Vaejovis troupi Ayrey et Soleglad, 2015
|
publication ID |
https://doi.org/ 10.18590/euscorpius.2015.vol2015.iss194.1 |
|
publication LSID |
lsid:zoobank.org:pub:B5D24928-5D29-40D8-8448-4953099A75EB |
|
DOI |
https://doi.org/10.5281/zenodo.5508066 |
|
persistent identifier |
https://treatment.plazi.org/id/8B5C2249-188E-4AAC-A26F-956F272DAEED |
|
taxon LSID |
lsid:zoobank.org:act:8B5C2249-188E-4AAC-A26F-956F272DAEED |
|
treatment provided by |
Carolina |
|
scientific name |
Vaejovis troupi Ayrey et Soleglad |
| status |
sp. nov. |
Vaejovis troupi Ayrey et Soleglad View in CoL , sp. n.
Figs. 1–7 View Figure 1 View Figures 2-6 View Figure 7 , 10 View Figure 10 ; Tables 1–2
http://zoobank.org/urn:lsid:zoobank.org:act:8B5C22 49-188E-4AAC-A26F-956F272DAEED
References:
Vaejovis sp. cf. vorhiesi: Ayrey, 2013a: 3 View in CoL ; Ayrey, 2013b: 2; Ayrey, 2014: 3; Vaejovis sp., vorhiesi group View in CoL : Bryson et al., 2013: 5.
Diagnosis: Medium sized species, 26 mm in length (only female known); light brown in color with variegated patterns on the carapace, lighter mottlings on the mesosoma, pedipalps, and legs. Pectinal tooth counts 11–12. Chelal fingers with six inner denticles (ID) on both the movable and fixed fingers. Patellar external trichobothria est–et 3 –v 3 form an angle, et 3 occurring more proximally.
Etymology. This species is named in honor of Robert Troup who found the only specimen.
Distribution. Known only from the type locality, Whetstone Mountains, Cochise County, Arizona, USA.
Type material. Holotype female, Whetstone Mountains , Cochise County, Arizona, USA (31.81093, -110.39370; 1607 m asl), 14 October 2009, leg. R. Troup, specimen #RA223, deposited in USNM. GoogleMaps
FEMALE. Description based on holotype female. See Table 1 for measurements.
COLORATION. Color is medium brown, slightly lighter on the legs, telson orange. Weak underlying mottling is visible on the carapace, mesosoma, pedipalps, and legs.
CARAPACE ( Fig. 3 View Figures 2-6 ). Anterior margin of carapace with a conspicuous wide emargination. Carapace moderately granular occurring primarily in the subtle mottled areas. Three lateral eyes on each side, the most proximal the smallest. Ratio of median eyes position from anterior edge/carapace length 0.340; carapace length/width at median eyes 1.442.
MESOSOMA. Tergites moderately granular on proximal half on segments I– VI. Tergite VII with strong crenulated median and lateral carina. Sternites III– VI smooth. Sternite VII with rough surface with weak ventral lateral carinae. Stigma small and ovoid with median side rotated 35 degrees from posterior sternite margin.
STERNUM. Sternum conforms to type 2, lateral lobes and apex subtly defined. Sclerite is wider than long.
GENITAL OPERCULUM. Sclerites separated on posterior one-third.
PECTINES. With three anterior lamellae, 8/8 middle lamellae, and 11/12 teeth. Sensorial areas present on all teeth and fulcra are present.
METASOMA ( Fig. 2 View Figures 2-6 ). Segments I–IV: dorsal and dorsolateral carinae strong and serrate with distal denticle of I–III enlarged and spinoid, and spinoid on the dorsal carinae and expanded and flared on the dorsolateral carinae for segment IV. Lateral carinae strong and serrate on I, present on posterior 2/3 of II, posterior 1/3 of III, and obsolete on IV. Ventrolateral carinae strong and serrate, ventromedian moderately granular on I and crenulate to serrate on II–IV. Segment V: Dorsolateral carinae strong and irregularly granulate. Lateral carinae granular on basal 3/5. Ventrolateral and ventromedian carinae serrate. Anal arch with approximately 16 small denticles. Dorsal intercarinal spaces irregularly granular.
TELSON ( Fig. 2 View Figures 2-6 ). Smooth with several setae on ventral surface. Vesicular tabs with one to two small pointed granules. Subaculear tubercle present but small. LAS present exhibiting 5–6 serrations.
CHELICERAE. Dorsal edge of movable cheliceral finger with two subdistal (sd) denticles. Ventral edge is smooth, with well-developed serrula on distal half.
PEDIPALP ( Figs. 4, 6–7 View Figures 2-6 View Figure 7 ). Femur. Dorsointernal and dorsoexternal carinae serrated, and ventrointernal crenulated, ventroexternal rounded. Dorsal and ventral surfaces very rough, internal surface with scattered granules, and external with line of serrated granules. Patella. Dorsointernal and ventrointernal carinae crenulated, dorsoexternal and ventroexternal carinae granulated. Dorsal patellar ( DPS) and ventral patellar ( VPS) spurs formed with a pointed granule, DPS c carina well developed with approximately 14 serrated granules. Chela. Digital (D1) carina weak, with small irregularly placed granules, subdigital (D2) represented with a single rounded granule, dorsosecondary (D3) rounded with slight median granules, dorsomarginal (D4) rounded with scattered granules, dorsointernal (D5) rounded and irregularly granulated with pointed granules, ventroexternal ( V 1) and ventromedian ( V 2) carinae rounded and smooth, ventrointernal ( V 3) rounded with minor granulation, and external (E) carina weak to obsolete. Chelal finger median denticle ( MD) rows in straight line. Fixed finger median denticles ( MD) divided into 6 groups by 5 outer ( OD) denticles, and 6 ID denticles are found on the inner edge. Movable finger with 6 MD groups, 5 OD denticles and 6 ID denticles. Trichobothrial pattern type C orthobothriotaxic (see Figure 7 View Figure 7 ). Chelal ib and it trichobothria located at fixed finger’s base, considerably proximal of sixth ID denticle; Dt on chela is proximal of palm midpoint; dt and dst are proximal to et and distal of est; patellar v 3 is located on external surface and positioned distally of et 3. Trichobothrium et 3 is located proximal of a line connecting est and v 3.
LEGS ( Fig. 5 View Figures 2-6 ). Ventral surface of tarsomere II with single median row of spinules terminating distally with one spinule pair.
Reproduction. This species is not represented in the Vaejovis “vorhiesi” group study (Ayrey, 2013a) but is presumed to have behavior similar to the rest of the group. The “vorhiesi” group average clutch size is 22.87 first instars (n=100 births, 2,287 first instars). Being one of the larger species it would have a relatively large clutch size, estimated to be approximately 25 first instars. Birth and postpartum behavior would be as described in Ayrey (2013a).
Comparison of Species
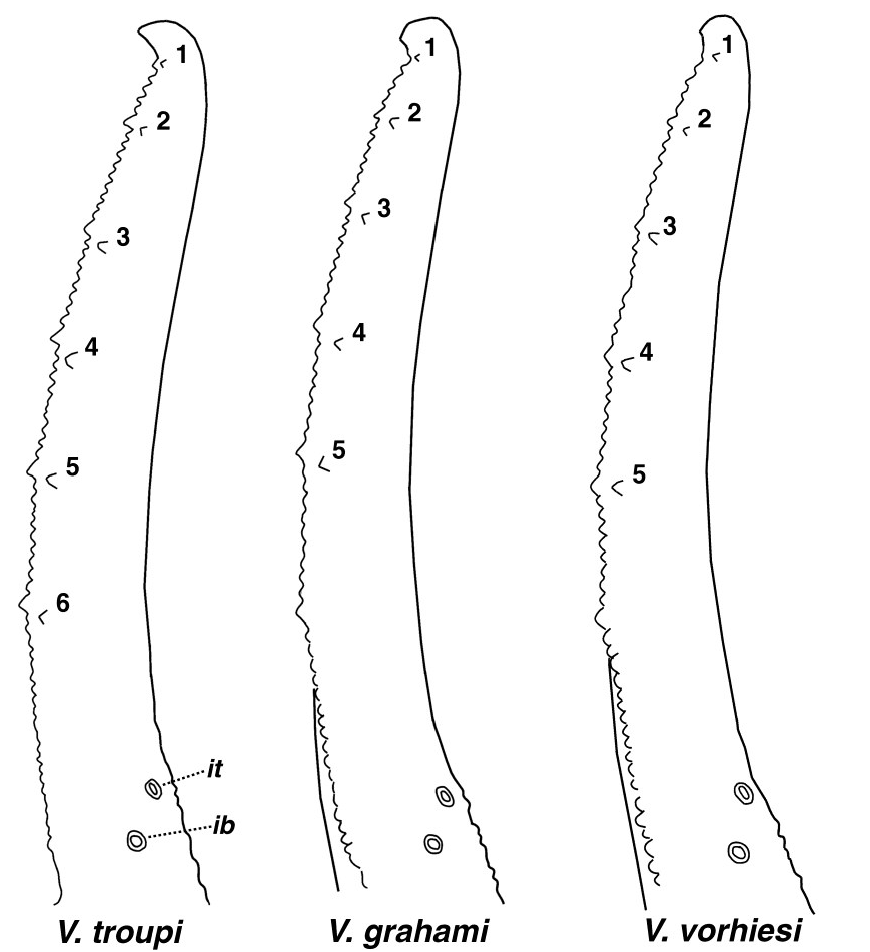
With the description of Vaejovis troupi presented herein, 18 species are now currently placed in the “ vorhiesi ” group of Vaejovis . Morphologically, these species are roughly partitioned into two presumably phylogenetic groups, those with seven inner denticles (ID) on the chelal movable finger (seven species), found primarily in central to northern Arizona, and those with six ID (eleven species) located primarily in southeastern Arizona, southwestern New Mexico, and northern Mexico (see Map in Fig. 11 View Figure 11 ).
In this contribution we compare V. troupi to two other species, V. grahami and V. vorhiesi , both which are found in adjacent sky island mountain ranges in southeastern Arizona. The choice of these two species is based, in part, on the recent phylogenetic analysis based on molecular considerations (see Bryson et al., 2013), which suggests that these two species are the closest relatives of V. troupi (see below).
Geological considerations. Weldon Heald (1951) coined the term “Sky Islands” to describe the distinct isolated groups of Madrean pine-oak woodlands found at higher elevations in a complex of small mountain ranges in southern and southeastern Arizona, southwestern New Mexico, and northwestern Mexico. These “Sky Islands” are surrounded at lower elevations by the Sonoran and Chihuahuan Deserts thus essentially being isolated by different extreme xeric climates and different microhabitats. Here we use an expanded definition that includes all isolated, high elevation habitats in Arizona, New Mexico and Mexico. Many habitats in northern Arizona and northern New Mexico are high elevation habitats isolated by the intervening Colorado Plateau. Graham (2007: 1) states: “...Faunas adapted to these isolated mountain environments are therefore disconnected from similar habitat on adjacent mountain ranges by extreme climates of xeric lowlands. ...”. This paper applies to the isolated, elevation habitats on the Colorado Plateau as well as it does the Madrean pineoak woodlands of the south.
These three species are found in three separate geographically close, but distinctly isolated, mountain ranges, V. troupi found in the Whetstone Mountains, V. grahami from the Santa Rita Mountains, and V. vorhiesi from the Huachuca Mountains (see Fig. 10 View Figure 10 ). These three isolated mountain ranges are situated quite close to each other, only 45 – 66 km separate the type localities of these three species. The Huachuca Mountains are the most southern and highest, their Miller’s Peak reaching 2878 m. It extends roughly 32 km in a north to southeast direction reaching Mexico. The Santa Rita Mountains are the largest mountain range, extending roughly 42 km, with its highest point Mount Wrightson at 2863 km. The Whetstone Mountains, which are located directly north of the Huachuca Mountains, is the smallest range, only 18 km long in a north to south direction, its highest point is the Apache Peak at 2346 m. Figure 10 View Figure 10 states the altitudes of the type localities of the three species found in these three mountain ranges.
Molecular considerations. In an important study conducted by Bryson et al. (2013) using multilocus data (mtDNA and two nuclear genes), they explored the phylogeographic structure of the “ vorhiesi ” group. The dataset was generated from 63 samples of “ vorhiesi ” group scorpions including 11 of the then described species as well as many other populations. Important to this paper, the dataset included species V. vorhiesi and specimens from two populations, the Santa Rita Mountains, now named V. grahami , and the Whetstone Mountains, the location of V. troupi , the new species described in this paper. In their study divergence times were estimated across the mtDNA dataset and the multilocus species tree, and the temporal distribution of divergence events was modeled across southwestern North America. The result of their study placed V. grahami , V. troupi , and V. vorhiesi in a terminal clade, implying that they were the most closely related. Both the multilocus species tree and mtDNA gene tree analyses supported this result. Their chronograms (Bryson et al., 2013: fig. S2) showed the estimated divergence times (in millions of years, Mya) as follows: for the multilocus species tree, 3.25–8.48 (5.75) Mya for V. grahami and V. vorhiesi + V. troupi , and for the mtDNA gene tree, 3.3– 8.07 (5.45) Mya for V. grahami and V. vorhiesi + V. troupi . Similarly, multilocus species tree, 1.05–5.12 (2.98) Mya for V. vorhiesi and V. troupi , and mtDNA gene tree, 1.53–5.80 (3.53) Mya for V. vorhiesi and V. troupi .
It should also be noted that the Bryson et al. (2013) study placed species V. deboerae and V. tenuipalpus in the same basic clade as our three subject species, but earlier chronologically, exhibiting 4.45–11.08 (7.71) Mya and 8.07–18.19 (12.78) Mya, respectively.
Morphological considerations. We have isolated three morphology-based characters that distinguish V. troupi from species V. grahami and V. vorhiesi : 1) number of inner denticles (ID) on the chelal fingers; 2) comparative positions of certain patellar trichobothria; and 3) morphometric ratios of the metasomal segments. Of course, it must be emphasized here that only one specimen of V. troupi , a female, is currently available for examination, and the male is unknown. Therefore, some variability should be expected as more specimens are collected and studied.
All three species exhibit six ID on the chelal movable finger, also common to eight other species in the “ vorhiesi ” group (see map in Fig. 11 View Figure 11 ). Unusual, however, are the six ID also found on the fixed finger in V. troupi (found on both chelae), somewhat unusual in this species subgroup that only have six ID on the movable finger. In contrast, V. grahami and V. vorhiesi , on an average, only have five ID on the fixed finger (see Fig. 8 View Figure 8 ). Other species contained in the species set with six ID on the movable finger, only V. tenuipalpus, Sissom et al. (2012: fig. 4D) and V. electrum Hughes (2011: fig. 8d) match V. troupi with six ID on the fixed finger, as these authors illustrated for the type specimens. It should be stated here, however, that variability does occur on the number of ID found on the fixed finger for “vorhiesi” group species. For example, Sissom (2012: 12) reported 4–6 (5.732) [56] for V. tenuipalpus ; for V. grahami , six specimens, we encountered significant variability, 5–6 (5.417) [12]; however, in stark contrast, V. vorhiesi exhibited 5 ID on the fixed finger for four specimens (including lectotype as described by Graham (2007: 6)).
We compared the relative positions of five external patellar trichobothria et 1 –et 3, est, and v 3 in species V. troupi , V. grahami , and V. vorhiesi (see Fig. 9 View Figure 9 ). Both patellae of V. troupi and thirteen patellae of V. grahami and V. vorhiesi were included in this analysis, demonstrating little variability in the relative positions of these five trichobothria. In Figure 9 View Figure 9 we plotted the five trichobothria by anchoring each patellar pattern to trichobothrium et 1, allowing the other trichobothria to form clusters. It is apparent that these trichobothria in general form fairly tight clusters, thus demonstrating minimal variability in their relative positions. In Figure 9 View Figure 9 , we see that et 3 in V. troupi is noticeably proximal to a line connecting est and v 3, forming an angle of approximately 130 degrees. In the other two species et 3 is essentially located in this connecting line, thus the angle is quite obtuse, over 165 degrees. This difference in the relative pattern can be attributed to a couple of observations. In general the distance between et 1 and et 2 is approximately the same as between et 2 and et 3 in V. troupi . For the other two species, the distance between et 2 and et 3 is shorter. Trichobothria et 1 –et 3 are essentially in a straight line in V. troupi , whereas et 3 is out of line, closer to the ventral edge in the other two species. Based on the analysis of published trichobothrial patterns of other sky island species which exhibit six ID on the movable finger, we see that species V. deboerae and V. halli have patterns similar to that described for V. troupi (see Ayrey, 2009: fig. 14; and Ayrey, 2012: fig. 10).
We compared several morphometric ratios between the V. troupi female and V. grahami (three females) and V. vorhiesi (four females, including the lectotype measurements contained in Graham (2007: 3–4)). These measurements are presented in Table 1. The V. troupi female has a relatively thinner metasoma than female V. grahami and V. vorhiesi (see Table 2), ranging from 8 to over 16 percent mean value differences ( MVD). In particular, the basal segments I–III exhibit the largest differences.
| R |
Departamento de Geologia, Universidad de Chile |
| USNM |
Smithsonian Institution, National Museum of Natural History |
| VI |
Mykotektet, National Veterinary Institute |
| V |
Royal British Columbia Museum - Herbarium |
| MD |
Museum Donaueschingen |
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
SubFamily |
Vaejovinae |
|
Genus |
Vaejovis troupi Ayrey et Soleglad
| Ayrey, Richard F. & Soleglad, Michael E. 2015 |
Vaejovis sp. cf. vorhiesi: Ayrey, 2013a: 3
| Thorell 1876: 3 |
Vaejovis sp., vorhiesi group
| Thorell 1876 |