Uropeltis dindigalensis ( Beddome, 1877 )
|
publication ID |
https://doi.org/ 10.11646/zootaxa.5209.1.6 |
|
publication LSID |
lsid:zoobank.org:pub:36C6DA66-C341-46EA-8FAF-4EA66F0ACEC1 |
|
DOI |
https://doi.org/10.5281/zenodo.7330384 |
|
persistent identifier |
https://treatment.plazi.org/id/03D787AB-7658-FF91-3891-FE03FBC3FC7A |
|
treatment provided by |
Plazi |
|
scientific name |
Uropeltis dindigalensis ( Beddome, 1877 ) |
| status |
|
Uropeltis dindigalensis ( Beddome, 1877)
( Figs. 1–8 View FIGURE 1 View FIGURE 2 View FIGURE 3 View FIGURE 4 View FIGURE 5 View FIGURE 6 View FIGURE 7 View FIGURE 8 ; Tables 1–3 View TABLE 1 ; Appendix 2)
Silybura dindigalensis Beddome, 1877
Silybura dindigalensis — Beddome 1886; Boulenger 1890, 1893; Sclater 1891; Sarasin 1910; Wall 1923
Uropeltis dindigalensis — Smith 1943; Gans 1966; Rajendran 1985; McDiarmid et al. 1999; Vanak et al. 2001; Sharma 2004; Ganesh & Asokan 2010; Wallach et al. 2014; Ganesh & Arumugam 2015a, b, 2016a, b; Pyron et al. 2016
Uropeltis (Siluboura) dindigalensis — Mahendra 1984
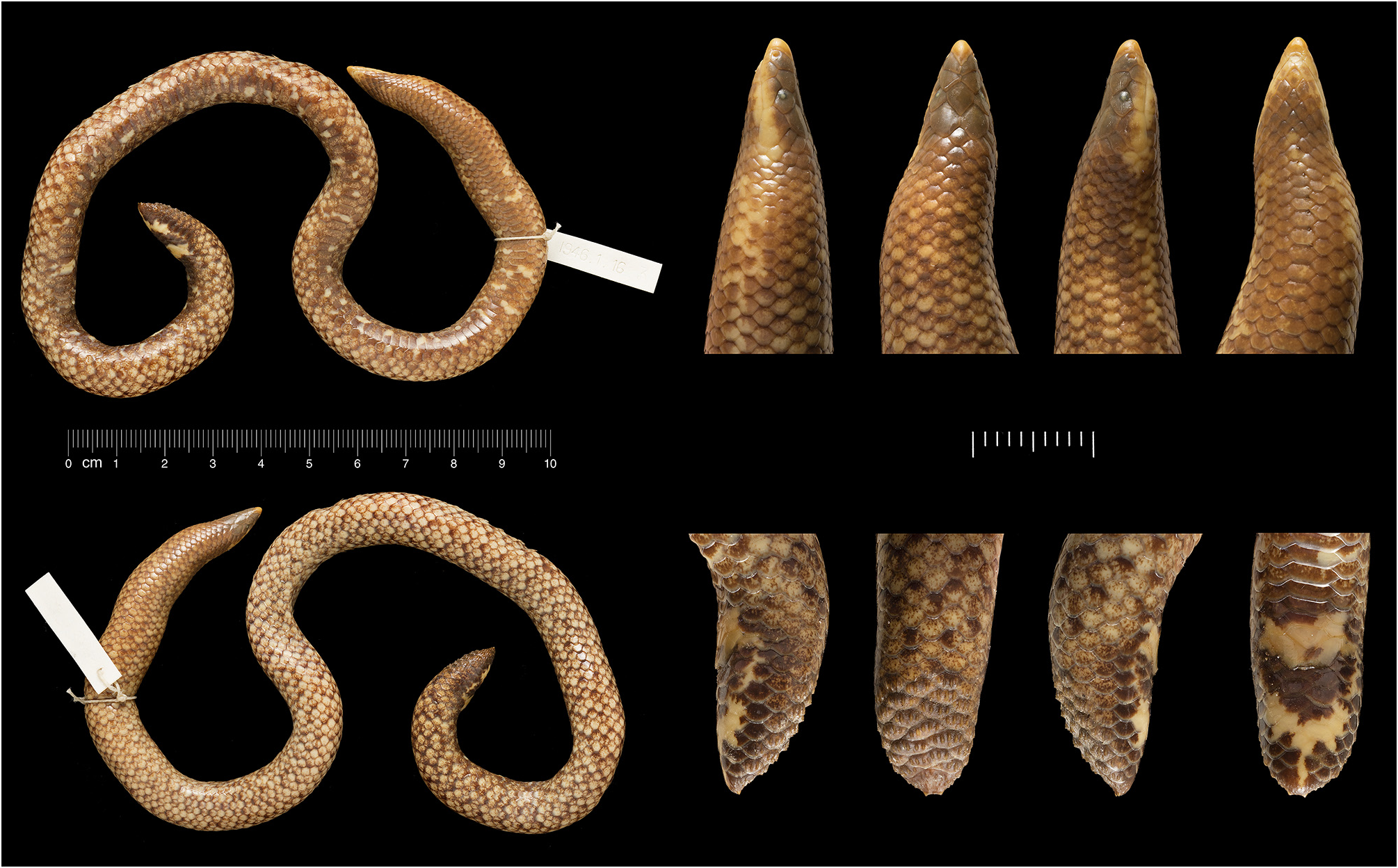
Lectotype (here designated). BMNH 1946.1 .16.3 (formerly 83.1.12.6: Fig. 2 View FIGURE 2 ) from Sirumallays, near Dindigal, 4,500 ft, collected before 1883 (based on accession date: the “83.1.12” part of the original number indicates 12 January 1883) and very probably before 1878 (given that the species was described in 1877), R. H. Beddome.
Paralectotypes. BMNH 1946.1 .16.2 (formerly 83.1.12.5: Fig. 3 View FIGURE 3 ) , BMNH 1946.1 .16.4 (formerly 83.1.12.7: Fig. 4 View FIGURE 4 ) and MNHN 1895.88 About MNHN ( Fig. 5 View FIGURE 5 ) from Sirumallays , near Dindigal, 4,500 ft, before 1883 and very probably before 1878 , R.H. Beddome .
Other Material Examined. BMNH 1946.1 .16.37 (formerly 77.8.10.3), Sirumallays , near Dindigal (according to mid-20 th Century catalogue and jar label, but no locality information provided in 1877 accessions register), 1877 or earlier , R.H. Beddome , BNHS 3277 View Materials , Kandige Estate , Sirumalai Hills, S.P. Vijayakumar . CAS 244756–763 About CAS (8 specimens). All of these CAS specimens except CAS 244756 About CAS are reported in the CAS catalogue as from 1,500 m at Sirumalai, collected 11 May, 1972. CAS 244756 About CAS is reported as from 6,500–7,500 ft (1,981–2,286 m) in or near Kodaikanal, collected 5 November 1972. All CAS specimens collected by or for C. Gans .
Remarks. Because we (i) are revisiting the taxonomy of U. dindigalensis , (ii) note that a congener U. broughami (Beddome, 1878) with the same reported type locality shares a similar tail shield and colour pattern ( Pyron et al. 2016), and (iii) recognize that there are reported variations in colour pattern among the syntypes of U. dindigalensis such that they might not all match historical accounts in the literature (see Pyron et al. 2016), we designate a lectotype. As per Article 74.7 of the 4 th Edition of the International Code of Zoological Nomenclature ( ICZN 1999), we select BMNH 1946.1.16.3 (formerly 83.1.12.6), an adult female, as the lectotype of Silybura dindigalensis Beddome, 1877 . We select this specimen as lectotype because it is the best-preserved of the BMNH types, the material of which we have been able to examine extensively (Recommendation 74D). The other three syntypes hereby become paralectotypes (Recommendation 74F).
It is unclear whether any of the CAS specimens (non-types) are the same as those that Rajendran (1985) reported collecting in Sirumalai on a single day (11 May 1972). However, we consider that as possible because Gans’ Indian uropeltid material was typically obtained with or by Rajendran and family and/or colleagues (Albert Rajendran, pers. comm.), and the CAS specimens fall within the size range of the 19 specimens reported by Rajendran (1985: table 13).
Description of lectotype. An adult female; specimen well-preserved, retaining much of its colour and pattern; forebody mildly thicker than the rest of trunk; head narrower than neck; snout pointed in profile and especially in dorsal view; eyes small compared to ocular scale (ca. one quarter of the length); tail with an incompletely flattened caudal disc (tail shield) bearing matt, multicarinate scales. A relatively large specimen, total length 349 mm, of which tail length 12.2 mm (3.5 % of total length). Body subcylindrical, midbody width 10.2 mm; head (9.7 mm long) shorter than tail shield (11.9 mm), which is longer than broad (7.9 mm). Midbody ventral scales 4.6 mm wide, approximately 1.4 times wider than scales in adjacent first row of dorsals (3.2 mm).
Rostral visible from above, partly separating opposite nasals; part of rostral visible from above slightly longer than its distance from frontal; nostril piercing anteroventral corner of nasal; frontal hexagonal, longer than broad, ocular margins slightly converging posteriorly; parietals large, each longer and wider than frontal. Supralabials 4,4, first the smallest of all head shields, rectangular; second supralabial taller than long, third longer than tall, taller anteriorly, shorter posteriorly; fourth supralabial much the largest. Infralabials 3,3, second the largest, third the smallest. Body scales macroscopically smooth and glossy, imbricate, cycloid; dorsal scale rows: 19: 17: 17 (see Appendix 2 for scale row reductions); ventrals 168; subcaudals 7,6; caudal scales across length of tail shield 6; caudal scales across width of tail shield 4; matt shield scales with 2–5 keels per scale; terminal scute dorsoventrally flattened, with tubercles, low keels and two pointed, posteriorly and slightly upwardly projecting terminal spurs.
Dorsum fawn brown overall; tail shield, head and anterior one seventh of body somewhat darker and browner (versus paler and more off-white). Most scales on dorsum pale (off-white), some of which have dark (brown) flecks or speckles; fewer scales on dorsum with larger dark areas (especially towards scale bases) with pale flecks; these darker scales arranged mostly in a series of transverse whorls that fail to continue across the venter; ventral side instead brown with larger pale yellow to off-white irregular and irregularly distributed spots. Darker dorsal whirls zigzagging and approximately one-scale wide, interspersed by generally 1–2 pale scales. Rostral paler and more orange than other head scales; labials, gular and venter, pale yellow, extending posteriorly as irregular, poorly developed stripes along the lateral surface of anterior end of body to approximately 3–4 head lengths behind head. Underside of tail brown with pale yellow marks on pre-anal region, central part of underside of tail, and narrow midline of posterior of ventral surface of tail.
Variation. Including the lectotype, the examined museum specimens comprise seven females (198–353 mm) and seven males (156–213 mm), which are clearly sexually dimorphic in numbers of ventrals (161–169 versus 151–158, respectively) and subcaudals (5–7 versus 8–11) ( Tables 1–2 View TABLE 1 ). In addition, females have relatively shorter tails (3.3–3.7 % of TL versus 4.9–6.1 %) with relatively less-elongate tail shields, and typically fewer keeled, matt scales on the shield (22–28 versus 28–31). The sample size is small, but available data suggest that females also attain greater total length. There are invariably four supralabials per side and three infralabials per side except for four infralabials on the left of CAS 244757. In the four BMNH specimens, dorsal scales reduce from 19 rows behind the head to 17 rows by the 26 th to 39 th ventral (Appendix 2); most specimens maintain this number of rows to near the vent but with a very minor fluctuation (one side only in BMNH 1946.1.16.2 and 4) or a brief return to 19 rows (BMNH 1946.1.16.32), though in the lectotype and on one side of each of BMNH 1946.1.16.2 and 4 there is instead a larger third row scale and smaller fourth row scale between the 53 rd and 60 th ventral (see Sampaio et al. 2020 for a discussion of similar scale row irregularities in Rhinophis spp. ). See below (penultimate paragraph of Distribution section) for a discussion of the morphology of the tail shield of U. dindigalensis with respect to the shield types designated by Smith (1943) and Pyron et al. (2016).
The MNHN paralectotype resembles the other types in having yellow lip margins ( Fig. 5 View FIGURE 5 ), contra Pyron et al. (2016). The BMNH paralectotypes are generally slightly darker than the lectotype, with more than half of the scales on the dorsum being more than half brown (versus very few in the lectotype). The BMNH and MNHN paralectotypes have more clearly apparent (more strongly contrasting) darker zigzag transverse markings on the dorsum, and also have a slightly better developed pale yellow lateral stripe behind the head, which runs along dorsal scale row 4 and the margins of rows 3 and 5 (versus along the junction between rows 3 and 4 in the lectotype).
At least some of Rajendran’s (1985: table 13) scalation data reported for U. dindigalensis differ substantially from our observation of all specimens known to us. The way the first (anteriormost) ventral scale was identified in that work ( Rajendran 1985) was not reported, but even allowing for differences in that (see Gower & Ablett 2006), it is difficult to reconcile our ventral counts of 151–169 with Rajendran’s counts of 139–164 (with 12 of 19 specimens reported by Rajendran as having counts of <151), and the much less clearly bimodal (sexually dimorphic) relationship between numbers of ventrals and numbers of subcaudals for Rajendran’s data. Nevertheless, we do not doubt the identity of these topotypical specimens being U. dindigalensis . We are satisfied that no other morphologically diagnosable uropeltid species has definitely been recorded in Sirumalai, at least in the higher elevations from where Rajendran collected this lot ( Beddome, 1886; Vanak et al. 2001; Ganesh & Arumugam 2016a; Pyron et al. 2016; this work).
Ganesh & Arumugam (2016a) presented ranges for some scale counts and measures for nine uncollected specimens observed in the field. Here ( Table 3 View TABLE 3 ) we present those field-collected data individually and add the ventral counts. The previous report of single rather than paired anal shields and four rather than three infralabials on each side ( Ganesh & Arumugam 2016a) were in error. Otherwise, the scalation data for the uncollected specimens are within the ranges recorded for the preserved museum specimens, except for female subcaudal counts of 8 rather than 5–7. Small differences in the range of relative tail length for males and females might be caused by minor differences in how tail length and total length were measured by different observers and/or in differences between live and preserved material.
Natural-history observations. Uropeltis dindigalensis , as most other shieldtails, are probably mostly fossorial— Rajendran (1985) reported digging them out from moist manure heaps and from compost near graveyards. This species is probably more active on the surface during the monsoons, when live individuals were found, often on or close to roads. However the studies reported by both Vanak et al. (2001) and Ganesh & Arumugam (2015a, b; 2016a, b) did not include sampling during the dry season (February–April). Surface activity in this species appears to be largely nocturnal or crepuscular because many roadkill specimens were recorded in the early morning ( Ganesh & Arumugam, 2015b). However, at least on one occasion an adult was observed actively foraging and feeding during broad daylight.
Rajendran (1985) reported collecting 19 specimens of Uropeltis dindigalensis in 1972 from Sirumalai at 1,500 m, although the highest peak of Sirumalai is at an elevation of 1,390 m. Rajendran found specimens in plantain cultivations, an abandoned graveyard, under rotting leaves, on the edge of manure, and in rubbish (“dump”) heaps. He adds that the soil hardness measured with a penetrometer read 0.5–1.5 (units unspecified), the temperature was 22–23°C, and that the soil and leaf litter fauna at these digging sites included centipedes, millipedes, termites, earthworms, and the elapid snake Calliophis nigrescens . Rajendran (1985) also reported anecdotal information from farmers that small, possibly newborn U. dindigalensis are observed during June.
A total of 13 U. dindigalensis were observed during monsoon (June to September) and post-monsoon (October to December) surveys undertaken between May 2014 and January 2015 in Sirumalai, as reported by Ganesh & Arumugam (2015a, b; 2016a, b). These surveys comprised 500 hours of visual encounter and time-constrained searching. No dedicated digging was deployed because the study was focused on all herpetofaunal species. Eight live U. dindigalensis were observed, the other five individuals being roadkills, 8.6% of all roadkill herpetofauna encountered during these surveys ( Ganesh & Arumugam 2015b). The following natural history observations were made by one of us (SRG) during the surveys reported by Ganesh & Arumugam (2015a, b; 2016a, b).
On 20 th May 2014, an adult ( Fig. 6D View FIGURE 6 ) was sighted under a fallen log in disturbed forest, at 11:20 h in Gowmari Amman Koil (10.236˚N, 78.011˚E; 1,130 m elevation). On 21 st May 2014, a male and female ( Fig. 6B View FIGURE 6 ) were found next to each other under a log in a coffee plantation adjoining wet forests, at 10:30 h in Agasthyapuram (10.222˚N, 77.978˚E; 1,215 m). The male (10 pairs of subcaudals) was darker and smaller (total length 207 mm) than the female (250 mm; 7 pairs of subcaudals); the male’s hemipenes were slightly visible when first examined. On 25 th May 2014, the smallest recorded individual (TL 130 mm) was encountered ( Fig. 6C View FIGURE 6 ), found under a rock in a bean and areca nut plantation, at 13:00 h in Kannadiparai (10.180˚N, 78.007˚E; 1,170 m). On 27 th May 2014, an adult ( Fig. 6A View FIGURE 6 ) was sighted under a fallen log in a matrix of secondary forest and coffee plantation, at 09:10 h near Namasthe Estate (10.181˚N, 77.969˚E; 970 m).
On 12 th June 2014, an individual in ecdysis was found under a flowerpot in a fruit orchard, at 10:10 h in Siluvamalai (10.250˚N, 77.987˚E; 1320 m). Pieces of its cast-off slough were visible as it was shedding. Portions of forebody skin had already shed and sloughing was occurring on posterior parts of the body when sighted ( Fig. 6E View FIGURE 6 ). On 24 th June 2014, a presumably adult (not small) individual was sighted under a rock near a stream inside a matrix of moist deciduous forests and bean and bitter-gourd plantation at 13:10 h in Karuppusamy Koil (10.159˚N, 77.996˚E; 1,210 m).
On 18 th December 2014, a freshly dead, roadkill specimen was found at 8:30 h near Sirumalai View point (10.257˚N, 77.998˚E; 1,060 m). It had parts of an earthworm’s body inside its gut ( Fig. 6F View FIGURE 6 ). On the same day, two much more squashed roadkills were sighted near the same site, at 09:15 h and 12:30 h. On 22 nd December 2014, a roadkill was sighted on a tar road bordering a temple pilgrimage site at 09:00 h near Vellimalayan Koil (10.240˚N, 77.994˚E; 1,180 m).
On 29 th December 2014, a 250 mm long individual ( Fig. 6G View FIGURE 6 ) was observed feeding on a roadkill earthworm at 10:45 h in VSN Estate, Pazhapannai (10.184˚N, 77.994˚E; 1,090 m). The dead earthworm was approximately as long as the snake, was fresh and limp and probably not long dead. At 10:48 h the snake approached the dead earthworm from the side of the road, where the habitat was fruit orchards and disturbed moist deciduous forest. At 10:49, the snake’s snout was in contact with the earthworm, which it tongue-flicked five times. At 10:50–10:51, the snake bit the earthworm at one end of its body and started feeding. The snake swallowed approximately 40% of the earthworm in 14 minutes. At 11:03 the snake started to break the earthworm’s body midway by biting and shaking its head to either side repeatedly. The snake finished feeding at this point and by 11:05 h slowly moved away from the middle of the tar road back to the roadside vegetation. A part of the worm remained uneaten on the road. On 30 th December 2014, a heavily squashed roadkill U. dindigalensis was sighted on a tar road passing through coffee estates at 08:55 h near JMA Gardens (10.251˚N, 77.991˚E; 1,150 m).
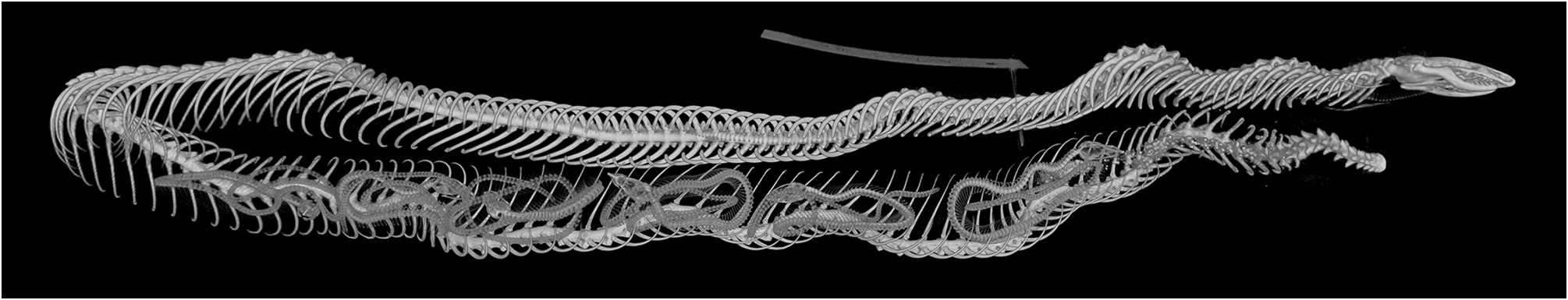
The large (353 mm TL) female specimen BMNH 1946.1.16.37 is gravid, as could be seen from a longitudinal ventral incision into the coelom. The microCT scan data show six foetuses in the posterior half of the body ( Fig. 7 View FIGURE 7 ). This matches closely Rajendran’s (1985: 70) report of five to seven foetuses in three dissected specimens. In BMNH 1946.1.16.37, the foetuses are all loosely coiled, three with their heads pointed anteriorly, three posteriorly.
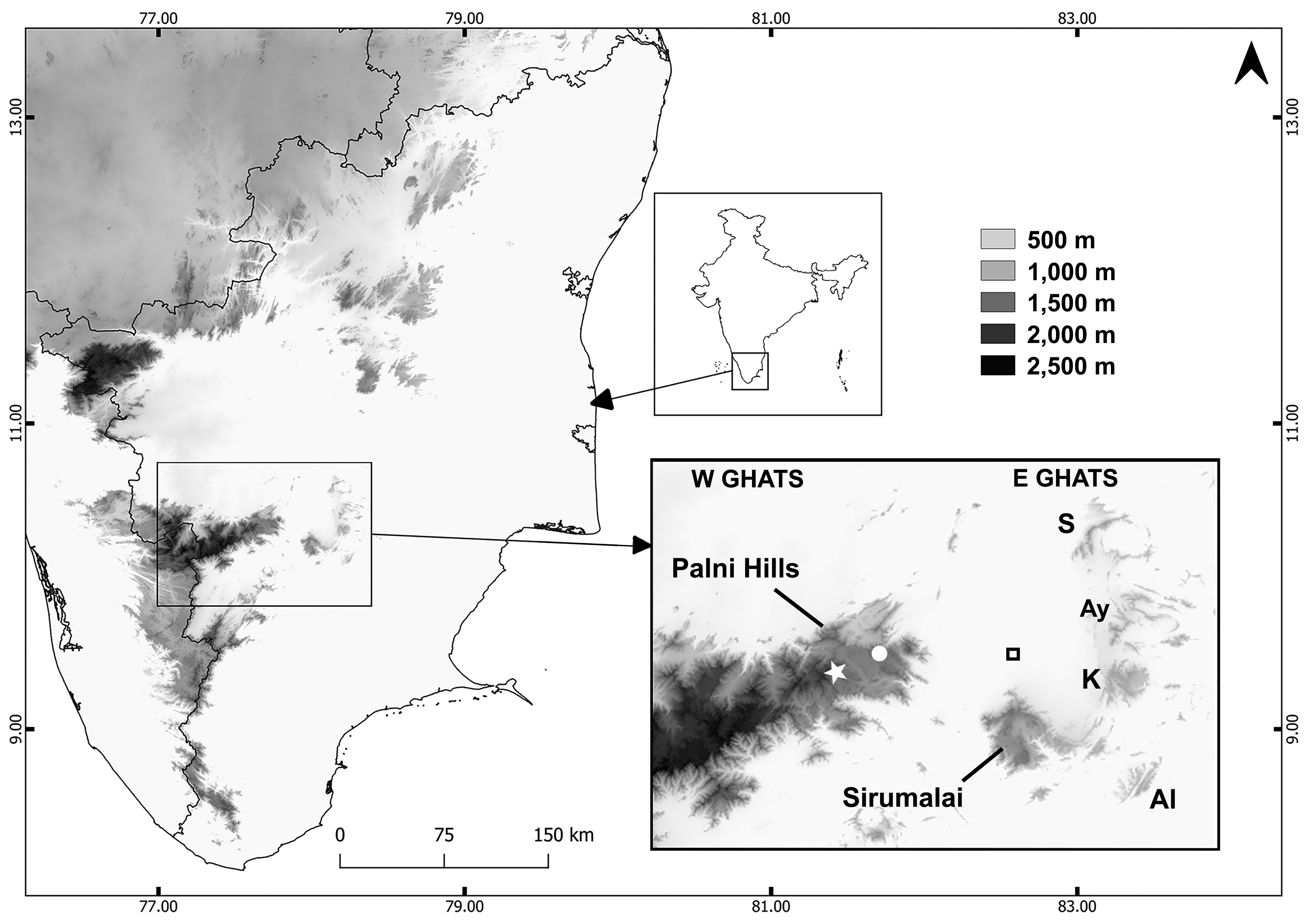
Distribution. The type specimens of Uropeltis dindigalensis were reportedly ( Beddome 1877) collected between ca. 1,200 –1,500 m elevation at Sirumalai (ca. 10.17–10.28˚N, 77.94–78.10˚E), in the southernmost part of the Eastern Ghats. This hill range is now known to also harbour other endemic reptiles, such as the recently described rock gecko Hemidactylus sirumalaiensis ( Khandekar et al. 2020) . As far as we are aware, all other U. dindigalensis museum specimens and records are from the same small hill range ( Beddome 1877; Rajendran 1985; Vanak et al. 2001; Ganesh & Asokan 2010; Ganesh & Arumugam 2015a, b; 2016a, b), except for CAS 244756 from Kodaikanal, according to catalogue data that we consider questionable. The individuals reported by Ganesh & Arumugam (2015a, b; 2016a, b) were found between 970–1,320 m elevation. Local people and Forest Department staff reported to one of us (SRG) in 2014 that the species has been sighted in Sirumalai at elevations as low as 650– 700 m. If confirmed, this might open the possibility of U. dindigalensis occurring in some nearby, lower elevation ranges such as Ayyalur (10.42˚N, 78.21˚E; 950 m elevation), Karandhamalai (10.31˚N, 78.20˚E; 920 m), Semmalai (10.58˚N, 77.20˚E; 1,000 m) and Alagarmalai (10.12˚N, 78.23˚E; 790 m) hills ( Fig. 1 View FIGURE 1 ).
Ganesh et al. (2008) and Chandramouli & Ganesh (2010; also Ganesh et al. 2014b) listed U. dindigalensis and U. cf. dindigalensis , respectively, as part of the fauna of the High Wavy Mountains (Meghamalai), of the Western Ghats, highlands that are approximately 65 km southwest of the type locality of U. dindigalensis . These reports were based on four animals, none of which were collected, but one of which was photographed in life ( Chandramouli & Ganesh 2010: fig. 16; Ganesh et al. 2014b: fig. 6a) and two dead animals that were examined for scalation. Upon further consideration, the colour pattern, possession of a rostral scale that separates the nasals, a tail shield most similar to Smith’s (1943) Group III strongly that this is not U. dindigalensis . Instead, these specimens are much more similar to U. pulneyensis (Beddome, 1863) (except for their higher ventral count of approximately 190) or U. grandis (Beddome, 1867) (except for their lower ventral count and 17 versus 19 dorsal scale rows at midbody). These specimens are currently better considered as U. cf. pulneyensis . There is no evidence that U. dindigalensis occurs in Meghamalai.
No other uropeltid species were found at Sirumalai during the surveys reported by Rajendran (1985), Vanak et al. (2001) and Ganesh & Arumugam (2015a,b; 2016a,b). Uropeltis broughami was described on the basis of an unspecified number of specimens from “Sirumullay hills (Madura district), 5500 feet elevation”, but the lack of variation reported for ventral and subcaudal scales is consistent with there being a single type. There have been no subsequent reports of U. broughami from Sirumalai. All reported non-type specimens of U. broughami , and the type and only reported specimens of its junior synonym Silybura levingii Beddome, 1878 , are instead from the Palni (= Palani) Hills (e.g., Roux 1928; Smith 1943; Pyron et al. 2016), which are part of the nearby (ca. 20 km) Western Ghats, separated by low-lying (<300 m asl) plains from the edge of Sirumalai in the Eastern Ghats: Fig. 1 View FIGURE 1 ). We note that one specimen in the London collection (BMNH 1914.1.26.4) that generally agrees with the types of U. broughami and S. levingii is, according to the accessions register, from Seaforth, in the Nilgiris, which we consider very surprising.
Uropeltis dindigalensis and U. broughami are readily distinguished, the former has 17 dorsal scale rows at midbody (versus 19), substantially fewer ventral scales (<170 in females and <158 in males versus>195 and>185, respectively), and a shorter rostral (relative to the rostral–frontal gap) and less pointed head in dorsal view. In addition, there are colour pattern differences: the dorsum of preserved specimens of U. dindigalensis is dominated by pale, off-white scales with flecks of brown, while in U. broughami dorsal scales are more typically brown with darker and paler markings; the darker approximately zigzag lines on the dorsum of U. broughami differ from those in U. dindigalensis in typically (especially posteriorly) having also whitish, sometimes ocellus-like dots; U. dindigalensis has a fairly short and narrow lateral yellowish stripe behind the head, whereas in U. broughami this is often a series of disconnected and taller blotches that continue further along the body posteriorly (see Boulenger’s 1893 drawings of the two species, reproduced here in Fig. 8 View FIGURE 8 ). Smith (1943) distinguished between a larger, flat or concave upper (posterodorsal, matt and keeled) tail shield surface in U. broughami versus a smaller, “feebly convex” and “never quite flat” shield surface in U. dindigalensis (Smith’s Type II versus Type I shield, respectively). The shield is very slightly flatter in the specimens of U. broughami we have examined than in U. dindigalensis , but we agree with the alternative views presented by Boulenger (1890, 1893) and Pyron et al. (2016) that the tail shields in the two species are rather similar. However, we observe that the upper surfaces of the tail shields of both U. broughami and U. dindigalensis are less flat (longitudinally) than Boulenger (1890, 1893) reports but flatter than Pyron et al. (2016) report, being somewhat intermediate between Pyron et al.’s illustrated Types 4 and 5 (their figs. 3D and E, respectively).
Although the description of U. broughami was published in 1878, the holotype was not accessioned into the London (BMNH) collection until 12 January 1883, along with other uropeltids collected by R.H. Beddome. The accession register entry is accompanied by a handwritten note, presumably by Beddome, that lists the number of specimens of each taxon and some locality information. On the handwritten note, the information for the single U. broughami specimen is “Cumbum Hills, Madura” rather than Sirumallay (as in Beddome 1878), and the accession register repeats “Cumbum hills”, which indicates possible confusion in the precise locality for this specimen. A probable case of mixed up distribution involving the Western Ghats (Travancore) and the Eastern Ghats (Sirumalai) exists for at least one other reptile species, also of Beddome’s collecting―the scincid lizard Ristella guentheri Boulenger, 1887 (see remarks in Ganesh & Arumugam 2016a). Thus, we suggest it is also possible that the holotype (as for all other reports) of U. broughami is from the Western Ghats rather than Sirumalai.
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.