Characidium barbosai, Junior & Lima & Machado & Melo, 2020
|
publication ID |
https://doi.org/ 10.11646/zootaxa.4816.3.5 |
|
publication LSID |
lsid:zoobank.org:pub:31128F69-9DCA-4A85-A887-6F993EBEAFC7 |
|
DOI |
https://doi.org/10.5281/zenodo.4323951 |
|
persistent identifier |
https://treatment.plazi.org/id/DE7DBDB1-4F86-466F-A57A-0D8EDCF99C7B |
|
taxon LSID |
lsid:zoobank.org:act:DE7DBDB1-4F86-466F-A57A-0D8EDCF99C7B |
|
treatment provided by |
Plazi |
|
scientific name |
Characidium barbosai |
| status |
sp. nov. |
Characidium barbosai , new species
( Figs. 1-2 View FIGURE 1 View FIGURE 2 , 3A View FIGURE 3 )
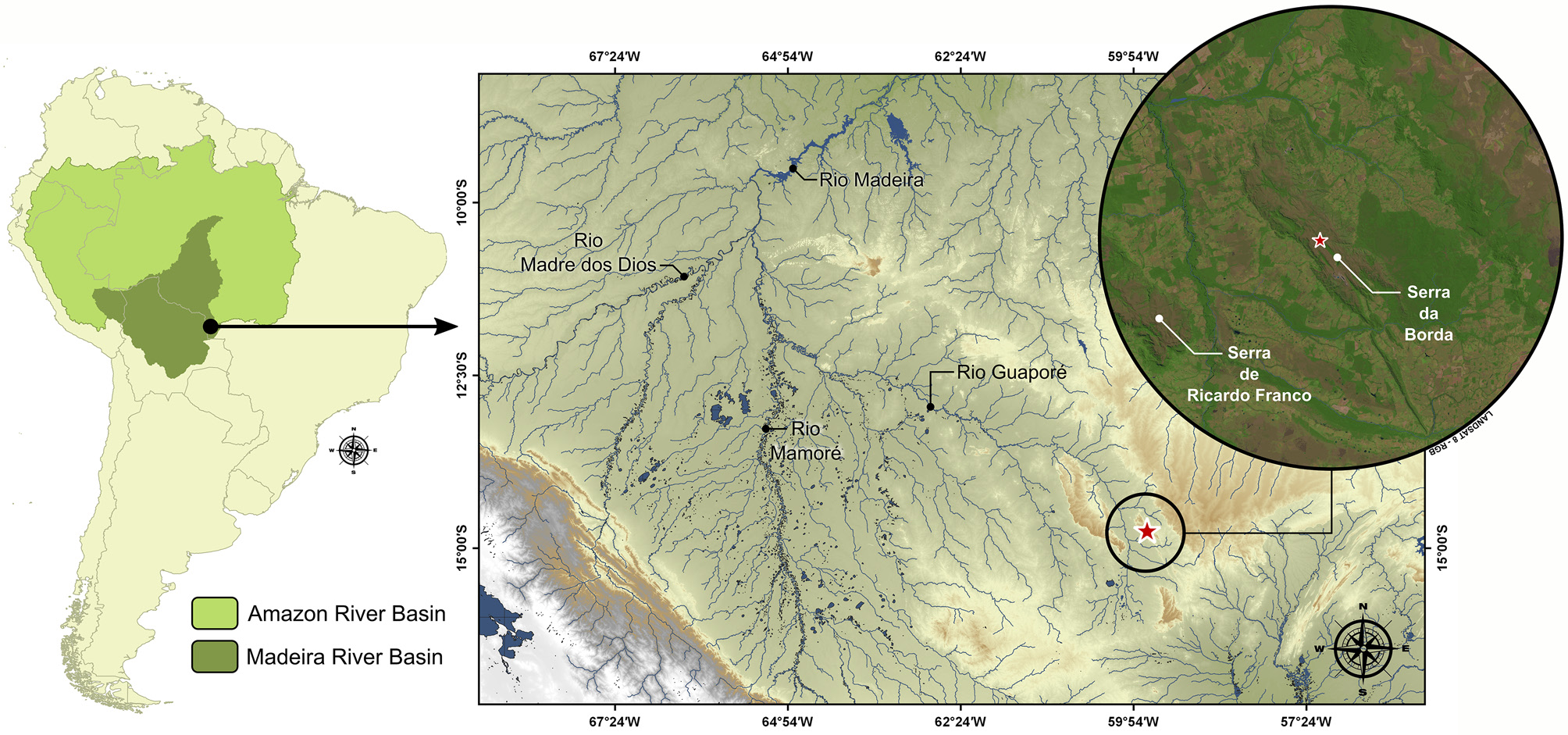
Holotype: ZUEC 17139 View Materials (42.5 mm SL): Brazil, Mato Grosso, Vila Bela da Santíssima Trindade, córrego Longa Vida, tributary of rio Guaporé , Serra da Borda , 14º45’35’’S, 59º42’14’’W; F.A. Machado, N.E. Silva & C.M.C. Leite, 3 June 2005. GoogleMaps
Paratypes: All from Brazil, Mato Grosso, Vila Bela da Santíssima Trindade, rio Guaporé basin, Serra da Borda . ZUEC 171140 View Materials (17, 21.0– 43.7 mm SL) ; ANSP 207590 View Materials (5, 32.3–42.2 mm SL) ; CAS 247209 View Materials (5, 28.8–37.7 mm SL): same data as holotype GoogleMaps . ZUEC 17145 View Materials (17, 21.3–39.8 mm SL) ; FMNH 144645 View Materials (5, 26.3–33.5 mm SL) ; MCZ 174158 View Materials (5, 30.8–36.4 mm SL): Córrego Longa Vida , 14º45’31’’S, 59º42’28’’W, 580 meters a.s.l.; F.A. Machado, N.E. Silva & C.M.C. Leite, 3 June 2005 GoogleMaps . ZUEC 17144 View Materials (194, 8 CS, 17.3–40.9 mm SL); CPUFMT 6778 (5, 29.6–35.7 mm SL) ; INPA 59043 View Materials (5, 28.5–36.1 mm SL); LBP 29207 (5, 27.0– 35.4 mm SL) ; MCP 54289 (5, 25.9–36.4 mm SL) ; MHNG 2784.070 View Materials (5, 30.4–33.5 mm SL) ; MNRJ 51745 View Materials (5, 32.0– 38.1 mm SL) ; MZUSP 125671 View Materials (5, 28.4–38.1 mm SL): Córrego Longa Vida , immediately below dam, 14º48’42’’S, 59º39’43’’W, 635 meters a.s.l.; F.A. Machado & N. Flausino Jr., 11 Sept 2005 GoogleMaps . MZUSP 84775 View Materials (91, 24.1–55.1 mm SL) ; UF 245019 (10, 26.4–48.6 mm SL): Córrego Cabeceira , c. 14º50’S, 59º40’W; F.A. Machado & N.E. Silva, 8 May 2004 GoogleMaps . ZUEC 17149 View Materials (4, 32.2–34.9 mm SL): Córrego Cabeceira, Mineradora Santa Elina , c. 14º50’S, 59º40’W; F.A. Machado, N.E. Silva & C.M.C. Leite, 2 June 2005 GoogleMaps . ZUEC 14735 View Materials (9, 18.5–43.5 mm SL): Córrego Cabeceira , c. 14º50’S, 59º40’W; F.A. Machado, N.E. Silva & C.M.C. Leite, 2 Jun 2005 GoogleMaps . ZUEC 14736 View Materials (49, 16.7–35.4 mm SL): Córrego Longa Vida (trib. rio Guaporé ), 14º48’42’’S, 59º39’43’’W, 635 meters a.s.l.; F.A. Machado, N.E. Silva & C.M.C. Leite, 2 Jun 2005 GoogleMaps .
Diagnosis. Characidium barbosai can be diagnosed from all congeners, except from C. summus , by lacking a preorbital stripe. It can be diagnosed from C. summus by the presence of 10–12 bars along the body (vs. bars absent), basicaudal spot inconspicuous (vs. basicaudal spot conspicuous), humeral blotch inconspicuous (vs. conspicuous), by having 12 scales around caudal peduncle (vs. 14), and by an adipose fin present (vs. absent). In addition, C. barbosai further differs its congeners except C. bahiense Almeida , C. chicoi da Graça, Ota & Domingues , C. clistenesi Melo & Espíndola , C. interruptum Pellegrin , C. lagosantense Travassos , C. lanei Travassos , C. laterale (Boulenger) , C. litorale Leitão & Buckup , C. mirim Netto-Ferreira, Birindelli & Buckup , C. nana Mendonça & Netto-Ferreira , C. nupelia da Graça, Pavanelli & Buckup , C. orientale Buckup & Reis , C. rachovii Regan , C. satoi Melo & Oyakawa , C. stigmosum Melo & Buckup , C. vestigipinne Buckup & Hahn , and C. xavante da Graça, Pavanelli & Buckup by the presence of a single row of dentary teeth (vs. two rows); among those species, it differs from C. bahiense , C. chicoi , C. interruptum , C. lagosantense , C. lanei , C. laterale , C. mirim , C. nana , C. nupelia , C. orientale , C. rachovii , C. stigmosum , C. vestigipinne , and C. xavante by the presence of the parietal branch of the supraorbital canal (vs. absent); it differs from C. clistenesi , along with C. bahiense , C. bimaculatum , C. laterale , C. nana , C. nupelia and C. xavante , by the absence of a conspicuous peduncular blotch, and from C. litorale and C. satoi by having one or two scales between anus and anal-fin origin (vs. four or five in C. litorale and four to seven in C. satoi ). See the Discussion for further comments on the diagnosis of the new species.
Description. Morphometric data summarized in Table 1. Largest specimen examined reaching 45.2 mm SL. Body moderately elongated. Dorsal profile convex between tip of snout and posterior naris, gently convex from latter point to dorsal-fin origin, straight along dorsal-fin basis, slightly convex to straight from dorsal-fin terminus to anteriormost dorsal procurrent dorsal-fin ray. Ventral profile gently convex between tip of dentary and isthmus, gently convex to straight between latter point and anal-fin origin, slightly convex at anal-fin base; almost straight between anal-fin terminus and anteriormost ventral procurrent caudal-fin ray. Greatest body depth at dorsal-fin origin.
Snout short, roughly triangular in lateral view. Mouth small, terminal. Tip of maxilla reaching level of anterior margin of orbit. Margin of orbit free, with narrow eyelid. Cheek narrow, its depth less than one third of eye diameter. Nares distinctly separated. Dermal flap surrounding anterior naris, and at anterior margin of posterior naris. Temporal and parietal branches of supraorbital laterosensory canal present; parietal branch elongated, reaching the parietal bone. Fontanel limited anteriorly by frontals, laterally by parietals and posteriorly by supraoccipital.
Dentary teeth 9 (3), 10(4), 12(1), arranged in single row; teeth crown triangular, lacking noticeable cusps, increasing in size toward symphysis. Premaxillary teeth 5(1), 6(11), 7*(42), 8(12) arranged in single row; teeth crown triangular, lacking noticeable cusps, increasing in size towards symphysis. Maxillary teeth absent. Ectopterygoid teeth arranged in single row; teeth minute and conical, numbering 1(2), 3(2), 4(1), 5(1), 6 (1), 7(1). Mesopterygoid teeth absent. Branchiostegal rays 4 (8); three attached to ceratohyal (8), one attached to epihyal (8). Total gill rakers on first arch 10(1), 11(2), 12 (1), 13(1), 14(1); gill rakers attached to epibranchial 4(2), 5 (5), 6(1); gill rakers attached to ceratobranchial 4(1), 5(4), 6 (1), 7(2); gill rakers attached to basibranchial 0(2) or 1(6).
Scales cycloid; 11-15 parallel radii present on posterior field of scale, circuli restricted to anterior field of scales. Lateral line complete, all scales perforated; lateral-line scales 30(1), 31(1), 32(2), 33(5), 34*(42), 35(17), or 36(1). Scales above lateral line 4*(69). Scales below lateral line 4*(69). Scales around caudal peduncle 12*(66) or 13(2). Pre-dorsal scales series regularly arranged, except at its anterior portion, where often misaligned; scales on pre-dorsal series 9(3), 10(34), 11*(23), 12(6). Scales between anus and anal fin 1(39), 2*(26). Isthmus scaled.
Pectoral fin short, not reaching vertical that pass through dorsal-fin origin. Origin of dorsal fin at level slight anterior to pelvic-fin origin. Pectoral-fin rays iii,7,i* (5), iii,8,i (62), iii,9,i (2). Pelvic-fin rays i,6,i (3), i,7,i* (64), i,8,i (1). Dorsal-fin rays iii,8 (1), ii,9* (67); supranumerary element on first pterygiophore of dorsal fin 1 (8). Analfin rays iii, 6(1), ii,7* (65); supranumerary element on first pterygiophore of anal fin 1 (8). Principal caudal-fin rays i,8,7,i (1), i,8,9,i* (55); upper procurrent rays 8 (1), 9(2); lower procurrent rays 8(1), 9(1). Adipose fin present.
Precaudal vertebrae 20 (3), 21 (5); total vertebrae 33 (5), 34(3). Supraneurals 5 (1), 6(6), 7 (1). Hypurals numbering 6, hypurals 4, 5, and 6 not fused (8). Epurals 2(4), 3 (4). Posterior chamber of swim bladder larger than anterior chamber, 25.2–25.8 % in SL (2).
Coloration of preserved specimens. Ground color of head and trunk light brown, darker dorsally, lighter ventrally. Sides of head and opercle light brown, with minute, numerous evenly-spaced melanophores. Dorsal portion of snout and head light brown. Preorbital and postorbital stripes absent. Humeral blotch present, vertically elongated, situated immediately behind upper corner of opercle, extending vertically one scale row and half above lateral line dorsally, reaching or falling short of reaching, lateral line, ventrally. Midlateral stripe conspicuous, narrow, extending from immediately behind humeral blotch to caudal peduncle, diffuse anteriorly and posteriorly not reaching end of caudal peduncle. Midlateral stripe anteriorly at level of lower portion of scale row immediately above lateral line and dorsal portion of lateral line scale row, gradually lowering to central portion of lateral-line scale row. Basicaudal spot present, small and inconspicuous, more noticeable in small specimens, but little discernible or absent in specimens larger than 41.0 mm SL. Peduncular spot absent. Bars alongside body 10-12, relatively broad dorsally, tapering ventrally; bars on midbody more conspicuous, first and second bars typically not extending ventrally from longitudinal stripe, middle bars not extending to abdominal region, last two bars very narrow. Bars limits becoming blurred ontogenetically and absent from specimens equal or larger than 41.0 mm SL, which have a reticulated color pattern, with pigmentation concentrated on distal portion of scales. Pectoral, pelvic, dorsal, anal, and caudal fins mostly hyaline; melanophores present only on fin-rays lepidotrichia, and forming a subdued medial stripe at dorsal fin.
Sexual dimorphism. Tiny hooks are present on the pelvic-fin rays of a few specimens (ZUEC 17144, 7, 29.7–35.3 mm SL), on first to fifth branched rays, numbering 3–12 per fin ray. No fin hooks were observed on the pectoral-, anal-, dorsal- or caudal-fin rays. One of the specimens bearing hooks was dissected and identified as a mature male with well-developed testicles. Only three females could be positively identified by the possession of well-developed ovaries (discernible through the abdominal cavity wall, and confirmed through dissection; ZUEC 17144, 3, 31.6–34.7 mm SL). These females do not have hooks on pelvic-fin rays.
Distribution. Characidium barbosai is only known from the headwaters of two tributaries of the Rio Guaporé, the Córrego Longa Vida and the Córrego Cabeceira, at the Serra da Borda, an isolated plateau with altitudes ranging between 300–800 meters a.s.l., rio Madeira drainage, Amazon basin, Mato Grosso, Brazil ( Fig. 3 View FIGURE 3 ).
Ecological notes. The streams from Serra da Borda plateau possess clearwater and range from intermittent small streams only flowing during the rainy season (November to April), to the relatively large and perennial Córrego Longa Vida. Riparian vegetation at these streams range from open cerrado savannah, buritizais – wetlands dominated by the buriti palm, Mauritia flexuosa –, and semideciduous, submontane forest. Characidium barbosai occurs at the intermittent streams and become restricted to the remaining water pools during the dry season, but is more abundant at the perennial Córrego Longa Vida. These streams located in the upper Serra da Borda Plateu are virtually isolated from their lower sections in the surrounding lowlands by steeps waterfalls. Unlike its congeners, Characidium barbosai is a midwater fish, picking food items drifted by the current or at the surface, much like several characid fishes as Astyanax spp. with a similar pelagic feeding behavior. During the night, specimens were observed motionless at the water column or, more rarely, at the bottom. Only a few fish species, all silurifoms, were collected syntopically with C. barbosai at the Córrego Longa Vida: Callichthys callichthys (Callichthyidae) , an unidentified species of Ancistrus (Loricariidae) , and an unidentified species of Trichomycterus (Trichomycteridae) . Gut contents of eleven examined specimens (ZUEC 14735, 1; 14736, 2; 17144, 8) were mostly empty, but a single specimen possessed a few arthropods remains.
Conservation status. Characidium barbosai is so far only known from a restrict area, the stretches of the Córrego Longa Vida and Córrego Cabeceira crossing the Serra da Borda plateau, where the species seems to be common. Gold mining in the Serra da Borda (originally called Serra de São Vicente) started in the 1730’s with the establishment of the Arraial de São Francisco Xavier, one of the first colonial settlements in the Mato Grosso state, and was abandoned by the end of the XIX century with the demise of the alluvial gold in the region ( Póvoas, 1995). Intensive gold mining using modern techniques was resumed in the last twenty years in the area. The upper portion of the Córrego Longa Vida was dammed in 2005 forming a small reservoir, related to the gold mining activities in the area. It is unclear how the damming impacted C. barbosai , but specimens were collected in both the lake above the dam and in the stream stretch below it in 2005 (F.A Machado and N. Flausino Junior, pers. obs.). Besides the damming, it seems that little disturbance has taken place so far at the Córrego Longa Vida. On the other hand, the Córrego Cabeceira, the other headwater tributary from where C. barbosai is known, was intensely disturbed since 2005, with large tailing dams build at its upper course, where untreated sewage is also being disposed of. Unregulat- ed, intensive illegal gold mining has been occurring intermittently at the eastern versant of the Serra da Borda since 2015 and has completely ravaged some headwater streams draining into the rio Galera (a tributary of rio Guaporé), but the Córrego Longa Vida and its tributaries, which drain the western versant of the Serra da Borda, directly into the rio Guaporé, seem to have been spared so far from the devastation. As the extent of occurrence of C. barbosai is not well documented, nor the potential impacts at its habitat, we prefer at the moment to consider C. barbosai as Data Deficient (DD) following IUCN’s criteria (International Union for Conservation of Nature, 2012).
Etymology. The specific epithet honors Gerson Natalício Barbosa, a District Attorney from Mato Grosso state, for his commitment in the enforcement of the environmental laws, and for being one of the conceivers of the project “Água para o Futuro”, which is surveying, protecting and restoring springs in the urban area of Cuiabá, the capital of Mato Grosso. A patronymic adjective.
| CS |
Musee des Dinosaures d'Esperaza (Aude) |
| MCP |
Pontificia Universidade Catolica do Rio Grande do Sul |
| UF |
Florida Museum of Natural History- Zoology, Paleontology and Paleobotany |
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |