Triozocera paulistana Kogan, 1958: 422
|
publication ID |
https://doi.org/ 10.11646/zootaxa.3779.1.7 |
|
publication LSID |
lsid:zoobank.org:pub:D546C69E-631E-4CB3-ADEF-7C71A0459163 |
|
DOI |
https://doi.org/10.5281/zenodo.5056534 |
|
persistent identifier |
https://treatment.plazi.org/id/275ECD60-3462-FFBF-3EF4-FA626D3C3097 |
|
treatment provided by |
Felipe |
|
scientific name |
Triozocera paulistana Kogan, 1958: 422 |
| status |
|
Triozocera paulistana Kogan, 1958: 422
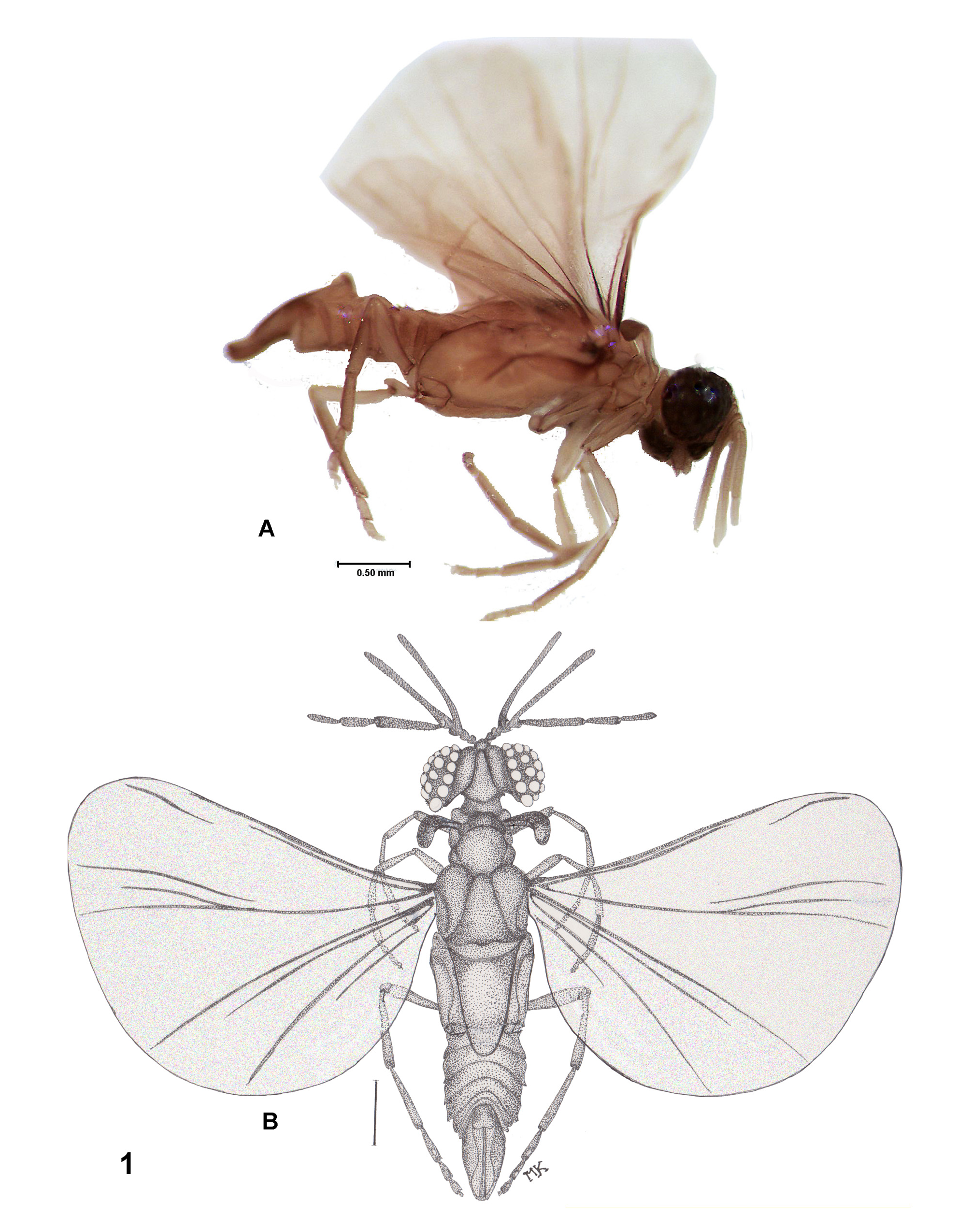
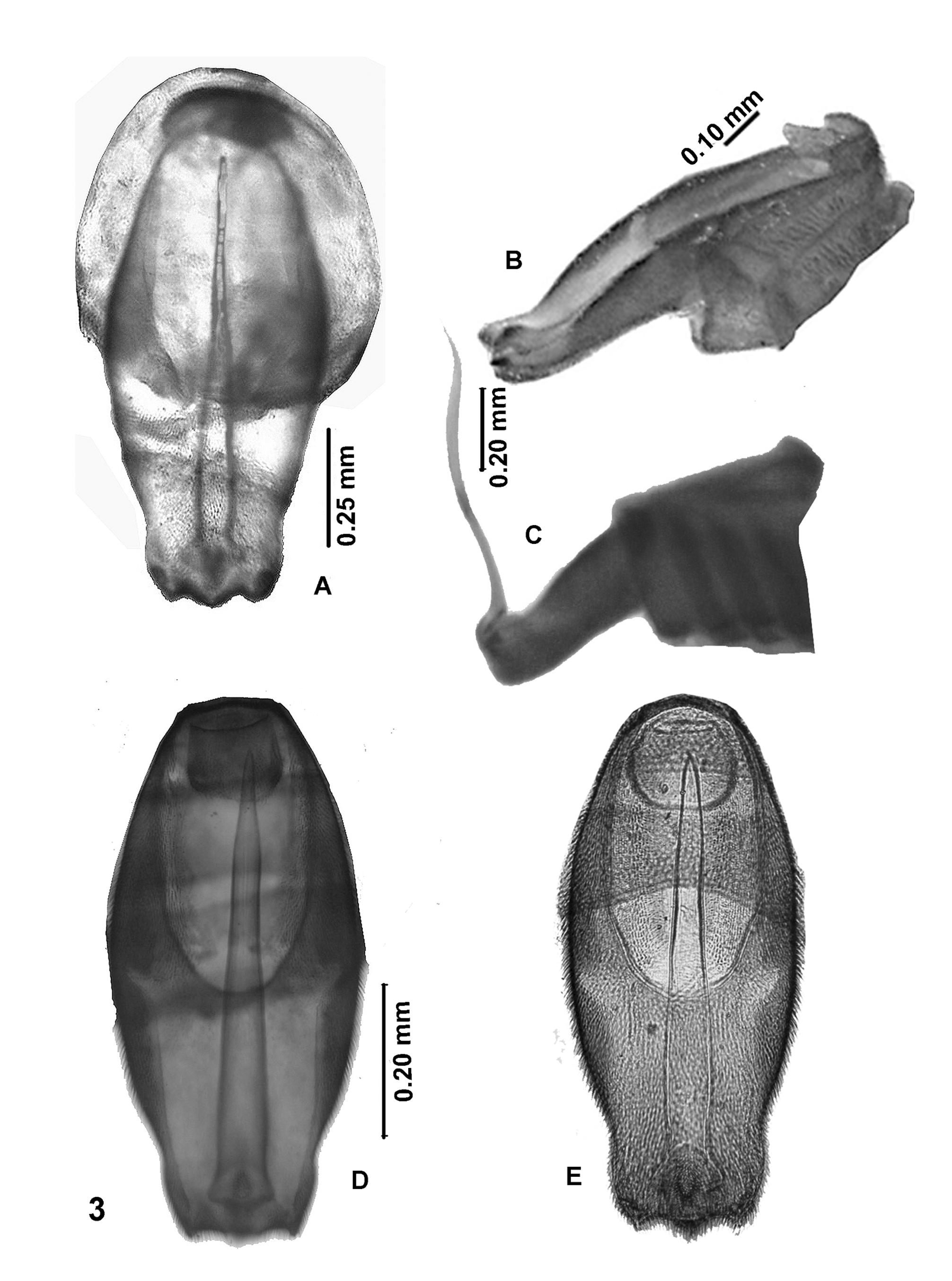
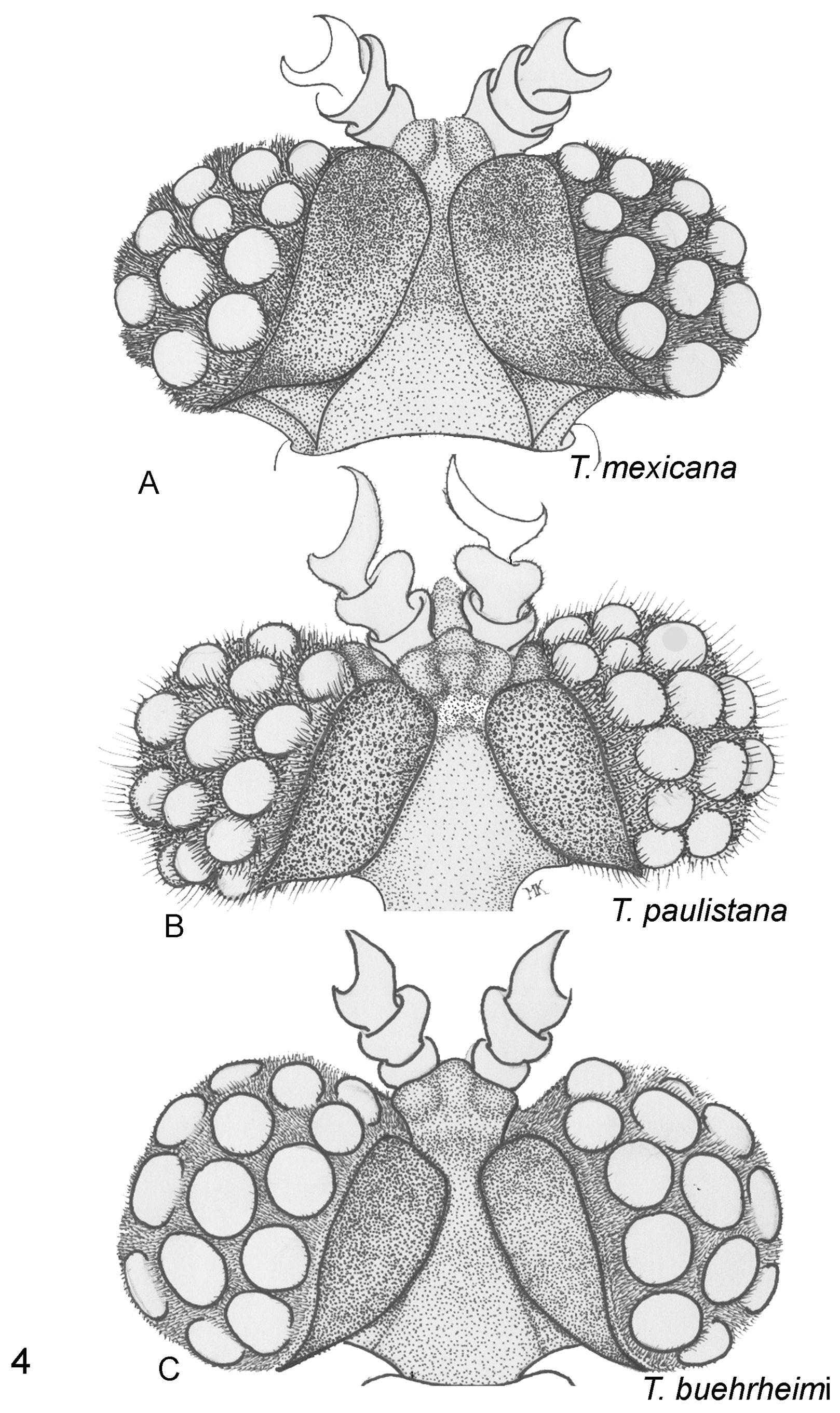
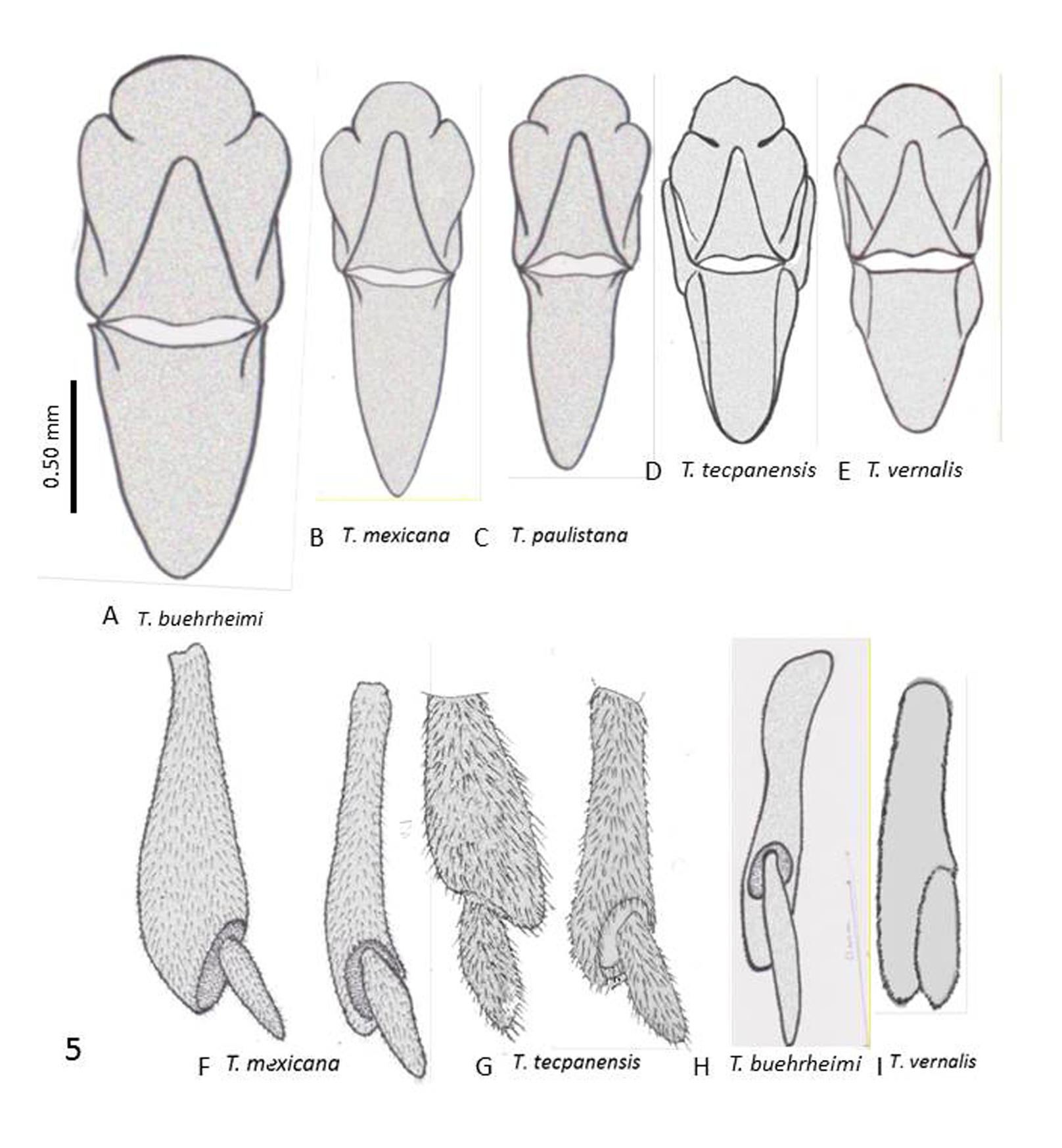
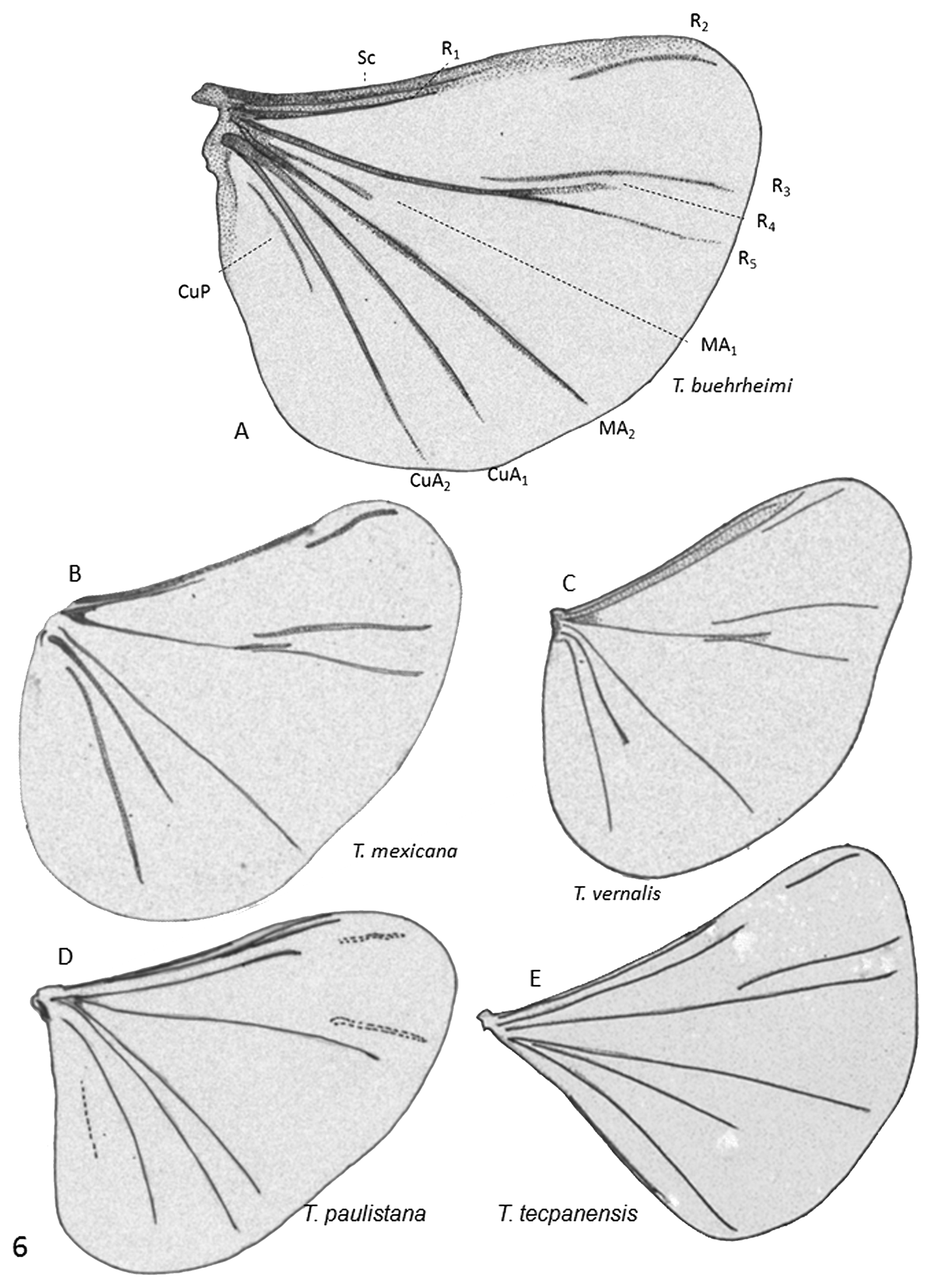
( Figures 3E View FIGURE 3 , 4B View FIGURE 4 , 5C View FIGURE 5 , & 6I View FIGURE 6 )
Triozocera paulistana, Oliveira and Kogan, 1959: 231 ; Luna de carvalho, 1967: 29
Triozocera mexicana View in CoL , p.p. Kinzelbach, 1971 b: 150; Luna de Carvalho, 1978: 353
Triozocera paulistana, Kifune and Hirashima, 1979: 64 ; Kifune and Brailovsky, 1987: 134; Kathirithamby, 1990: 470; 2005: 8
T. paulistana was the first species of Strepsiptera originally described from Brazil. A single specimen was collected in a primitive light trap and kept dry for over five years before it was hot KOH treated and mounted in Canada balsam for study. Although the type specimen was damaged, all major diagnostic characters were intact. The species was listed by Kinzelbach (1971) as synonymous of T. mexicana , without the criteria used for the synonymy. Since then, Kifune and Brailovsky (1987) argued against the synonymy based on the difference in size and disjunct geographic distribution of T. mexicana and T. paulistana . They questioned, as well, the synonymy of T. mexicana and T. texana Pierce, 1911 , based on an assessment by Luna de Carvalho (1967). Kathirithamby (1990) retained the synonymy of T. mexicana and T. texana , but listed T. paulistana as a separate species; she later accepted that synonymy ( Kathirithamby, 1993).
Triozocera paulistana distinguishing characters from T. mexicana in the original description were confirmed and expanded in this study. Those differences are reiterated here based on a reassessment of the holotype and Pierce’s original descriptions; examination of 4 specimens identified as T. mexicana from Puerto Rico, Cuba, and Southern USA (Gainesville, Mississippi) (see Table 1 View TABLE 1 ); as well as photos posted on the Web of representatives of a large cohort of male Triozocera , likely to be T. mexicana , collected in light traps near Austin, Texas (Quinn, 2008). The list of those diagnostic characters follows and a key to the species of Nearctic and Neotropical species is included at the end of the paper:
1. Vertex (epicraneal) plates narrower in T. paulistana ( Fig. 4B View FIGURE 4 ) and widely separated anteriorly ( Kogan, 1958; Oliveira and Kogan, 1959) while the plates are wider and nearly touch each other frontally, as seen in all illustrations of T. mexicana (see Pierce, 1913, Plate 1 Fig. 1 View FIGURE 1 ; 1918, Plate 54 fig. 10 ( T. texana ), Plate 55 fig. 1 ( T. mexicana )); observed in the four specimens used in this comparative study ( Fig. 4A View FIGURE 4 ); and seen in photos published in the Web (e.g., Quinn, 2009).
2. Antefrons (frontal tubercle) in T. paulistana with 2 lateral- and one medial lobe projecting anteriorly, in shape of a triangle with rounded angles; postfrons extending posteriorly separating the vertex plates; antefrons in T. mexicana rounded anteriorly ( Figs. 4A &B View FIGURE 4 ).
3. Profuse, long inter-eyelet trichomes in T. paulistana ( Fig. 4B View FIGURE 4 ); profuse but much shorter trichomes in T. mexicana ( Fig. 4A View FIGURE 4 ).
4. Although the wings of the T. paulistana holotype were damaged, it was possible to reconstitute the key elements of the venation. It seems that R 4 does not fork out of the extension of R 5, a character apparently shared with T. tecpanensis that differentiates them from the other species.
5. Subtle differences were also observed in the terminalia (9 th abdominal sternite, 10 th tergite), and base of aedeagus in dorsal view ( Figs. 3D & E View FIGURE 3 ).
6. The only known host of T. mexicana is Pangaeus bilineatus Say ( Heteroptera : Cydnidae ). The species does not seem to occur in Brazil (Lis et al., 2000), but the genus is represented by two other species P. aethiops (Fabr.) and P. neogeus Froeschner (ESALQ-USP, 2004) . Although host specificity in Triozocera has not been documented, the absence of the T. mexicana host in Brazil, may offer an additional argument for the separation of the two species.
As for the arguments raised by Kifune and Brailowsky (1987), the following considerations are in order. Differences in size ( T. paulistana much larger than T. mexicana ) resulted from artifact of the mounting procedure of T. paulistana . Boiling the specimen in 70% KOH caused the abdomen to expand. Using the illustration in Kogan (1958), Fig. 1 View FIGURE 1 , the area corresponding to the inter-segmental membranes of the abdomen was deleted and the length of the specimen reassessed. The length was reduced from the originally reported 3.08 mm, to 2.89 mm, well within the range of T. mexicana , T. vernalis and T. tecpanensis , but smaller than T. buehrheimi ( Table 2 View TABLE 2 ). The importance of body size in Strepsiptera should be considered in light of the size variation of the host, as demonstrated by Kathirithamby and Johnston (1992) and Cook (2000) for Caenocholax fenyesi Pierce , the males of which are parasitic on ants. Pangaeus bilineatus , the host of T. mexicana , is a ubiquitous cydnid in North America, and body length variability has been observed but not measured (Lis et al., 2000). Intraspecific size variability in Heteroptera has been studied in a few species, but the range of variability seems to be considerably less than that observed in Formicidae . For instance, body length variability in Pyrrhocoris apterus L. ( Heteroptera : Pyrrhocoridae ), as affected by rearing conditions, was under 10% ( Honĕk, 1987). In Nezara viridula L. ( Heteroptera : Pentatomidae ), in a family that contains Strepsiptera hosts, male size variability may reach 20% ( McLain, 1985). Length variability in Formicidae , however, is much greater. Cushman et al. (1993) reported a 52% difference in body size among ants in a species of Camponotus . We infer from this that body size variability is expected in Triozocera , but the range of that variability probably is significantly smaller than that found in Caenocholax . Unless the various body proportions show tendency to gigantism, as in T. buehrheimi , smaller differences, particularly in total body length, probably are less significant.
Finally, the argument of the disjunct geographic distribution of the two species needs to be reconsidered. Caenocholax fenyesi Pierce, 1909 is an example of introgression of a Neotropical Strepsiptera species both ways into North and South America. C. fenyesi was originally described from males collected in Cordoba, Mexico. Its recorded range has since been extended north to nine Southern USA States, and south to 10 Central American countries and the Antilles, and to three and possibly five countries in South America ( Cook et al., 1997; Kathirithamby and Hughes, 2002). This expansive distribution may, perhaps, involve a complex of cryptic lineages the nature of which is beginning to be unraveled ( Hayward et al., 2011). What seems certain, however, is that Strepsiptera distribution depends on suitable host availability ( Kathirithamby, 2009).
We conclude that T. paulistana differs from T. mexicana in various morphological characters (apomorphies), but we deemphasize the importance of differences in body size and disjunct distribution of the two species. The key differential characters are: a) profuse and long inter-eyelets pubescence; b) shape and structure of the vertex plates with wide separation of the plates anteriorly; c) shape of the antefrons (frontal tubercle); d) apparent lack of an R 4 vein off of the R 5; and, e) subtle differences in the terminalia. The absence in South America of Pangaeus bilineatus , host of T. mexicana , may also be of significance in support of the revalidation of T. paulistana .
TABLE 1. Specimens used in the comparative study of South American Triozocera males; all mounted in balsam. Type localities for Pierce’s
| Triozocera sp. | Specimen | Locale | Date | Collector | Det. | Label Remarks | Collection |
|---|---|---|---|---|---|---|---|
| T. mexicana | A | Gainesville, MS , USA | 25-IV-1966 | Khalaf | L. de Carvallho, 1972 | LC-1085 | J. Cook, Huntsville, TX, USA |
| T. mexicana | B | Gainesville, MS , USA | 25-IV-1966 | Khalaf | L. de Carvallho, 1972 | LC-1085 | J. Cook, Huntsville, TX, USA |
| T. mexicana | C | Mina Carlota, Trinidad Mts. (1500 ft) Cuba | 06-VI-1959 | ? | ? | LC-1086 – INNS, C10 ; Acc.no. 49681 | J. Cook, Huntsville, TX, USA |
| T. mexicana | D | Maricao, Puerto Rico | VII-1960 | J. Maldonado Capriles | Kogan, 1963 | IOC # 56 View Materials | Inst. Oswaldo Cruz. Rio de Janeiro, Brazil |
| T. mexicana | Holotype | Cordoba, Vera Cruz, Mexico | ? | A. Finyes | Pierce, 1909 | Holotype & 1 paratype | US Nat. Museum Cat. No. 10080 |
| T. texana | Holotype | Victoria, TX USA | 4-VII-1908 | J.D.Mitchell | Pierce, 1911 | Holotype | US Nat. Museum Cat. N. 13713 |
| T. paulistana | Holotype | Monte Mor , São Paulo, Brazil | IX-1953 | M. Kogan | Kogan, 1958 | IOC # 1 View Materials | Inst. Oswaldo Cruz. Rio de Janeiro, Brazil |
| US |
University of Stellenbosch |
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |
Triozocera paulistana Kogan, 1958: 422
| Kogan, Marcos & Cook, Jerry L. 2014 |
Triozocera paulistana, Kifune and Hirashima, 1979: 64
| Kathirithamby, J. 2005: 8 |
| Kathirithamby, J. 1990: 470 |
| Kifune, T. & Brailovsky, H. 1987: 134 |
| Kifune, T. & Hirashima, Y. 1979: 64 |
Triozocera mexicana
| Luna de Carvalho, E. 1978: 353 |
Triozocera paulistana
| Luna de Carvalho, E. 1967: 29 |
| Oliveira, S. J. D. & Kogan, M. 1959: 231 |
Triozocera paulistana
| Kogan, M. 1958: 422 |