Ameerega shihuemoy, Serrano-Rojas, Shirley J., Whitworth, Andrew, Villacampa, Jaime, May, Rudolf Von, Gutiérrez, Roberto C., Padial, José M. & Chaparro, Juan C., 2017
|
publication ID |
https://doi.org/ 10.5281/zenodo.246758 |
|
publication LSID |
lsid:zoobank.org:pub:0BBAE8E3-9B03-4FCB-AA60-C775DFC86459 |
|
DOI |
https://doi.org/10.5281/zenodo.6038387 |
|
persistent identifier |
https://treatment.plazi.org/id/038687B6-FFAC-E833-4FB2-F93EFB54FE69 |
|
treatment provided by |
Plazi |
|
scientific name |
Ameerega shihuemoy |
| status |
sp. nov. |
Ameerega shihuemoy View in CoL sp. nov.
( Figures 1–3 View FIGURE 1 View FIGURE 2 View FIGURE 3 , 6 View FIGURE 6 )
Cryptophyllobates sp: Chaparro & Ochoa 2005 p.7 ( MHNC 4779 View Materials collected on 0 7 December 2004 by J. C. Chaparro & J. A. Ochoa at Erika Lodge, Departamento Madre de Dios).
Ameerega View in CoL gr. pictus: Chaparro et al. 2016 p. 2 (from Amarakaeri Communal Reserve, Departamento Madre de Dios). Ameerega View in CoL sp1: Whitworth & Villacampa 2014 p. 3 (from Manu Learning Centre, Departamento Madre de Dios).
Holotype. MHNC 15488 View Materials ( Fig. 1 View FIGURE 1 ), an adult female, from near Cupudnoe river , 440 m elevation, Peru (Coordinates: 12°47'26.70" S, 70°58'21.30" W), Distrito Madre de Dios, Provincia Manu, Departamento Madre de Dios, collected on 25 April 2010 by R. C. Gutiérrez. GoogleMaps
Paratopotypes. Seven specimens: Five adult females (MUSA 3178, MUSA 3180–3182, MHNC 12988), and two immature females (MUSA 3177, MUSA 3 179), collected with the holotype by R. C. Gutiérrez.
Paratypes. Fifteen specimens, all from Provincia Manu, Departamento Madre de Dios, Peru. Two adult females ( MHNC 10406–10407 View Materials ), and one adult male ( MHNC 10408 View Materials ), from the Reserva Comunal Amarakaeri, 32 km east of Huepetuhe , near the headwaters of Colorado river , 846 m elevation, (Coordinates: 12°59'59.25'' S, 70°50'31.32'' W), collected on 0 7 November 2010 by J. Delgado and J. G. Estrada. Two adult females ( MHNC 10525–10526 View Materials ), and two adult males ( MHNC 10536 View Materials , MHNC 10542 View Materials ), from the Reserva Comunal Amarakaeri, 21.5 km southwest of Shintuya, near Azul river GoogleMaps , 480 m elevation, (Coordinates: 12°48'50.8'' S, 71°06'07.8'' W), collected on 23 May 2011 by J. Delgado. An adult male ( MHNC 15863 View Materials ), from the Reserva Comunal Amarakaeri, Pad A, Lote 76, near Dahuene river GoogleMaps , 530 m elevation, (Coordinates: 12°58'38.47'' S, 71°01'30.73'' W), collected on 20 July 2016 by R. Coronel and G. Valencia. Two immature specimens ( MHNC 10806 View Materials , MHNC 10811 View Materials ), from 3.5 km south of Shintuya, near Serjali river GoogleMaps , 450 m elevation, (Coordinates: 12°42'49.02'' S, 71°15'17.51'' W), collected on 20 January 2011 by J. G. Estrada. Two immature specimens ( MHNC 10816 View Materials , MHNC 10826 View Materials ), from 10 km southwest of Itahuania, near Shilive river GoogleMaps , 450 m elevation, (Coordinates: 12°43'02.93'' S, 71°09'07.56'' W), collected on 26 January 2011 by J. G. Estrada. An immature female ( MHNC 14561 View Materials ; Figs. 2A View FIGURE 2 , B), from the Reserva Comunal Amarakaeri, Pad A, Lote 76, near Colorado river GoogleMaps , 825 m elevation, (Coordinates: 12°59'17.97'' S, 71°0'52.67'' W), collected on 0 4 February 2015 by T. Gregory. An adult specimen ( MHNC 5012 View Materials ), from Aguas Calientes, 2.75 km east (downstream) from Shintuya, (Coordinates: 12°40'7.27" S, 71°16'12.20'' W), collected on 0 9 July 2014 by S. J. Serrano. An immature specimen ( MHNC 4779 View Materials ; Figs. 2 View FIGURE 2 C, D), from Erika Lodge GoogleMaps , 500 m elevation, (Coordinates: 12°45'23.53" S, 71°22'48.68" W), collected on 0 7 December 2004 by J. C. Chaparro and J. A. Ochoa.
Referred specimens. Five specimens: MUSM 31611, MUSM 31664, MUSM 31691, MUSM 31692 and MUSM 31730, from Manu Learning Centre Research Station, 460 m elevation, Peru, (Coordinates: 12°47'21.849" S, 71°23'28.06" W), Distrito Manu, Provincia Manu, Departamento Madre de Dios, Peru, collected on 12 February, 0 5 July, 21July, and 25 July 2012, and on 0 3 February 2013, respectively, by A. Whitworth.
Etymology. The specific name shihuemoy (English pronunciation: shee-way-moy) corresponds to the Harakmbut word for "poison dart frog". The Amarakaeri are aboriginals from Amazonian Peru and their language belongs to the Harakmbut linguistic group. They coexist with the new species.
Diagnosis and comparisons with other species. Following Grant et al. (2006), the new species is assigned to the genus Ameerega on the basis of the following characters: dorsal skin finely granular; Finger I> Finger II when adpressed; toe and finger webbing absent; presence of bright flash marks and absence of ventrolateral line. This is a medium species of Ameerega with an adult SVL of 23.3 ± 2.7 (19.3–30.4 mm, N = 25). Vocal slits present; snout subacuminated, short and protruding. The head and dorsal surfaces are blackish with bronze-brown shades. The flanks are black with metallic bluish-green blotches, with a distinct bright orange or coral stripe extending from groin to above the eye and forward along the canthus rostralis to join around the snout. A flash spot is observed on the hip and upper surface of thighs, and a pale orange spot is located on the anterodorsal base of the thighs. It lacks concealed axillary and calf spots. The ventral surfaces have black reticulations with blue. The maxillary arch has teeth and the dentigerous process of vomers is lacking. The choanae are partly concealed by the palatal shelf of maxilla. The finger discs are weakly to moderately expanded, and when adpressed the first finger is slightly longer than second, and the fourth is shorter than first and second fingers, while the third is longer than all others. The finger discs are weakly to moderately expanded. The finger disc of the third finger of adults is about 1.2 times wider than the immediately adjacent part of the finger. The third finger shows no sexual dimorphism. Basal webbing is present on toes II–IV.
Morphologically, divergence of A. shihuemoy from other species is supported by a unique combination of characters: the lack of the conspicuous axillary, thigh and calf flash marks, characteristics of many other species of Ameerega ; black granular dorsum with cream to light orange dorsolateral lines, metallic blue venter and underside of extremities with black reticulations and spots. Within Ameerega , it shows the general appearance of A. altamazonica Twomey & Brown 2008 , A. boliviana ( Boulenger 1902) , A. hahneli ( Boulenger 1883) , A. ignipedis Brown & Twomey 2009 , A. petersi ( Silverstone 1976) , A. picta ( Bibron 1838) , A. pongoensis ( Schulte 1999) , A. pulchripecta ( Silverstone 1976) , A. simulans ( Myers, Rodriguez & Icochea 1998) , A. smaragdina ( Silverstone 1976) , and A. yungicola ( Lötters, Schmitz & Reichle 2005) , with which it shares a granular black to brown dorsum, a light labial bar, a conspicuous dorsolateral line running from the snout to the groin, and a metallic blue belly and underside of arms and hind limbs. From all these species it can be distinguished by lacking flash marks on the axillae, thighs, and calves (absent only in A. boliviana , A. simulans , A. smaragdina , most A. petersi , and some A. pongoensis ), by having bright cream to orange dorsolateral stripes (white, pale yellowish-green, intense yellow, or green in all other species but A. picta ), and by its blue belly reticulated with black (bluish white and black in A. boliviana , green and blue with black marbling in A. petersi , and green and blue lacking black marbling in A. smaragdina ).
Measurements (in mm) of the holotype. The female holotype ( Fig. 1 View FIGURE 1 ) has SVL, 25.7; TL, 12.3; GBW, 8.2; HW, 7.9; HL, 8.0; ED, 5.2; IOD, 3.0; TSCN, 2.0; NED, 2.6; IND, 2.9; EL, 3.3; HDT, 2.0; MTD, 0.8; HaL, 7.0; WTFD, 0.7; WTF, 0.5; WTTD, 0.8; WTT, 0.4; WFTD, 0.9; WFT, 0.4.
Description of the type series. External morphology: A small to medium-size Ameerega , with adult males attaining approximately 19.2–21.8 mm SVL and adult females approximately 21.5–25.7 mm SVL (measurements and proportions summarized in Table 1 View TABLE 1 ). Dorsal skin of head, body, shank, thigh, and hind limbs coarsely and conspicuously granular; skin smooth or nearly smooth on forelimbs, and smooth on sides of head, body and on ventral surfaces. Head slightly wider between jaw articulations than between outer edges of upper eyelids; head usually narrower than body or about as wide. Head width between jaws 29–33% of SVL. Snout sloping, bluntly pointed or rounded in profile, truncate to rounded (usually) or bluntly pointed in dorsal and ventral view. Nares situated near tip of snout, directed slightly posterolaterally; nares visible from front and from below but not from above. Canthus rostralis short, protruding; loreal region nearly vertical, slightly concave to flat. Interorbital distance wider than upper eyelid. Eye shorter than snout length; distance from center of naris to eye 60–87% of eye length. Tympanum 35–65% of eye size. Hand ( Fig. 1 View FIGURE 1 F) moderately large, its length 23–28% of SVL and 74–90% of head width between angles of jaws. Relative lengths of adpressed fingers IV> I> II> III; fingers I, II, and IV approximately similar in size when adpressed, with overlapping discs; adpressed first finger varies from slightly shorter to slightly longer than second. Finger discs weakly to moderately expanded; third finger disc 1.2–1.8 times wider than distal end of adjacent phalanx. A large outer metacarpal (= palmar) tubercle on the base of the palm and a smaller inner metacarpal tubercle on the base of first finger, these being relatively small, with rounded surfaces. One subarticular tubercle on fingers I & II, and two subarticular tubercles on fingers III & IV; subarticular tubercles well developed and prominently raised, although distal one on finger III and both in IV sometimes weaker; supernumerary tubercles, finger keels, and outer metacarpal fold absent; tibia length 47–54% of SVL; relative lengths of adpressed toes, IV> III> V> II> I; first toe usually reaching base of subarticular tubercle of second toe; basal webbing on toes II–IV; toe fringes absent; outer metatarsal fold absent; a large inner metatarsal fold located on the distal half of tarsus and extended near the inner metatarsal tubercle ( Fig. 1 View FIGURE 1 G); ventrolateral side of tarsus relatively smooth, not especially rugose or tubercular; toes with moderately expanded discs, wider than finger discs ( Fig. 1 View FIGURE 1 G); one to three moderately raised subarticular tubercles (one each on toes I & II, two each on III & V, and three on IV); two large metatarsal tubercles with low, rounded surfaces; inner metatarsal tubercle slightly larger than outer metatarsal tubercle; supernumerary tubercles absent.
Color pattern in life. (Based on living specimens and photographs; Figs. 2 View FIGURE 2 , 3 View FIGURE 3 ). The noticeably granular body is blackish with bronze shades middorsally, turning black and blue dorsolaterally. The flanks are mostly black with metallic bluish green, with a distinct bright orange, coral, or cream stripe extending obliquely from groin to above the eye, and forward along the canthus rostralis to join around the snout. A pale whitish-bronze labial stripe commences between naris and eye, and extends posteriorly under the eye and tympanum to the base of the upper arm. A bright orange to coral spot is present on the hip and upper thigh; there is no calf spot and no pale markings in axilla or groin. The limbs are bronze-brown with shades of green and black. The ventral surfaces are overall blue with variable black reticulation, while in some individuals the ventral surfaces are overall black with blue reticulation.
Color in preservative ( Fig. 1 View FIGURE 1 ). The bright orange and coral colors fade to pale grey; the blue ventral surfaces fade to grey in a dark reticulum of variable distinctiveness.
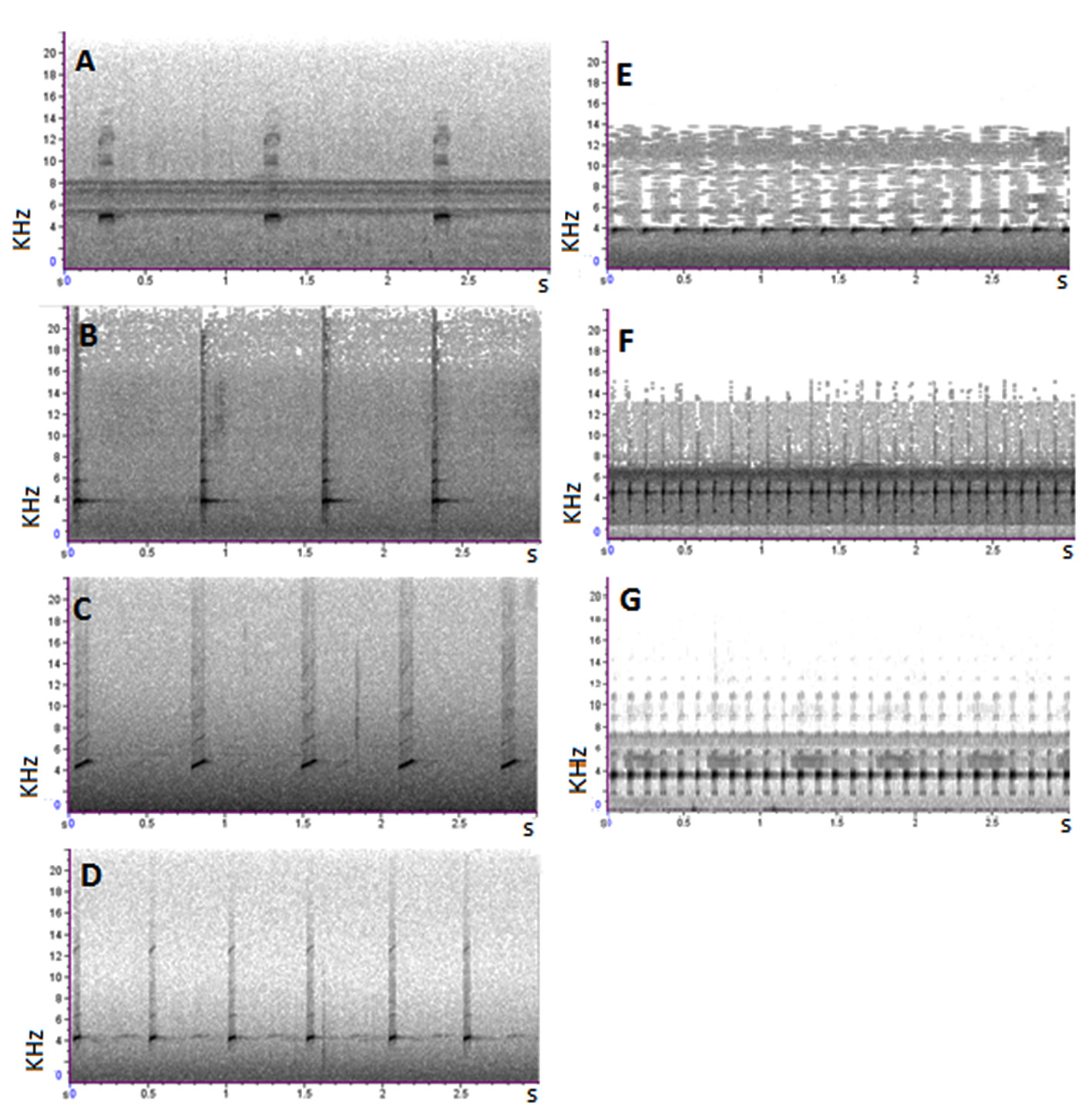
Vocalization. Recordings of three A. shihuemoy males were taken at the Manu Learning Centre reserve. The advertisement call of this new species can be characterized as a series of chirp-like, pulsed notes. Notes are repeated at a rate of 0.8–1.0 notes per second (0.9 ± 0.1), duration of individual notes range from 84–109 ms (98.4 ± 6.8 ms), spaced 969–1196 ms apart (1042.5 ± 186.6 ms), with eight pulses (8.2 ± 0.8) per note. Dominant frequency ranges from 4478.9–4909.6 Hz (4672.72 ± 251.0 Hz) and is not frequency-modulated. Calling activity happens most frequently in the early mornings between 05:00 to 09:00 and late afternoon between 16:00 to 18:00. We also recorded a second call in A. shihuemoy consisting of three notes in quick succession (within 174.9 ± 16.6 ms of each other), repeated once every four to five seconds. The three notes in this call have different duration; the first note (87.2 ± 2.5 ms) typically has a longer duration than the second (69.4 ± 2.6 ms) and the third note (72.8 ± 2.8 ms). This three-note call seems to function as an aggressive or territorial call.
We compared the call of the following species with Ameerega shihuemoy : two recordings of A. simulans (74 advertisement calls) by J. M. Padial from Marcapata, Quispicanchis Province , Department Cusco, Peru, were obtained from Fonoteca Zoologica (Museo Nacional de Ciencias Naturales 2016); two recordings of A. boliviana (45 advertisement calls) by J. Bosch & I. De la Riva from Correo-Apolo , La Paz, Bolivia ; one recording of A. yungicola (30 advertisement calls) by M. Pacheco-Suarez from Caranavi, Yungas, Bolivia obtained from “Guía fotográfica de los anfibios de la region de las yungas Bolivia ” ( Pacheco-Suarez 2015); two recordings of A. hahneli (46 advertisement calls) by J. Serrano-Rojas from Shintuya , Madre de Dios, Peru ; two recordings of A. picta (63 advertisement calls) by J. M. Padial from Madidi National Park, Northern Bolivia and from Paractito , Cochabamba Department, Central West Bolivia deposited at Fonoteca Zoologica (Museo Nacional de Ciencias Naturales 2016); two recordings of A. macero (55 advertisement calls) by J. Serrano-Rojas from Manu Learning Centre , Madre de Dios, Peru. The advertisement call of A. shihuemoy is easily distinguished ( Fig. 4 View FIGURE 4 ) from the morphologically similar Ameerega species of the Ameerega picta group (for measurable call parameters and intra- and interspecific variation see Table 2).
Parameters A. shihuemoy A. simulans A. picta A. hahneli
Mean SD Mean SD Mean SD Mean SD Calls/N° of individuals 80/3 - 74/2 - 63/2 - 46/2 -
Notes/min 52.8 5.4 79.2 3.6 130.2 6.0 511.8 4.2 Notes/s 0.9 0.1 1.3 0.1 2.2 0.1 8.5 0.1 Duration of the note (ms) 98.4 6.8 104.9 10.2 45.8 2.4 13.4 2.8 Break between calls (ms) 1042.5 186.6 691.4 162.7 429.9 44.6 107.4 12.6 Fundamental frequency (Hz) 4237.0 281.9 4060.9 74.6 3770.7 76.7 2516.8 83.7 Dominant frequency (Hz) 4672.7 251.0 4460.3 157.7 4044.2 94.7 4550.0 49.1
continued.
Parameters A. boliviana A. yungicola A. macero
Mean SD Mean SD Mean SD Calls/N° of individuals 45/ 2 - 30 - 55 /2 -
Notes/min 72.0 4.2 313.2 0.0 519.4 2.2 Notes/s 1.2 0.1 5.2 0.0 8.7 0.0 Duration of the note (ms) 80.9 8.6 47.8 4.7 37.6 1.3 Break between calls (ms) 783.2 88.6 148.1 7.3 76.1 2.7 Fundamental frequency (Hz) 3416.1 68.2 3475.7 43.5 3353.7 38.1 Dominant frequency (Hz) 3846.0 46.3 3703.7 0.0 3617.6 0.0 The note duration of the new species (84–109 ms) is longer than A. hahneli (11–18 ms), A. macero (36–40 ms), A. picta (41–52 ms) and A. yungicola (40–57 ms), but similar to A. boliviana (75–94 ms) and A. simulans (91–118 ms). The space between calls in A. shihuemoy is longer than in the other six species. The number of notes per second in the advertisement call of A. shihuemoy is lower than the other six species. The call of the new species has a higher fundamental frequency than all other species but similar to A. simulans , and a higher dominant frequency than all other species but similar to A. hahneli and A. simulans , except the latter species has pronounced upward frequency-modulated notes by about 950 Hz ( Fig. 5 View FIGURE 5 ). It is important to take into consideration that this comparison was performed on recordings from three individuals of A. shihuemoy from a single location.
Tadpoles. Ontogenetic variation of 11 characteristics measured for 16 tadpoles in stages 24–46 ( Gosner 1960) are summarized in Table 3 View TABLE 3 . The tadpole of A. shihuemoy sp. nov. belongs to the exotrophic ecomorphological guild, benthic type as defined by Altig & Johnson (1989). This description is based on a tadpole (MHNC lot 12987) at developmental Stage 25 ( Gosner 1960; Figs. 3 View FIGURE 3 A, C) and measuring a total length 17.2 mm; body length 6.2 mm; maximum width 4.1 mm; depth 3.0 mm; internarial distance 0.6 mm; eye to nares distance 0.5 mm; eye diameter 0.6 mm; interorbital distance 1.0 mm, oral disc width 1.2 mm; tail length 11.0 mm. The body is globular, compressed in lateral view, and ovoid in dorsal view. The snout is rounded in dorsal view ( Figs. 6 View FIGURE 6 A and C). The mouth is located anteroventrally and is surrounded by a small oral disc ( Figs. 6 View FIGURE 6 B and D). Papillae are laterally emarginated, simple and conical. Marginal papillae absent on anterior labium, present in one complete row on posterior labium. Anterior jaw sheath has a medial indentation with reduced serration, posterior jaw sheath Vshaped and has serration throughout. Lateral processes long, extending well past lower jaw. Labial Tooth Row Formula (LTRF) is 2(2)/3(1). A-1 complete, A-2 with medial gap, same width as A-1. P-1, P-2, and P-3 complete; P-1 and P-2 equal width, P-3 shorter. Nares are oval, small, without projections and inflexions, and are located dorsolaterally. Eyes are small and oriented dorsolaterally. The spiracle is single, sinistral, and located just before mid-body. Fins are concave and the posterior end is rounded, reaching their maximum height at the last third of the tail; the dorsal fin does not extend onto the body. The maximum tail height is reached at about the end length and is as high as body height. In preservative, the body is dark grey, the belly is translucent with intestines slightly visible; caudal musculature creamy white with small, irregular grey flecks and slightly translucent fins. In life, the body is dark brown with black spots, the belly is transparent but slightly pigmented posteriorly, intestines well visible through skin, caudal musculature poorly pigmented and transparent tail fin with melanophores in small and irregular clusters along the tail.
Gosner stages In addition, we described observations in the field following Gosner (1960). We found two clutches of eggs in small rocky cavities; they were monitored every two days in order to take notes about the development of eggs. The first set was found on 25 June 2013, with 22 eggs at stage 13; a stage in which the neural plate develops on the dorsal surface, and eggs were covered by a transparent orange mucilage. After nine days, eggs become tadpoles, where they reach stage 17 (tail bud). The same embryos were abandoned by the father after two days on 0 5 July 2013. The embryos were getting dirty and dry, and inside we could observe the larvae of an insect, which could likely prey upon the embryos.
The second set was found on 10 July 2013, containing 25 eggs in stage 13 covered by a transparent orange mucilage. After ten days, eggs were observed at stage 19 (heart beat), which is represented mainly by the development of the gills and tail. Seven days later tadpoles were at stage 21 (cornea transparent), in which the tail becomes increasingly transparent. Finally, three days later (30 July 2013) only five tadpoles were found at stage 22 (tail fin circulation); we assumed that the father was carrying the rest of tadpoles.
One year later we found two other tadpoles at stage 26 (limb bud), on 11 June 2014. After four days, these reached stage 38 (toe development XIII). Another group of four tadpoles were found at stage 40 (cloacal tail piece XV–XVII), which changed to stage 42 (nose development XXI) after four days. Two more tadpoles were found at stage 42 (nose development XXI) on 15 June 2014; the stage 46 (metamorphosis complete) was reached after three days. In summary, we suggest that the development between stages 13–22 takes twenty days, stages 26–38, four days, and stages 40–46, seven days. However, the time of development of stages 22–26, and stages 36–40 remains unknown.
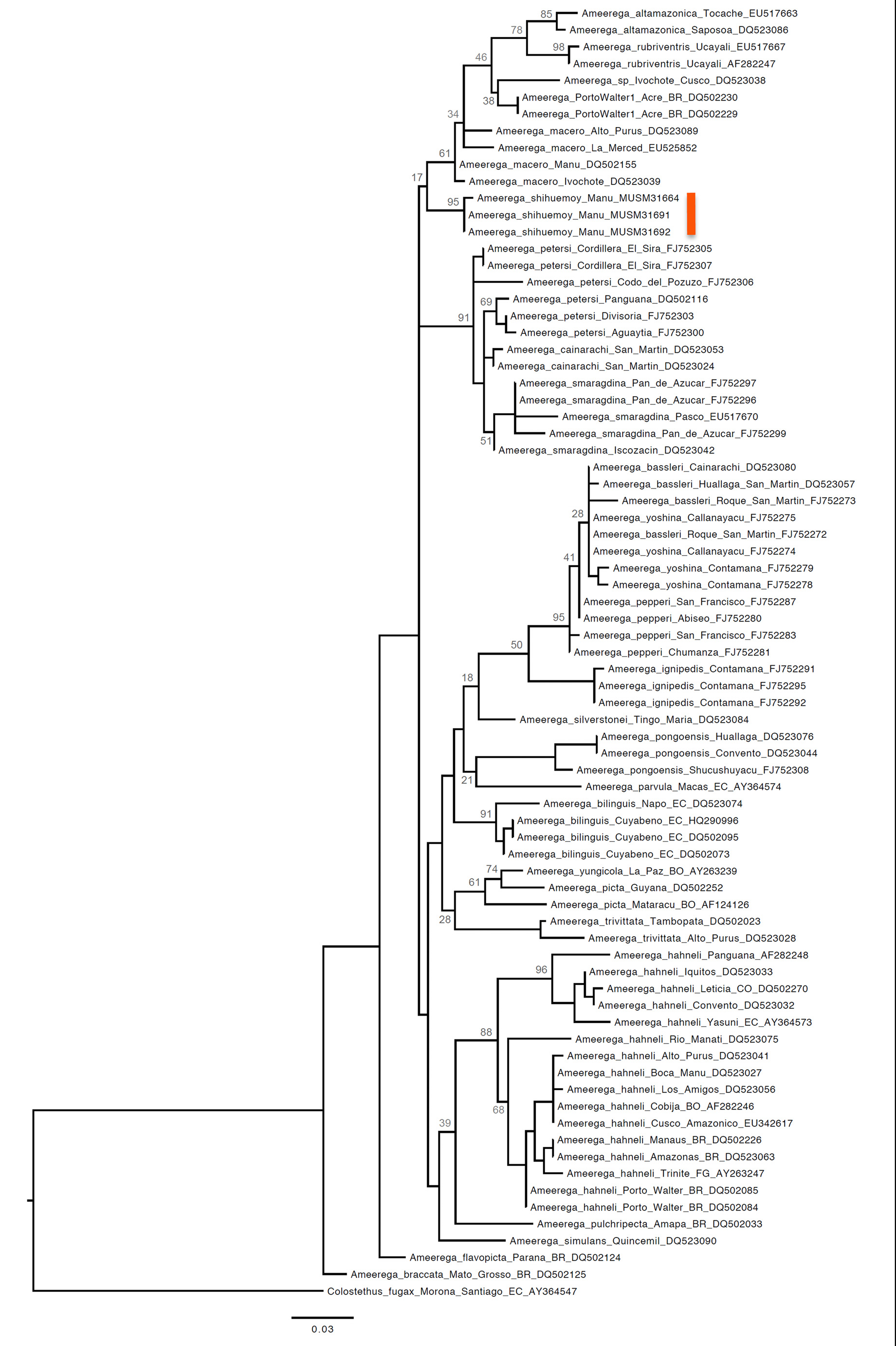
Phylogenetics. Our Maximum Likelihood (ML) tree was generally congruent with previous molecular phylogenies based on mitochondrial DNA ( Twomey & Brown 2008, Brown & Twomey 2009) and supported the distinctiveness of the Ameerega shihuemoy from other closely related taxa. The new species was most closely related to Ameerega macero , A. altamazonica , A. rubriventris ( Lötters et al. 1997) , and two undescribed species— Ameerega sp. from Porto Walter, Acre, Brazil, and Ameerega sp. from Ivochote, Cusco, Peru ( Fig. 7 View FIGURE 7 ). In general, the topology we recovered is similar to the ones obtained by Twomey & Brown (2008) and Brown & Twomey (2009), although there were some differences. These differences were likely a result of using only one mitochondrial gene (our study) versus three mitochondrial genes. Nevertheless, in addition to determining the relationship between the new species and other closely related taxa, our analysis inferred three main cladesbassleri, hahneli and petersi —identified in these previous studies ( Twomey & Brown 2008, Brown & Twomey 2009). Our analysis also inferred several species pairs that were inferred to be closely related according to the previous analyses (e.g., A. picta and A. yungicola ; A. rubriventris and A. altamazonica ).
Distribution and natural history. Ameerega shihuemoy is distributed in southeastern Peru at the transition between the montane forest and the lowlands. Its altitudinal range spans from 340 to 850 m above sea level. This species is known from nine localities; three in the buffer zone of the Manu Biosphere Reserve (The Manu Learning Centre, Erika lodge, and Aguas Calientes, Shintuya) and another six locations in the Amarakaeri Communal Reserve. The area is humid and hot: during the wet season, the average daily temperature is 24.78°C, the average humidity is 90.58% and the rainfall is 3098 mm; during the dry season the average temperature is 23.74°C, the average humidity is 84.89% and the average monthly rainfall is 1557 mm (climate information from Whitworth et al. 2016b).
Ameerega shihuemoy is most commonly found in low disturbance forest. During the dry season it is frequently found amongst boulders along or near forest streams ( Fig. 8 View FIGURE 8 ). During the rainy season it moves away from the streams into the forest interior. The activity patterns appear to be distinctly crepuscular, being most active in the early morning (05:30 to 09:00) or evening (16:00 to 18:00) when males call vigorously. Males typically call from exposed positions of rocks, leaf litter, or woody debris. Calling has been heard throughout the year. Crevices or holes made of boulders or roots are used as refuges. During the night, individuals rest on low vegetation between 0.1 to 0.5 m above the ground.
Reproduction takes place near both permanent and seasonal streams. On 25 June 2013 and 10 July 2013 two clutches of eggs were found inside of small shelters next to a stream. These clutches contained 22 and 25 eggs respectively, and were guarded by males. On 30 July 2013, one uncollected male was observed transporting ten tadpoles along a slow moving stream. We detected free-living A. shihuemoy tadpoles and metamorphs co-occurring with metamorphs of A. macero in the same streams; with shallow, slow moving, clear water and bottoms of sand and dead leaves.
Results of the GLM analysis in both forest types (CCR and SLR) showed that six environmental variables were the most important habitat features to explain the species presence near streams (frog presence ~ canopy cover + leaf litter cover + number of potential refuges + presence of large rocks + presence of a still body of water + stream flow; Table 4 View TABLE 4 , Appendix IV). The best supported model explained 89.05% of the variation in the data. When we ran the same analysis just in streams where the frogs were found (SLR forest), the GLM analysis presents two top models with ∆AICc <2 ( Table 5 View TABLE 5 , Appendix V). The best supported model explained 83.41% of the variation in the data. Model averaging was carried out in these two models. The relative importance for leaf litter cover, number of potential refuges, presence of large rocks, presence of a still body of water and stream flow were 1.0, signalling that they were important predictors. Canopy cover had some support (0.69), suggesting lower importance when comparing just within the SLR forest, and was therefore excluded from the top-preferred, and more parsimonious model (Appendix V).
This top model suggests that a greater number of potential refuges (often created by the presence of a high quantity of large rocks), a great amount of leaf litter cover, the presence of still bodies of water, and a low stream flow (likely to benefit breeding strategy) are the most important predictors of the presence of A. shihuemoy near streams.
Conservation status. Applying IUCN Red List criteria (IUCN Red List of Threatened Species, 2 0 0 1 Criteria & Categories (version 3.1.); http://www.iucnredlist.org/info/categories_criteria2001, accessed 20 February 2016), which indicates that if a species occurs in less than ten threat-defined locations and the extent of occurrence is less than 20,000 km 2, it should be classified as Vulnerable or Endangered. Ameerega shihuemoy is known from nine localities distributed in the buffer zone of the Manu Biosphere Reserve and the Amarakaeri Communal Reserve ( Fig. 9 View FIGURE 9 ), with an estimated extent of occurrence of ca. 1,124 km 2, as such, we suggest that this new species might be classified as Vulnerable. However, due to the lack of intensive sampling effort in the Amarakaeri Communal Reserve, which may host a greater number of locations, we propose that A. shihuemoy should likely be categorized as Near Threatened (NT).
Predictor variables are canopy cover (A), leaf litter cover (B), number of potential refuges (C), percentage of large rocks (D), presence of a still body of water (E), stream flow (F), leaf litter depth (G) and Wood debris percent (H).
Predictor variables are canopy cover (A), leaf litter cover (B), number of potential refuges (C), percentage of large rocks (D), presence of a still body of water (E), stream flow (F), leaf litter depth (G) and Wood debris percent (H).
Remarks. The area that Ameerega shihuemoy inhabits is threatened by human disturbance associated with activities such as logging, agriculture, and gold mining, especially within the Biosphere Reserve ( Finer et al. 2015). Currently, these threats are increasing rapidly due to a draft law in favor of the construction of The Nuevo Eden– Boca Manu–Boca Colorado road that cuts through the buffer zones of the Amarakaeri Communal Reserve and Manu National Park ( Finer et al. 2016). This road has already begun to be illegally constructed with the purpose of fuel transport for illegal mining and logging. The approval of this road will trigger increased human disturbance within the buffer zone, resulting in a potential new deforestation hotspot in the Madre de Dios region. Changes affecting the habitat surrounding the reserve could ultimately lead to degradation of habitat within the nearby protected areas ( Laurance et al. 2012). This could therefore result in both a reduction of the number of known populations of A. shihuemoy and reduce the overall area of occupancy of the species. The discovery of a new species in Manu Province underscores the need of continued habitat protection in Madre de Dios region ( Jarvis et al. 2015), which is home to more than 114 amphibian species, and is one of the most biodiverse regions on the planet for herpetofauna (von May et al. 2009; Catenazzi et al. 2013) and a variety of other taxa.
TABLE 1. Measurements (in mm) of Ameerega shihuemoy type series.
| MHNC 15448 | MUSA 3181 | MUSA 3178 | MHNC 10525 | MHNC 12988 | MHNC 10526 | MHNC 10406 | MHNC 10407 | MUSA 3180 | MUSA 3182 | MHNC 10408 | MHNC 10536 | MHNC 10542 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| SEX | Female | Female | Female | Female | Female | Female | Female | Female | Female | Female | Male | Male | Male |
| SVL | 25.7 | 25.2 | 25.1 | 24.4 | 24.0 | 23.5 | 23.2 | 23.1 | 22.8 | 21.5 | 21.8 | 21.6 | 19.2 |
| TL | 12.3 | 12.9 | 11.4 | 11.8 | 12.2 | 11.2 | 11.5 | 11.2 | 11.6 | 11.7 | 11.0 | 10.9 | 9.0 |
| HaL | 7.0 | 6.8 | 6.3 | 6.3 | 6.5 | 6.0 | 6.2 | 6.0 | 6.1 | 5.5 | 6.1 | 5.8 | 4.5 |
| HL | 8.0 | 7.2 | 7.9 | 7.8 | 7.5 | 7.7 | 8.2 | 8.3 | 6.7 | 6.9 | 6.8 | 7.9 | 7.4 |
| HW | 7.9 | 7.6 | 7.8 | 7.4 | 7.6 | 6.8 | 7.3 | 7.4 | 6.8 | 7.2 | 6.8 | 6.7 | 6.0 |
| GBW | 8.2 | 8.4 | 7.9 | 8.5 | 7.2 | 7.6 | 8.3 | 7.4 | 6.8 | 6.7 | 7.9 | 7.6 | 7.6 |
| IOD | 3.0 | 3.1 | 3.0 | 2.3 | 2.7 | 2.1 | 2.3 | 2.3 | 2.8 | 2.3 | 2.1 | 2.3 | 2.1 |
| ED | 5.2 | 5.2 | 5.1 | 5.0 | 5.1 | 5.1 | 4.7 | 5.1 | 4.9 | 4.8 | 4.5 | 4.7 | 4.3 |
| HDT | 2.0 | 1.9 | 1.5 | 1.4 | 1.5 | 1.3 | 1.5 | 1.4 | 1.5 | 1.1 | 1.4 | 1.1 | 1.0 |
| EL | 3.3 | 3.0 | 3.3 | 3.1 | 3.1 | 3.1 | 2.9 | 2.9 | 3.1 | 3.0 | 2.7 | 2.7 | 2.9 |
| TSCN | 2.0 | 1.6 | 1.8 | 1.3 | 1.6 | 1.5 | 1.2 | 1.5 | 1.8 | 1.8 | 1.0 | 1.1 | 1.1 |
| NED | 2.6 | 2.6 | 2.3 | 2.2 | 2.1 | 2.0 | 1.9 | 2.1 | 2.1 | 1.8 | 2.3 | 1.8 | 2.0 |
| IND | 2.9 | 2.7 | 2.9 | 2.9 | 2.9 | 2.5 | 2.3 | 2.8 | 2.6 | 2.8 | 2.7 | 2.6 | 2.2 |
| MTD | 0.8 | 0.8 | 0.9 | 0.7 | 0.9 | 0.7 | 0.7 | 0.5 | 0.7 | 0.6 | 0.8 | 0.6 | 0.7 |
| W3FD | 0.7 | 0.8 | 0.6 | 0.7 | 0.8 | 0.7 | 0.6 | 0.7 | 0.7 | 0.5 | 0.6 | 0.5 | 0.7 |
| W3F | 0.5 | 0.5 | 0.3 | 0.4 | 0.4 | 0.4 | 0.3 | 0.4 | 0.4 | 0.3 | 0.4 | 0.4 | 0.4 |
| W4TD | 0.9 | 0.9 | 0.8 | 0.7 | 0.8 | 0.7 | 0.5 | 0.7 | 0.7 | 0.5 | 0.6 | 0.7 | 0.6 |
| W4T | 0.4 | 0.4 | 0.4 | 0.3 | 0.3 | 0.4 | 0.3 | 0.4 | 0.3 | 0.3 | 0.4 | 0.5 | 0.4 |
| W3TD | 0.8 | 0.8 | 0.7 | 0.7 | 0.7 | 0.5 | 0.7 | 0.7 | 0.7 | 0.6 | 0.4 | 0.6 | 0.6 |
| W3T | 0.4 | 0.5 | 0.4 | 0.4 | 0.4 | 0.4 | 0.3 | 0.4 | 0.3 | 0.3 | 0.3 | 0.4 | 0.3 |
TABLE 3. Morphometric measurements (mean ± SD, in millimeters) of developmental stages of tadpoles of Ameerega shihuemoy. Total length (TL), body length (BL), body height (BH), body width (BW), tail length (TAL), tail muscle height at the base of the tail (TMH), tail muscle width at the base of tail (TMW), eye-nares distance (END), internarial distance (IND), eye diameter (ED), interorbital distance (IOD), and oral disc width (OD).
| Character | 24 (N=1) | 25 (N=3) | 26 (N=2) | 27 (N=1) | 40 (N=2) | 41 (N=2) | 42 (N=1) | 43 (N=1) | 44 (N=1) | 45 (N=1) | 46 (N=1) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| TL | 17.8 | 16.9 ± 1.9 | 17 ± 2.8 | 18.3 | 22.6 ± 0.2 | 23.1 ± 0.6 | 16.6 | 14.5 | 12.1 | 10.5 | 10.4 |
| BL BW | 6.4 3.9 | 6 ± 0.9 3.6 ± 0.5 | 6.5 ± 0.8 3.9 ± 0.4 | 9.5 4.7 | 9.4 ± 0.1 5.3 ± 0 | 9.4 ± 0.4 6.8 ± 1.1 | 8.5 4.3 | 9.2 4.6 | 11.9 3.5 | 9.5 4.8 | 10.4 4.6 |
| BH TAL TMW | 2.2 11.4 1.1 | 2.6 ± 0.4 10.9 ± 1.1 1.5 ± 0.2 | 3 ± 0.2 10.5 ± 2 1.3 ± 0.1 | 3.5 11.8 2.0 | 3.8 ± 0.4 13.2 ± 0.1 2.2 ± 0.2 | 4.5 ± 0.1 13.7 ± 0.1 2.8 ± 0.1 | 3.2 8.1 2.2 | 4.2 5.3 2.7 | 3.3 0.2 1.4 | 2.4 1.0 1.9 | 2.4 _ _ |
| TMH END IND | 1.7 1.0 0.6 | 1.6 ± 0.2 0.6 ± 0.3 0.6 ± 0.1 | 2.1 ± 0.2 0.6 ± 0.2 0.9 ± 0.2 | 2.0 1.1 1.0 | 3.1 ± 0.2 0.9 ± 0.1 0.9 ± 0.2 | 3.5 ± 0.1 1 ± 0.3 1.2 ± 0.2 | 2.3 0.6 1.1 | 2.7 1.6 1.1 | 1.1 1.0 0.9 | 1.7 0.8 0.9 | _ 1.8 0.8 |
| ED IOD OD | 0.6 0.8 1.1 | 0.6 ± 0.2 0.9 ± 0.2 1.1 ± 0.4 | 0.7 ± 0.1 1.2 ± 0 1.2 ± 0 | 1.0 1.7 1.2 | 1.3 ± 0 1.7 ± 0.4 2.15 ± 0.1 | 1.3 ± 0.1 2 ± 0.1 2.2 ± 0 | 1.4 2.0 2.3 | 1.3 2.5 1.8 | 1.5 2.1 2.6 | 1.4 2.3 3.2 | 1.5 2.4 3.9 |
TABLE 4. Model selection for environmental variables potentially explaining habitat selection of Ameerega shihuemoy based on Akaike’s Information Criterion corrected for small sample size (AICc) showing the ten first candidates models and the null model: log likelihood (logLik), k (number of parameters), AICc values, AICc differences between the best model and each candidate model (∆ AICc) and Akaike weights (ωi).
| Model | K | AICc | ∆AICc | ωi |
|---|---|---|---|---|
| frog presence~ A + B + C + D + E + F | 6 | 39.015 | 0.000 | 0.395 |
| frog presence~ A + B + C + D + E + F + G | 7 | 41.169 | 2.154 | 0.135 |
| frog presence~ A + B + C + D + E + F + H | 7 | 41.210 | 2.194 | 0.132 |
| frog presence~ B + C + D + E + F | 5 | 41.567 | 2.552 | 0.110 |
| frog presence~ B + C + D + E + F + G | 6 | 43.303 | 4.288 | 0.046 |
| frog presence~ A + B + C + D + E + F + G + H | 8 | 43.388 | 4.372 | 0.044 |
| frog presence~ B + C + D + E + F + H | 6 | 43.593 | 4.578 | 0.040 |
| frog presence~ A + B + C + D + F | 5 | 45.232 | 6.217 | 0.018 |
| frog presence~ B + C + D + E + F + G + H | 7 | 45.414 | 6.399 | 0.016 |
| frog presence~ B + C + D + F | 4 | 46.134 | 7.118 | 0.011 |
| frog presence~1 | 1 | 224.474 | 185.459 | 0.000 |
TABLE 5. Model selection for environmental variables potentially explaining habitat selection of Ameerega shihuemoy, on streams within the SLR forest, based on Akaike’s Information Criterion corrected for small sample size (AICc), showing the ten first candidates models and the null model: log likelihood (logLik), k (number of parameters), AICc values, AICc differences between the best model and each candidate model (∆ AICc) and Akaike weights (ωi).
| Model | K | AICc | ∆AICc | ωi |
|---|---|---|---|---|
| frog presence~ A + B + C + D + E + F frog presence~ B + C + D + E + F frog presence~ A + B + C + D + E + F + H | 6 5 7 | 38.109 39.667 40.385 | 0.000 1.558 2.276 | 0.284 0.130 0.091 |
| frog presence~ A + B + C + D + E + F + G frog presence~ A + B + C + D + F frog presence~ B + C + D + F | 7 5 4 | 40.400 41.082 41.728 | 2.292 2.973 3.619 | 0.090 0.064 0.046 |
| frog presence~ B + C + D + E + F + G frog presence~ B + C + D + E + F + H frog presence~ A + B + C + D + E + F + G + H | 6 6 8 | 41.733 41.914 42.712 | 3.625 3.805 4.603 | 0.046 0.042 0.028 |
| frog presence~ B + D + E + F frog presence~1 | 4 1 | 42.793 164.154 | 4.684 126.045 | 0.027 0.000 |
| MHNC |
Musee d'Histoire Naturelle - La Chaux-de-Fonds |
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.