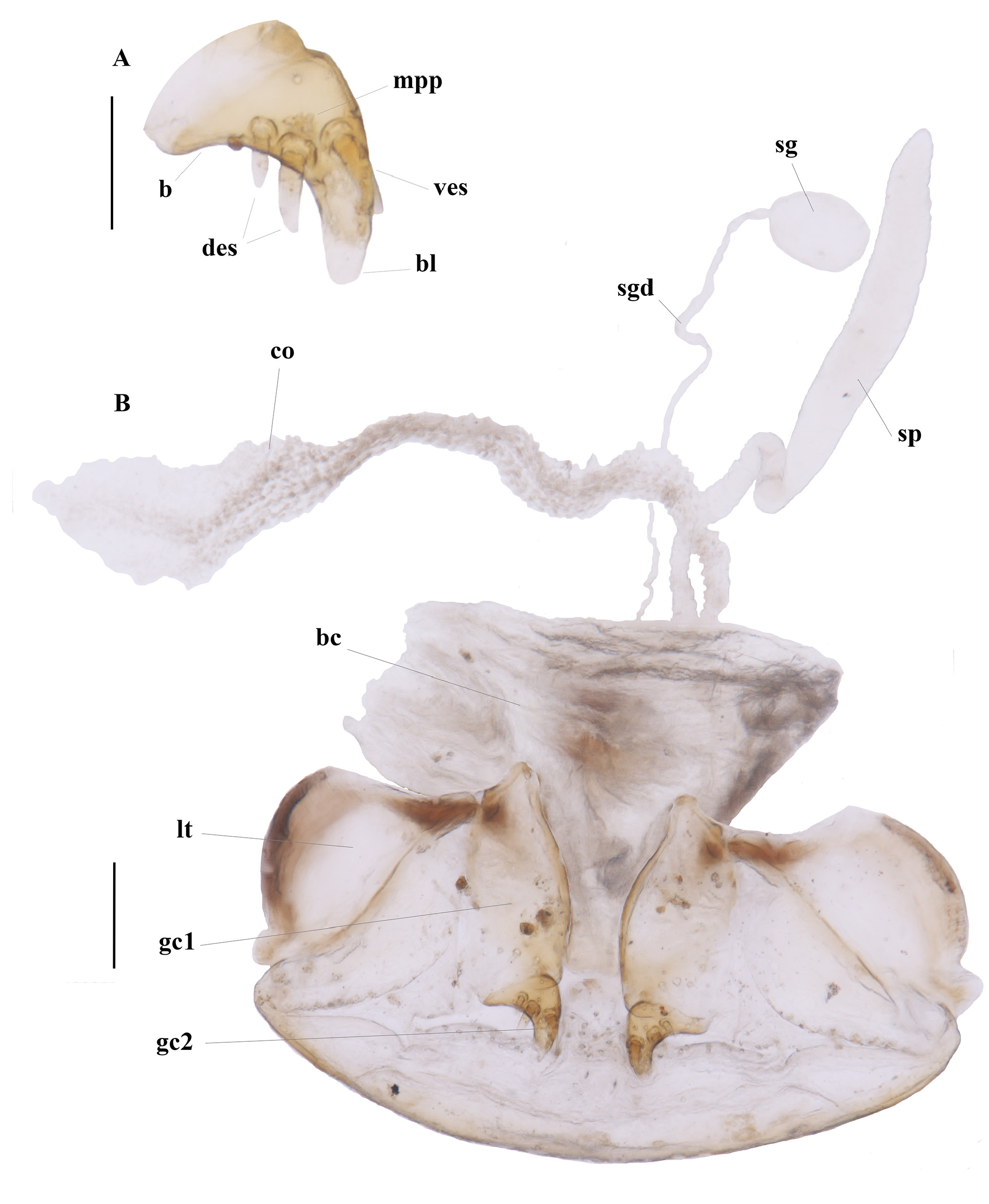
FEMALE GENITALIA. Ovipositor ( Fig. 4 View Fig ) with broad laterotergite (lt), basal gonocoxite 1 (gc1) longer than apical gonocoxite 2 (gc2); gonocoxite 1 apico-laterally bearing short and sturdy setae; gonocoxite 2 short, curved, in lateral aspect falciform, base (b) medium-size, narrow, blade (bl) rounded on the tip, with two dorsal ensiform setae (des), and one ventral ensiform seta (ves), all ensiform setae moderately long and robust; surface with many marginal pit pegs (mpp) ( Fig. 4A View Fig ). Female reproductive tract ( Fig. 4B View Fig ) proximally with short, broad bursa copulatrix (bc), continuous at its distal end with common oviduct (co) and long robust spermatheca (sp), latter slightly narrowed distally; spermathecal gland (sg) bulbous; spermathecal gland duct (sgd) long, slender, attachment not evident.
Habitat and ecological remarks
Caves are usually oligotrophic hypogean systems, with a nearly stable environment given the total absence of light and the humid atmosphere, reaching saturation ( Howarth 1980). Such features make the deep cave a unique environment. The environment that has characteristics most similar to the subterranean, is epigean leaf litter and edaphic soil in the cooler moist forests ( Howarth 1980) that maintain continually saturated atmospheres ( Bursell 1974). As a result, the arthropod fauna occurring in such habitats shares some of the features of hypogean fauna (e.g., brachyptery, lack of eyes and depigmentation) ( Poulson & White 1969), facilitating the colonization of the subterranean environment. The subterranean realm comprises the entire fissure network, the shallow subterranean habitats (i.e., MSS) and the human accessible macro-caves, making it an uninterrupted habitat, which is used by much of the specialized hypogean fauna ( Giachino & Vailati 2010). Still, specific subterranean microhabitats can also result in different morphologies for a highly specialized fauna, with specific characteristics related to the cave habitat, such as antenna length ( Trontelj et al. 2012).
Despite the common occurrence of species of Perigona in deep leaf piles during dry seasons, in forests ( Reichardt 1977; Baehr 2017), and associated with endogean compartments (e.g., P. gerardi Perrault, 1985 from French Guiana), the occurrence of the genus in the cave environment has not been documented. The only species registered close to a cave, P. nigriceps (Dejean, 1831) , was found in the MSS (subterranean superficial medium) in the surroundings of Movile Cave in Romania ( Nitzu 2001). This is a nearly cosmopolitan species ( Darlington 1953), with an edaphic habitat preference, which can be also found 6 m deep in the MSS ( Nitzu 2001). Although P. spelunca sp. nov. does not have remarkably elongated appendages, usually linked to cave habitat ( Trontelj et al. 2012), it does have elongated antennae that extend half the length of the body. Such elongation does not occur in its close microphthalmous relative, P. Neoperigona belloi .
Specimens of P. spelunca sp. nov. were observed in eight caves from the Arcos-Pains-Doresópolis speleological province (APD), in southeastern Brazil ( Fig. 5 View Fig ). This area has the highest density of caves in South America, with more than 2500 limestone caves registered. Despite the fact that more than 500 caves have been inventoried in the area (Ferreira R.L. pers. comm.), P. spelunca sp. nov. was only observed in eight caves, although some of those caves are located far from each other (18.65 km in a straight line in the case of the most distant pair of caves) ( Table 1 View Table 1 ). It is important to mention that all the known caves in the province are located within a polygon of about 900 square kilometres. Thus, the species can be considered widely distributed in the area. It is important to note that the wide distribution of a given subterranean species is possible by dispersal through small cracks in the karst and through the MSS. However, traps installed in the MSS between Brega and Santuário caves in a five-year long study never collected P. spelunca sp. nov. Furthermore, other traps installed at additional locations within the area also failed in capturing P. spelunca sp. nov in the last two years. This finding indicates that the species has caves as its preferred habitat, and that dispersion seems to occur occasionally, though this can be better assessed in more specific future studies. Hence, we prefer to consider the new species as a troglobitic, instead of generally subterranean or hypogean. Additionally, although such terms are to some extent equivalent, for practical purposes, the use of the term troglobitic may directly influence the species’ status, ensuring its protection according to the current Brazilian speleological legislation.
Most of the caves in the region are rather dry and may not provide a suitable habitat. All the caves in which specimens have been observed have water for at least part of the year, from percolating water, and from streams or phreatic levels. It is interesting to note that in most of the caves only a single individual was sampled (e.g., Serra Azul, Fazenda Amargoso, Cinderela and Brega caves), and in all cases they were males. The largest populations were found in Santuário, Éden and S1-GEC-035 caves, in which specimens are quite common.
In Santuário cave ( Fig. 5E View Fig ), specimens were generally observed near (or within) bat guano piles ( Fig. 5D View Fig ), apparently searching for prey (such as springtails). They did not show any reaction to light, indicating a potential absence of phototaxy. On some occasions (especially during rainy periods), up to three specimens were observed in the same guano pile. On the other hand, specimens from the Éden cave
( Fig. 5A View Fig ) were most frequently found close to decomposing plant material deposited along the stream banks, although some specimens were also observed near guano piles in the upper conduits of this cave. Unfortunately, no such data were provided for specimens from the S1-GEC-035 cave.
It is important to mention that even in caves with larger populations, specimens may not be encountered on every visit, especially during dry periods. Therefore, it is plausible that specimens migrate vertically, searching for suitably moist microhabitats during such periods. It is interesting to note that epigean species of Perigona usually occur in litter in dense forests, usually only being collected by specialized sampling methods, such as Berlese extraction or sifting ground litter ( Baehr 2017). For the endogean species P.gerardi , soil washing was the best method for collecting the specimens ( Perrault 1985). The caves lack typical external soils and also lack litter deposits, only having cave sediments, so specimens of P. spelunca sp. nov. are easier to find, especially due to their apparent lack of cryptic behavior. Furthermore, while most species of Perigona are capable of flight, being also encountered in flight intercept traps and at light ( Baehr 2017), P. spelunca sp. nov. has extremely reduced hind wings (which is considered to be an adaptation to life in caves) ( Fig. 1B View Fig ), being incapable of flight. However, according to Baehr (2017), little is known about the habits and ecology of almost all species of the genus.
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |