Cirrhilabrus sanguineus Cornic, 1987
|
publication ID |
https://doi.org/ 10.11646/zootaxa.4526.3.5 |
|
publication LSID |
lsid:zoobank.org:pub:FC4113BC-AE81-4163-B41C-9A4AD07729D3 |
|
DOI |
https://doi.org/10.5281/zenodo.5989618 |
|
persistent identifier |
https://treatment.plazi.org/id/03D487E6-FFFC-FFC9-69BD-2B8AA9398972 |
|
treatment provided by |
Plazi |
|
scientific name |
Cirrhilabrus sanguineus Cornic |
| status |
|
Cirrhilabrus sanguineus Cornic View in CoL
Blood-stained Fairy Wrasse
Figures 1–7 View FIGURE 1 View FIGURE 2 View FIGURE 3 View FIGURE 4 View FIGURE 5 View FIGURE 6 View FIGURE 7 , Tables 1–2
Cirrhilabrus sanguineus Cornic, 1987: 140 View in CoL , fig. (type locality, Mauritius); Randall, 1995: 24, figures 10–11 (description, distribution); Kuiter, 2002: 39, upper figures a,b (live photographs and brief description); Kuiter, 2010: 137, middle figures a–c (live photographs and brief description).
Diagnosis. Cirrhilabrus sanguineus shares similar meristic counts to other species in its complex, but is easily diagnosed by having a lanceolate caudal fin in males, and in the following live colouration details: head reddish orange to orange; body pale pink with two diffused oblique bands, the edges often dusky and not strongly delimited, the anterior most band greenish grey, the distal band yellow; anterior two-thirds of body with a broad, oblique, irregularly shaped magenta band.
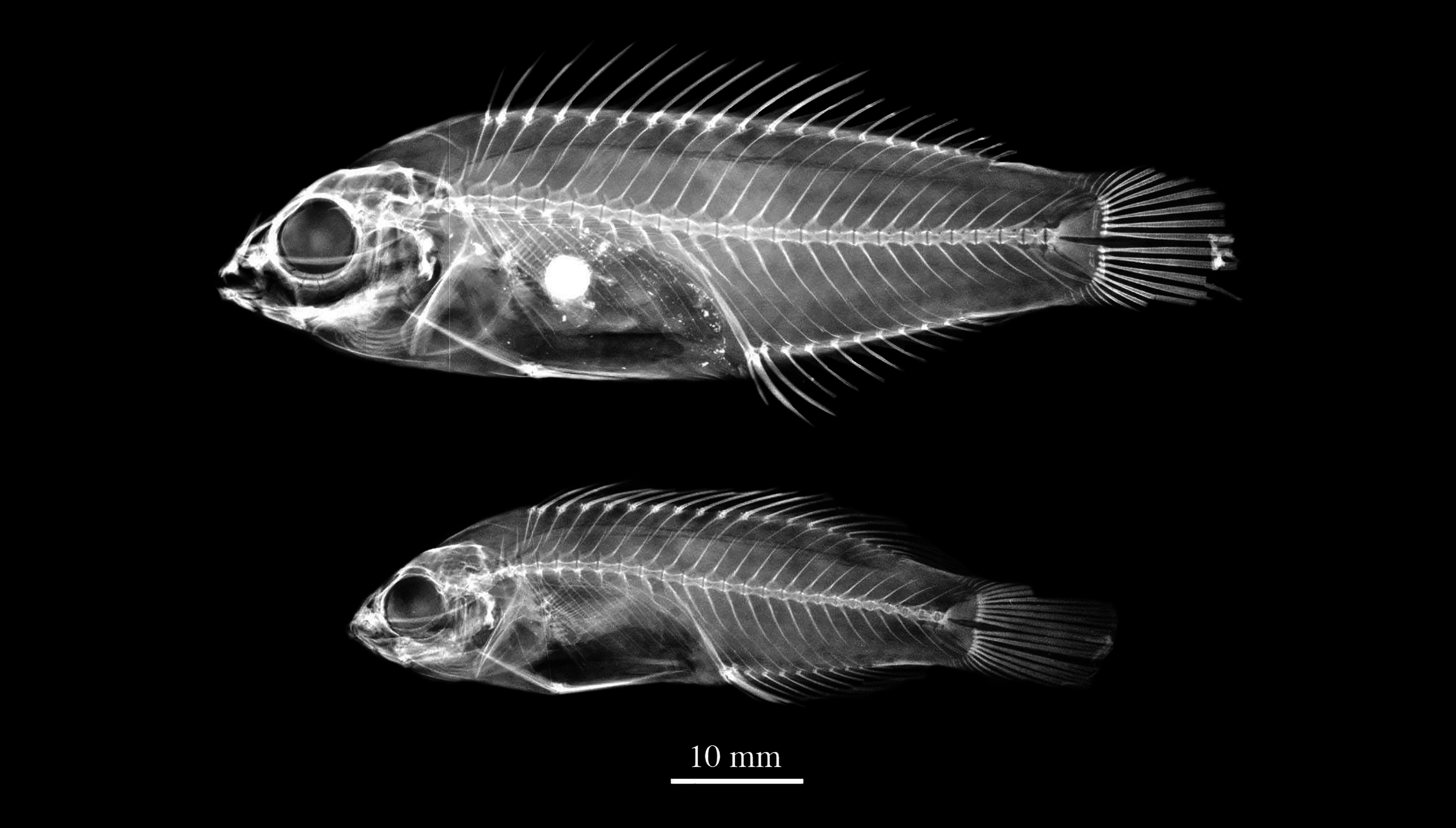
Description. Dorsal-fin rays XI,9; anal-fin rays III,9; dorsal and anal-fin soft rays branched except first ray unbranched; last dorsal and anal-fin ray branched to base; pectoral-fin rays 14–15 (14/14) (female specimen damaged on right side), upper two unbranched; pelvic-fin rays I,5; principal caudal-fin rays 7 + 6, uppermost and lowermost unbranched; upper procurrent caudal-fin rays 6, lower procurrent caudal-fin rays 6; lateral line interrupted, with dorsoanterior series of pored scales 16–18 (17/18) and midlateral posterior peduncular series 6–8 (7/7); scales above lateral line to origin of dorsal fin 2; scales below lateral line to origin of anal fin 6; median predorsal scales 4–5 (5); median prepelvic scales 6; rows of scales on cheek 2; circumpeduncular scales 14; gill rakers 6–7 (6) + 9–12 (11) = 15–19 (17); pseudobranchial filaments 11; vertebrae 9 + 16; epineurals 13.
Body moderately elongate and compressed, depth 3.2–3.5 (3.3) in SL, width 2.1–2.3 (2.2) in depth; head length 3.0–3.2 (3.0) in SL; snout pointed, its length 3.8–4.1 (3.8) in HL; orbit diameter 2.8–4.1 (4.1) in HL; depth of caudal peduncle 2.1–2.2 (2.2) in HL. Mouth small, terminal, and oblique, with maxilla almost reaching vertical at front edge of orbit; dentition typical of genus with three pairs of canine teeth present anteriorly at side of upper jaw, first forward-projecting, next two strongly recurved and outcurved, third longest; an irregular row of very small conical teeth medial to upper canines; lower jaw with a single stout pair of canines anteriorly which protrude obliquely outward and are slightly lateral to medial pair of upper jaw; no teeth on roof of mouth.
Posterior margin of preoperculum with 29–30 (29) very fine serrae; margins of posterior and ventral edges of preoperculum free to about level of middle pupil. Anterior nostril in short membranous tube, located nearer to orbit than snout tip; posterior nostril larger, roughly ovoid to rectangular, located just medial and anterior to upper edge of eye. Scales cycloid; head scaled except snout and interorbital space; 3 large scales on opercle; a broad naked zone on membranous edge of preopercle; a row of large, elongate, pointed scales along base of dorsal fin, one per element, scales progressively shorter posteriorly on soft portion of fin; anal fin with a similar basal row of scales; last pored scale of lateral line (posterior to hypural plate) enlarged and pointed; one scale above and below last pored scale also enlarged; a horizontal series of greatly enlarged scales extend two-thirds distance to central posterior margin of caudal fin; pectoral fins naked except for a few small scales at extreme base; a single large scale at base of each pelvic fin, about three-fourths length of pelvic spine.
Origin of dorsal fin above third lateral-line scale, predorsal length 2.7–3.1 (3.0) in SL; first 1–5 dorsal-fin spines progressively longer, sixth to ninth subequal, tenth to eleventh longest, 2.1–2.6 (2.6) in HL; interspinous membranes of dorsal fin in males extend beyond dorsal-fin spines, with each membrane extending in a pointed filament beyond spine; seventh dorsal-fin soft ray longest, 1.8–2.2 (1.8 as reported by Randall 1995; now broken in neotype) in HL, remaining rays progressively shorter; origin of anal fin below base of seventh dorsal-fin spine; third anal-fin spine longest, 2.4–3.1 (2.9 as reported by Randall 1995; now broken in neotype) in HL; interspinous membranes of anal fin extended as on dorsal fin; anal-fin soft rays relatively uniform in length, seventh longest, 1.6–2.1 (1.8 as reported by Randall, 1995; now broken in neotype) in HL; dorsal and anal-fin rays barely reaching caudal-fin base; caudal fin of males lanceolate (damaged in male neotype and additional male specimens); pectoral fins short, reaching vertical between bases of fifth or sixth dorsal-fin spines, longest ray 1.5–1.6 (1.6) in HL; origin of pelvic fins below lower base of pectoral fins; pelvic fins short, not reaching past anal fin origin, longest ray 1.5– 1.9 (1.9) in HL.
Colouration of males in life (based on colour photographs of the neotype and additional specimens when freshly dead, and aquarium photos of live individuals; Figures 1–3 View FIGURE 1 View FIGURE 2 View FIGURE 3 ): head reddish orange to orange; lower part of head pale pink; lilac stripe weakly present from behind upper orbit to upper edge of operculum; second stripe of same colour weakly present from behind lower orbit to lower part of cheek; lilac stripes continue past eye anteriorly on to snout, the upper stripe reaching mid-upper lip; upper part of nape, interorbital and upper part of snout reddish orange to orange, with a series of fine white stripes; iris bright orange, with yellow ring around pupil; body pale pink, fading to whitish cream ventrally; anterior one third of body with two diffuse oblique bands, the edges often dusky and not strongly delimited, the anterior-most band greenish grey, the distal band yellow; upper two-thirds of body from below seventh to tenth dorsal fin spines with a broad, oblique, irregularly shaped magenta band (variable between specimens, and very often asymmetrical on either side; Figure 3 View FIGURE 3 ); dorsal fin orange-yellow, increasingly hyaline posteriorly; posterior dorsal fin with a metallic blue medial stripe, often broken into spots; distal margin of dorsal fin narrowly bright blue; anal fin pale pinkish yellow, distal margin narrowly bright blue; caudal fin hyaline pink to pinkish yellow with a pair of prominent blue chevrons converging at lanceolate terminus of fin; pelvic fins chalky pink, narrowly bright blue on leading edge; pectoral fins pinkish hyaline.
Nuptial colouration of males (based on colour photographs of aquarium individuals; Figure 5 View FIGURE 5 ): head reddish orange to orange; lower part of head bright lilac to white; lilac stripe from behind upper orbit to upper edge of operculum intensifies, becoming metallic lilac-blue; second stripe of same colour from behind lower orbit to lower part of cheek intensifies; metallic stripes continue past eye anteriorly on to snout, the upper stripe reaching midupper lip; upper part of nape, interorbital and upper part of snout reddish orange to orange; body bright pinkishwhite, fading to pinkish anteriorly; anterior greenish grey band behind head and pectoral fins strongly intensifies, often dusky; the distal yellow diffused band disappears; oblique, irregularly shaped magenta band strongly intensifies; dorsal fin whitish pink; metallic blue medial stripe on posterior dorsal fin intensifies; anal fin whitish pink; caudal fin hyaline-magenta to pink, with chevrons markings intensifying to metallic blue; pelvic fins bright yellowish-orange, narrowly bright blue on leading edge; pectoral fins pinkish hyaline.
Colouration of females in life (based on colour photographs and aquarium photos of live individuals; Figures 6–7 View FIGURE 6 View FIGURE 7 ): similar to male, but body uniformly pink to pinkish orange, fading to pale white ventrally; head reddish orange to orange; dorsal edge of iris bright yellow; dorsal fin hyaline yellow, posterior with medial series of weak metallic blue spots; anal fin hyaline without any obvious markings; pelvic fins hyaline; caudal fin hyaline with weak metallic blue chevron markings; pectoral fins hyaline.
Colouration in preservative: head and body uniformly light tan; spines and soft rays of median and pelvic fins purple; interorbitals, preopercle and maxilla purple; oblique band in males light purple.
Etymology. An etymology for the specific epithet was not provided by Cornic (1987) in the original description of the species. However, the epithet sanguineus is the nominative, masculine form of the Latin “ sanguis ”, meaning “blood”. Presumably, this is in reference to the broad, dark magenta band present in the males of the species. A common name was not provided by Cornic (1987), nor by Randall (1995) in his later review of the species. Kuiter (2010) suggested the common name of “Blood-stained Fairy Wrasse”, which we follow.
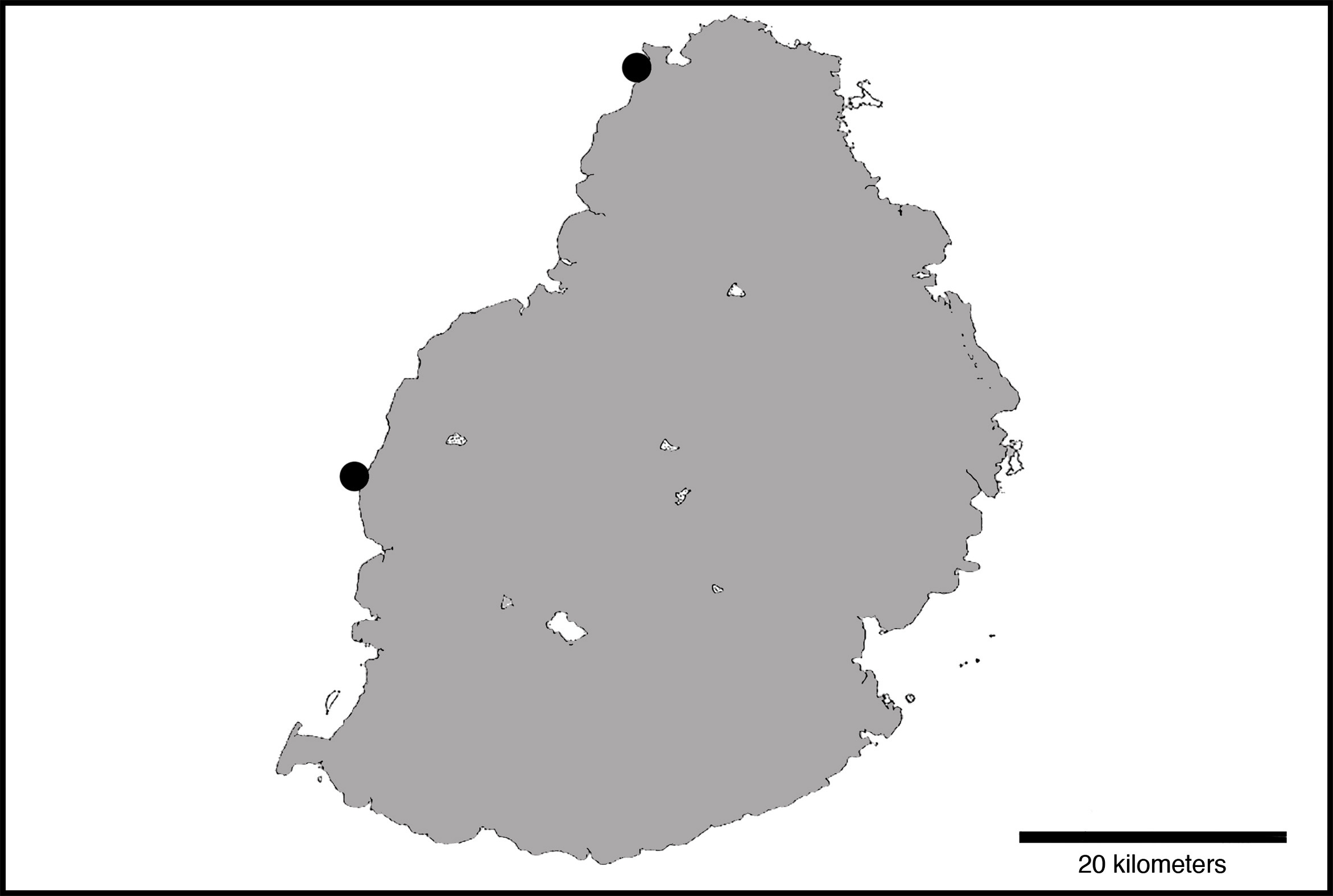
Distribution and habitat. Cirrhilabrus sanguineus is known only from the island of Mauritius in the western Indian Ocean ( Figure 8 View FIGURE 8 ). Randall (1995) reported that the neotype (BPBM 24779; Figures 1 View FIGURE 1 & 4 View FIGURE 4 ) and additional specimens (BPBM 22542 [ Figure 4 View FIGURE 4 ]; NSMT-P 34975) examined were collected from Flic en Flac, off the western coast of Mauritius, at depths of 42– 52 m. The new specimens examined in this study (ZRC 60168; Figure 7 View FIGURE 7 ) was collected off Cannoniers Point, off the northern coast of Mauritius, at depths of 70– 90 m. Given that Mauritius is a small island, it is likely that the species also occurs throughout the island and elsewhere in the Mascarene Archipelago, particularly at Réunion. The species inhabits low relief rubble slopes with little to no structure.
...Continued on next page
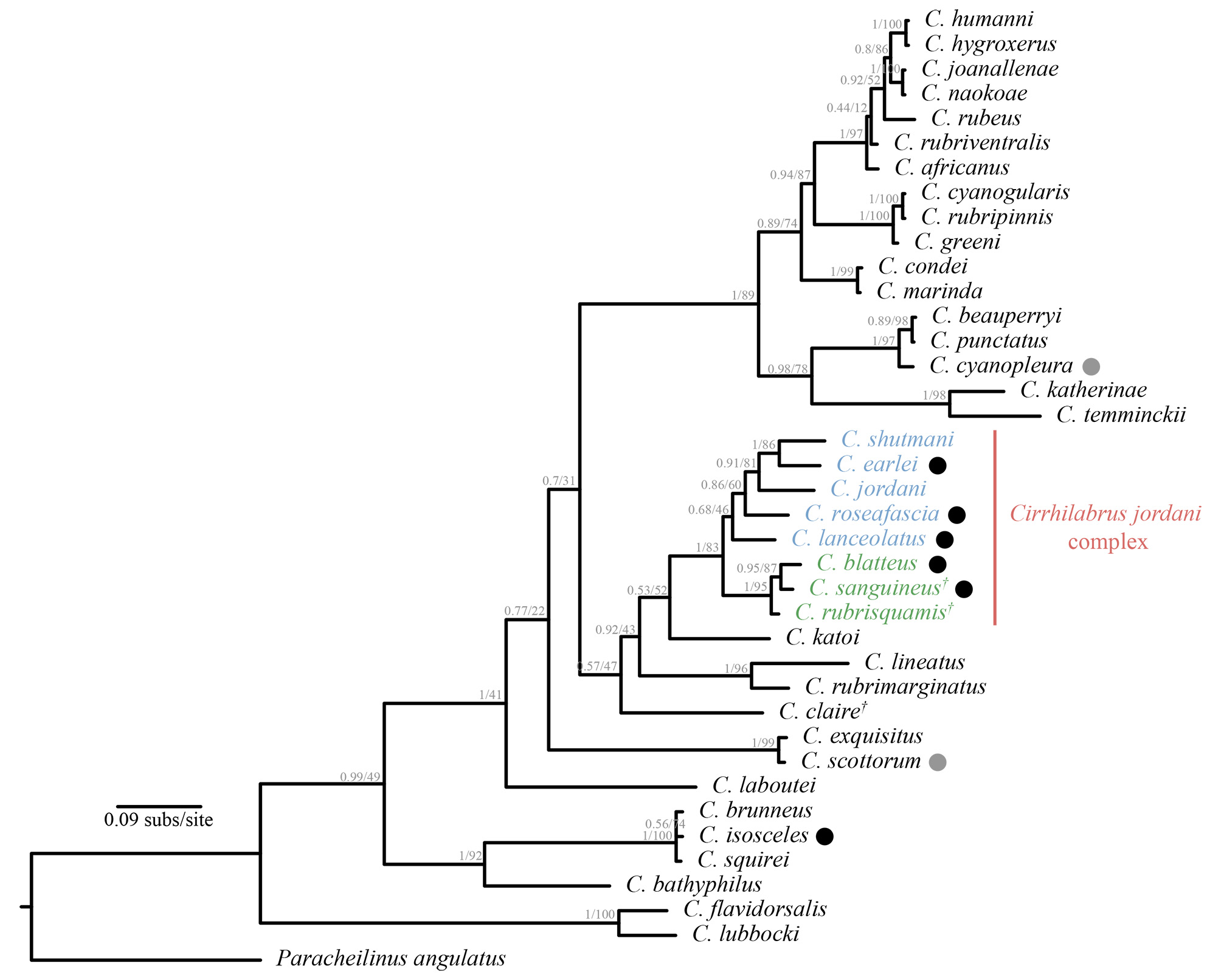
Comparisons and phylogenetic interpretation. Tea & Gill (2017) commented on the putative relationships for a set of Cirrhilabrus species. These were Cirrhilabrus jordani Snyder (1904) , C. blatteus Springer & Randall (1974) , C. roseafascia Randall & Lubbock (1982) , C. rubrisquamis Randall & Emery (1983) , C. sanguineus Cornic (1987) , C. lanceolatus Randall & Masuda (1991) , C. claire Randall & Pyle (2001) , C. earlei Randall & Pyle (2001) and C. shutmani Tea & Gill (2017) , herein referred to as the C. jordani complex. The species in this complex were hypothesised to be closely related based on the following combination of characters as defined by Tea & Gill (2017): relatively short pelvic fins (not or barely reaching anal-fin origin, except for C. claire with relatively long pelvic fins); a pair of stripes on head (in both sexes; strongly evident during nuptial display); and, dorsal and anal fins without obvious stripes or spots. Colour photographs of all nine species are provided in Tea & Gill (2017).
Members of the Cirrhilabrus jordani species complex preferentially inhabit deeper waters, at depths between 40– 90 m. These species are often strikingly coloured, with several possessing long, tapering caudal fins ( C. lanceolatus , C. roseafascia , C. earlei , C. blatteus and C. sanguineus ). However, this character has arisen independently in several species from apparently different lineages [eg., C. isosceles Tea, Senou & Greene (2016) ; C. rhomboidalis Randall (1988) ; C. melanomarginatus ( Randall & Shen 1978) ], and is therefore not phylogenetically informative ( Tea et al. 2016; Figure 9 View FIGURE 9 ). Nonetheless, it can be useful as a tool in identifying species. The males of Cirrhilabrus sanguineus cannot be confused with any of the currently valid species, and is easily distinguished from its congeners by its unique colour pattern in combination with its lanceolate caudal fin.
A phylogenetic analysis of the mitochondrial COI marker retrieved a monophyletic relationship for eight of the nine species in the C. jordani complex as defined by Tea & Gill (2017) ( Figure 9 View FIGURE 9 ). The members are further divided into two lineages—a western Indian Ocean lineage, comprising Cirrhilabrus sanguineus , C. blatteus and C. rubrisquamis , and a western Pacific Ocean lineage, comprising Cirrhilabrus earlei , C. shutmani , C. lanceolatus , C. roseafascia and C. jordani . Despite having a pair of prominent head stripes characteristic of members in the C. jordani complex (Figure 10), the phylogenetic position of C. claire did not conform to the relationships proposed by Tea & Gill (2017). Pending further investigation, we tentatively treat Cirrhilabrus claire as incertae sedis.
An additional mitochondrial marker (16S) was amplified for Cirrhilabrus sanguineus and C. rubrisquamis . However, since comparative data for the other species in the genus are lacking, only the COI marker was used in this phylogeny. It is worth mentioning, that phylogenetic relationships inferred using the mitochondrial COI marker in isolation should be treated with caution. In many Cirrhilabrus lineages, related species belonging to the same complex may exhibit high levels of mitochondrial introgression or shared haplotypes, leading to poor, or no phylogenetic signal ( Allen et al. 2015; Tea et al. 2016;). This is evident even between morphologically distinct species (e.g., C. isosceles and C. brunneus ; C. joanallenae and C. naokoae ; Figure 9 View FIGURE 9 ). In the case of Cirrhilabrus sanguineus , pairwise comparison of the COI marker reveals a difference of 1.5% in sequence data to that of C. rubrisquamis , and 1.9% to that of C. blatteus (Figure 10).
Nonetheless, we present the first molecular phylogeny which includes 38 of the 59 valid species of Cirrhilabrus . Previous estimates have relied on neighbor-joining as the primary method for phylogenetic inference, with a focus on specific lineages as opposed to evolutionary relationships at the generic level ( Allen et al. 2015; Tea et al. 2016; Victor 2016). Although some of the relationships at deeper nodes were not retrieved with strong support, the relationships within the C. jordani complex were well resolved, and while we expect relationships Remarks. Cornic described the species in 1987 in his field guide Poissons de I’lle Maurice, based only on a single colour photograph taken by Randall. A neotype (BPBM 24779; Figures 1 View FIGURE 1 & 4 View FIGURE 4 ) and two additional specimens (BPBM 22542 [ Figure 4 View FIGURE 4 ]; NSMT-P 34975) was later assigned and examined by Randall in his 1995 review of Cirrhilabrus from the western Indian Ocean, based on specimens collected by D. Pelicier, J.S. Dench and R.M. Bray in 1979. The female of the species has not been described or known, but Randall suggested that females might lack the oblique magenta band present in the males ( Randall, 1995). We confirm Randall’s initial postulation with new photographic records ( Figure 6 View FIGURE 6 ) and a single retained specimen (ZRC 60168; Figure 7 View FIGURE 7 ) of the female form.
The new specimen (ZRC 60168), as well as those pictured in Figures 2 View FIGURE 2 and 3 View FIGURE 3 , were collected off Cannoniers Point, off the northern coast of Mauritius at depths of 70- 90 m. This represents one of the deepest records of any known Cirrhilabrus , with only three other species documented to occur deeper— Cirrhilabrus earlei at depths to 110 m in Micronesia (B.D. Greene 2018, pers. comm.), C. Claire to 150 m in Moorea, French Polynesia (R. Kimura 2018, pers. comm.), and C. roseafascia to 155 m in the Great Barrier Reef ( Sih et al. 2017).
Cirrhilabrus sanguineus View in CoL is unique in having several of its osseus elements (interorbitals, preopercle, scales, and maxilla) and fin rays stain naturally purple in ethanol. In other species of Cirrhilabrus View in CoL , these are often uncoloured or are stained blue-green ( Springer & Randall 1974). Springer & Randall (1974) noted the same for C. blatteus View in CoL ( Figure 11 View FIGURE 11 ), alluding to this characteristic in their original description of the species. This purple quality is also observed in freshly preserved specimens of C. rubrisquamis— soft rays, spines, scales and preopercle ( Randall 1995), and C. earlei View in CoL —interorbitals, peropercle, soft rays, spines and scales ( Randall & Pyle 2001).
Material examined. Flic en Flac, off the western coast of Mauritius: BPBM 24779 View Materials , male, 66.5 mm SL (neotype) ; BPBM 22542 View Materials , male, 48.3 mm SL ; NSMT-P 34975 , male, 58.7 mm SL. Cannoniers point, off the northern coast of Mauritius : ZRC 60168, female, 41.2 mm SL.
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |
Cirrhilabrus sanguineus Cornic
| Tea, Yi-Kai, Frable, Benjamin W. & Wal, Cara Van Der 2018 |
Cirrhilabrus sanguineus
| Kuiter, R. H. 2010: 137 |
| Kuiter, R. H. 2002: 39 |
| Randall, J. E. 1995: 24 |
| Cornic, A. 1987: 140 |