Stoibocephalum, Cielocha, Joanna J. & Jensen, Kirsten, 2013
|
publication ID |
https://doi.org/ 10.11646/zootaxa.3626.4.9 |
|
publication LSID |
lsid:zoobank.org:pub:3F1600C4-23A9-4777-9410-0BA20D3E1C20 |
|
DOI |
https://doi.org/10.5281/zenodo.6160065 |
|
persistent identifier |
https://treatment.plazi.org/id/473787D9-FFF7-FFCF-B4A8-DC74AABEC00B |
|
treatment provided by |
Plazi |
|
scientific name |
Stoibocephalum |
| status |
gen. nov. |
Stoibocephalum n. gen.
Type species. Stoibocephalum arafurense n. sp.
Etymology. Stoibocephalum n. (stoibe, Greek, cushion; kephale, Greek, head) is named for its possession of a cushion-shaped apical organ.
Diagnosis. Worms apolytic; conspicuous longitudinal muscle bundles extending entire length of strobila, encircling reproductive organs. Scolex consisting of scolex proper with 4 acetabula, apical modification of scolex proper, and apical organ; cephalic peduncle absent. Acetabula in form of suckers. Apical modification of scolex proper bearing apical organ. Apical organ in form of muscular pad, retractable, non-invaginable. Proglottids craspedote. Testes numerous, several columns in frontal view, in multiple layers. Vas deferens expanded to form external seminal vesicle. Internal seminal vesicle absent. Cirrus sac pyriform. Cirrus spinitriches absent. Ovary margins lobulated, H-shaped in frontal view, bilobed in cross-section. Vagina medial, opening posterior to cirrus sac into genital atrium. Genital pores lateral, irregularly alternating. Uterus medial, tubular. Vitelline follicles lateral, in multiple irregular columns on each lateral margin, interrupted by ovary, post-ovarian vitelline follicles present. Excretory vessels in 3 pairs. Eggs singular, elliptoid, with bipolar filaments. Parasites of sharkrays ( Rhinidae ).
Remarks. Stoibocephalum n. gen. is clearly identifiable among orders of elasmobranch cestodes as a member of the Lecanicephalidea in its combined possession of a scolex consisting of 4 acetabula, an apical organ, a vagina opening into the genital atrium posterior to the cirrus sac, and the presence of an extensive external seminal vesicle.
Stoibocephalum n. gen. differs from all currently recognized lecanicephalidean genera except Hexacanalis by possessing 3 pairs of excretory vessels, rather than 1 or 2 pairs. The 3 pairs of excretory vessels can be observed in histological sections and most whole-mounts. Stoibocephalum n. gen. can be distinguished from Hexacanalis in its possession of a bilobed ovary in cross-section and eggs with bipolar filaments rather than a U-shaped ovary in crosssection and single eggs lacking filaments. Stoibocephalum n. gen. additionally differs from all other currently recognized lecanicephalidean genera with less than 3 pairs of excretory vessels as follows. It differs from Paraberrapex Jensen, 2001 and Aberrapex Jensen, 2001 in possessing an apical organ rather than lacking an apical organ. The presence of a large, muscular, retractable pad-like apical organ in Stoibocephalum n. gen. distinguishes it from Anteropora Subhapradha, 1955 , Eniochobothrium Shipley & Hornell, 1906 , Healyum Jensen, 2001 , Hornellobothrium Shipley & Hornell, 1906 , and Quadcuspibothrium Jensen, 2001 ; these genera possess small, internal, often glandular apical organs. The form of the apical organ of Stoibocephalum n. gen. also distinguishes it from Polypocephalus Braun, 1878 , in which the apical organ is divided into retractable tentacles, and from Rexapex Koch, Jensen , & Caira, 2012, in which the apical organ is in the form of an inverted cone with papilliform projections around its perimeter. In Stoibocephalum n. gen. the testes are numerous and arranged in several columns (and layers), while Sesquipedalapex Jensen, Nikolov , & Caira, 2011 possesses testes arranged in a single column, and Corrugatocephalum Caira, Jensen , & Yamane, 1997 possesses only 3 testes. Stoibocephalum n. gen. differs from Elicilacunosus Koch, Jensen , & Caira, 2012 in that the latter genus possesses a unique region of musculo-glandular depressions along the midline of the dorsal and ventral surface of the proglottids. Much like Stoibocephalum n. gen., Collicocephalus Koch, Jensen , & Caira, 2012, Lecanicephalum Linton, 1890 , Tetragonocephalum Shipley & Hornell, 1905 , and Tylocephalum Linton, 1890 possess large, mainly muscular apical organs. However, Stoibocephalum n. gen. can be distinguished from Collicocephalus in possessing craspedote proglottids and vitelline follicles arranged in irregular columns encroaching on the proglottid midline rather than possessing laciniate proglottids and vitelline follicles arranged in 4 distinct lateral columns; from Lecanicephalum in possessing testes arranged in several columns and layers, and lacking cirrus spinitriches rather than possessing testes arranged in 1–2 columns in a single layer and a cirrus conspicuously armed with spinitriches; and from Tetragonocephalum in possessing a tubular, unconstricted uterus and an ovary that is H-shaped in frontal view rather than a uterus that is constricted at its center and an ovary that is oval in frontal view. Stoibocephalum n. gen. is most similar to Tylocephalum . However, aside from the difference in number of excretory vessels, it differs from Tylocephalum in that its apical organ is fully retractable into the scolex proper resulting in an aperture at the scolex apex when retracted, while the apical organ of Tylocephalum is non-retractable.
Stoibocephalum n. gen. is described herein as being apolytic, as gravid proglottids were observed in almost all specimens. In a subset of whole-mounted type specimens used in this study (5 of 22), as many as 7 spent proglottids were observed retained on the strobila. In these spent proglottids, egg release presumably occurred through a dehiscence in the anterior of the proglottid (see Fig. 2 View FIGURE 2 F), approximately at the level of the genital pore.
Stoibocephalum arafurense n. gen., n. sp. ( Figs. 1–3 View FIGURE 1 View FIGURE 2 View FIGURE 3 )
Type host. Rhina ancylostoma Bloch & Schneider , sharkray (Rhinopristiformes sensu Naylor et al. 2012: Rhinidae ).
Type locality. East of Wessel Islands (11°17'44"S, 136°59'48"E), Arafura Sea, Pacific Ocean, Northern Territory, Australia.
Type material. Holotype (QM G233981; whole mount); 15 paratypes (QM G233982–G233996; 9 whole mounts, 1 scolex longitudinal section series and their whole-mounted vouchers, 1 scolex longitudinal section series stained with PAS and its whole-mounted voucher, 1 scolex cross-section series and its whole-mounted voucher, 1 mature proglottid cross-section series and its whole-mounted voucher, 1 lactophenol egg preparation and its wholemounted voucher, and 1 in situ longitudinal section series of scoleces [no voucher]); 10 paratypes (USNPC 106062.00–106064.00; 7 whole mounts, 1 scolex longitudinal section series [no voucher], 1 scolex longitudinal section series stained with PAS and its whole-mounted voucher, and 1 scolex cross-section series and its wholemounted voucher); 7 paratypes (LRP 7931–7952; 5 whole mounts, 1 scolex longitudinal section series stained with PAS and its whole-mounted voucher, and 1 whole-mounted voucher from which a lactophenol egg preparation and a mature proglottid cross-section series were prepared); 2 scoleces and a partial strobila prepared for SEM and their whole-mounted vouchers are retained in KJ’s personal collection at the University of Kansas.
Site of infection. Spiral intestine.
Prevalence. 100% (3 of 3).
Etymology. The specific epithet arafurense (n.) was chosen in recognition that this new species was found to parasitize sharkrays collected from the Arafura Sea, Australia.
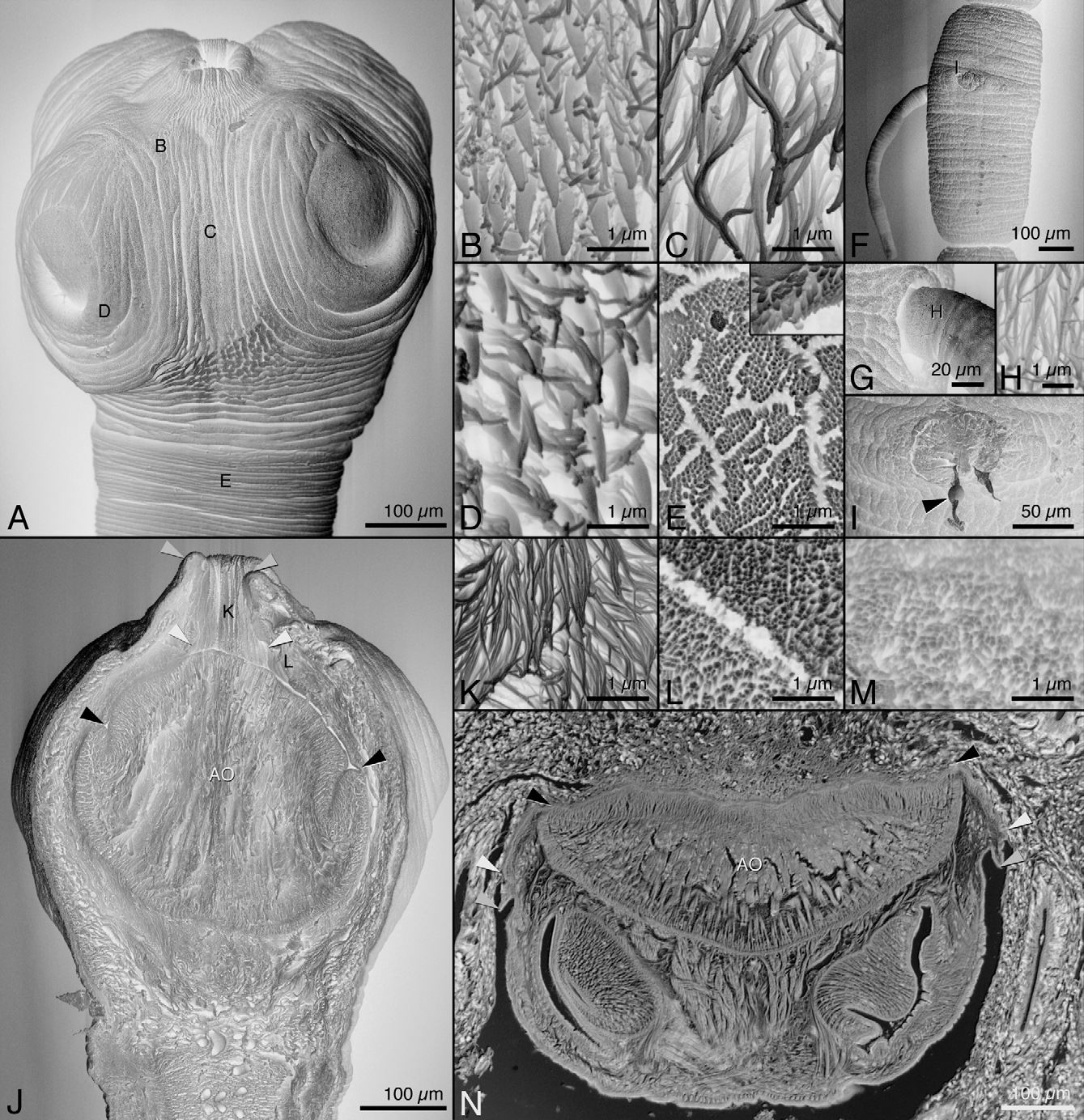
Description. (based on 22 whole worms; serial longitudinal sections of 5 detached scoleces and 1 piece of spiral intestine with multiple scoleces attached in situ, and serial cross-sections of 2 scoleces, and 2 mature and 1 immature proglottids; 2 scoleces and 1 partial strobila of mature proglottids prepared for SEM; and 2 lactophenol preparations of eggs). Worms 9–40 (20.7 ± 6.5; 22) mm long, apolytic; maximum strobilar width 383–667 (483 ± 74; 22) at posterior of strobila; proglottids 65–148 (110 ± 23; 22) in number. Scolex 427–657 (534 ± 57; 22) long by 558–782 (662 ± 56; 22) wide, consisting of scolex proper with 4 acetabula, apical modification of scolex proper, and apical organ ( Figs. 1 View FIGURE 1 A, 2A, J, N); cephalic peduncle absent. Acetabula in form of suckers, 168–216 (190 ± 11; 22, 44) in diameter. Apical modification of scolex proper bearing apical organ, partially invaginable, forming aperture with protruded rim when apical organ retracted ( Figs. 2 View FIGURE 2 A, J). Apical organ in form of thick muscular pad ( Figs. 2 View FIGURE 2 J, N), protrusible, 224–472 (319 ± 62; 22) long by 365–565 (467 ± 55; 22) wide when retracted.
Surface of scolex proper between and anterior to suckers ( Fig. 2 View FIGURE 2 C) and invaginated surface of the apical modification of the scolex proper ( Fig. 2 View FIGURE 2 K) covered with capilliform filitriches; apical modification of the surface of scolex proper adjacent to apical organ (internal when apical organ retracted) ( Fig. 2 View FIGURE 2 L) covered with acicular filitriches. Sucker surface ( Fig. 2 View FIGURE 2 D) and region of scolex proper surrounding suckers ( Fig. 2 View FIGURE 2 B) covered with scolopate spinitriches and acicular to capilliform filitriches. Apical organ surface ( Fig. 2 View FIGURE 2 M) covered with acicular filitriches. Surface of cirrus ( Fig. 2 View FIGURE 2 H) covered with capilliform filitriches; spinitriches absent. Strobilar surface ( Fig. 2 View FIGURE 2 E) covered with shorter capilliform filitriches throughout and small coniform spinitriches at posterior proglottid margin ( Fig. 2 View FIGURE 2 E, inset).
Proglottids slightly craspedote. Immature proglottids 56–133 (97 ± 22; 22) in number, wider than long, becoming longer than wide with maturity; posterior-most immature proglottid 353–691 (515 ± 110; 22) long by 236–410 (344 ± 44; 22) wide. Mature proglottids 3–11 (7 ± 2; 22) in number; posterior-most mature proglottid 438–1,180 (848 ± 168; 22) long by 312–454 (380 ± 39; 22) wide. Gravid proglottids 2–11 (5.7 ± 2.4; 22) in number, 945–2,322 (1,603 ± 381; 22) long by 323–627 (452 ± 82; 22) wide. Spent proglottids present in subset of worms, 1–7 (4 ± 2; 5) in number, posterior-most spent proglottid 938–1,364 (1,169 ± 168; 5) long by 339–363 (350 ± 12; 5) wide. Testes 26–42 (33 ± 4; 22, 66) in number, 32–101 (56 ± 14; 22, 66) long by 34–89 (60 ± 12; 22, 66) wide in posterior-most mature proglottid, extending from anterior margin of proglottid to level of Mehlis’ gland ( Figs. 1 View FIGURE 1 B, 3C), in several columns, multiple layers deep in cross-section ( Fig. 3 View FIGURE 3 B). Vas deferens expanded to form external seminal vesicle, medial in proglottid, extending from ootype region anteriorly to enter anterior margin of cirrus sac. Internal seminal vesicle absent. Cirrus sac pyriform to broadly pyriform, 119–261 (190 ± 35; 21) long by 102–178 (137 ± 21; 21) wide, tilted anteriorly, containing coiled cirrus ( Fig. 1 View FIGURE 1 B). Cirrus spinitriches absent ( Fig. 2 View FIGURE 2 H). Ovary 76–223 (161 ± 38; 21) long by 147–284 (240 ± 31; 21) wide in posterior-most mature proglottid, essentially H-shaped in frontal view ( Fig. 1 View FIGURE 1 B), with broadly lobulated margins, bilobed in cross-section ( Fig. 3 View FIGURE 3 C). Vagina straight, extending medially in proglottid from ootype to posterior margin of cirrus-sac, entering genital atrium posterior to cirrus sac. Genital pores lateral ( Figs. 1 View FIGURE 1 B, 2F), irregularly alternating, 55–79% (67 ± 6; 22) from posterior end in posterior-most mature proglottids. Uterus tubular, extending from anterior ovarian margin to anterior margin of proglottid, positioned centrally in proglottid posterior to genital pore (see Fig. 3 View FIGURE 3 B) and peripherally anterior to genital pore (see Fig. 3 View FIGURE 3 A); uterine duct entering uterus posteriorly; spent and most gravid proglottids with dehiscence in anterior half of proglottid ( Figs. 2 View FIGURE 2 F, I). Vitellarium follicular; vitelline follicles 25–64 (41 ± 9; 22, 66) long by 31–66 (49 ± 7; 22, 66) wide in posterior-most mature proglottid, lateral, in multiple irregular columns extending length of proglottid, encroaching on midline in mature ( Fig. 3 View FIGURE 3 B) and gravid proglottids, not interrupted at level of cirrus sac ( Fig. 3 View FIGURE 3 A), interrupted by ovary. Eggs in fibrous matrix in utero; individual eggs elliptoid, 15–18 (17 ± 1; 2; 15) long by 19–28 (23 ± 2; 2, 15) wide, with bipolar filaments ( Fig. 3 View FIGURE 3 D) of unequal length. Excretory vessels in 3 pairs ( Fig. 3 View FIGURE 3 A), interspersed among ovarian lobules at level of ovary ( Fig. 3 View FIGURE 3 C).
Remarks. Specimens of Stoibocephalum arafurense n. sp. available for study consisted of specimens found attached to the host’s intestinal mucosa with their apical organs protruded ( Fig. 2 View FIGURE 2 N) and those that had become detached during fixation; the latter specimens all had their apical organs retracted ( Figs. 1 View FIGURE 1 A, 2A), making reconciliation of particular landmarks on the 2 different scolex conditions difficult. In scoleces in situ with protruded apical organs, the apical organ surface is attached to the intestinal mucosa along its entire width (see Fig. 2 View FIGURE 2 N). The apical organ is flanked by a region of apical modification of the scolex proper (AMSP) that is also intimately connected to the host’s intestinal mucosa (region between white and black arrowheads in Fig. 2 View FIGURE 2 N); the more posterior regions of the AMSP do not interface directly with host tissue (see region between black and grey arrowheads in Fig. 2 View FIGURE 2 N). The frontally bisected scolex with a retracted apical organ prepared for SEM ( Fig. 2 View FIGURE 2 J) allowed comparison with the longitudinal sections of scoleces in situ ( Fig. 2 View FIGURE 2 N) and the determination of homologous regions as well as of the microthrix pattern of the surfaces of the apical organ and the AMSP. In specimens detached from the host spiral intestine, the apical organ was retracted and the AMSP had closed over the apical organ ( AO in Fig. 2 View FIGURE 2 J) forming an aperture with a protruded rim ( Figs. 2 View FIGURE 2 A and J). In this condition, the regions of the AMSP intimately connected to the host’s intestinal mucosa in attached scoleces (see region between white and black arrowheads in Figs. 2 View FIGURE 2 N and J) and the region that does not interface directly with host tissue in attached scoleces (see region between black and grey arrowheads in Figs. 2 View FIGURE 2 N and J) are invaginated into the scolex proper. Scanning electron micrographs of the frontally bisected scolex revealed distinct microthrix patterns on these apical regions correlating with attachment capabilities. The surface of the AMSP flanking the apical organ (regions between white and black arrowheads) and the surface of the apical organ (region between white arrowheads) were covered with shorter (i.e., acicular) filitriches ( Figs. 2 View FIGURE 2 L and M, respectively); these surfaces interface closely with the host’s mucosa (see Fig. 2 View FIGURE 2 N). The surface of the remainder of the invaginated region of the AMSP forming the internal walls of the aperture (regions between black and grey arrowheads) was covered with capilliform filitriches ( Fig. 2 View FIGURE 2 K); this surface does not interface directly with host tissue in attached scoleces (see Fig. 2 View FIGURE 2 N).
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |