Squalus albicaudus, De, Sarah T., De, Marcelo R. & Gomes, Ulisses L., 2016
|
publication ID |
https://doi.org/ 10.11646/zootaxa.4133.1.1 |
|
publication LSID |
lsid:zoobank.org:pub:E7276A63-67C8-4BC5-8419-2EBDAE4432B0 |
|
DOI |
https://doi.org/10.5281/zenodo.6075735 |
|
persistent identifier |
https://treatment.plazi.org/id/282F878E-FFC8-FFDD-14C0-2100FB555DB2 |
|
treatment provided by |
Plazi |
|
scientific name |
Squalus albicaudus |
| status |
sp. nov. |
Squalus albicaudus View in CoL sp. nov.
( Figs. 42–50 View FIGURE 42 View FIGURE 43 View FIGURE 44 View FIGURE 45 View FIGURE 46 View FIGURE 47 View FIGURE 48 View FIGURE 49 View FIGURE 50 ; Tables 3 View TABLE 3 , 10–11 View TABLE 10 View TABLE 11 )
Suggested common names: Brazilian whitetail dogfish; Cação-bagre-de-cauda-branca (Portuguese).
Squalus megalops View in CoL (not Macleay): Regan, 1908: 45, 47 (identification key, listed; global); Bigelow & Schroeder, 1948: 454 (cited); Bigelow & Schroeder, 1957: 29, 36, 37, figs. 3C, 4 (revision; Northwestern Atlantic); Bass et al., 1976: 11–13, 16– 18, figs. 6B, 7B, 8 (C, D), 11, pl. 3 (revision; Eastern South Africa); Cadenat & Blache, 1981: 51, 52 (revision; Mediterranean Sea); Compagno, 1984: 118, 119 (revision; global); Muñoz-Chápuli, 1985: 397, 398, fig. 1 (cited); Muñoz- Chápuli & Ramos, 1989: 1–21, figs. 1, 2 (E, F), 3 (D, E) (revision; Eastern Atlantic); Calderón, 1994: 1–104, fig. 5A (cited); Nion et al., 2002: 4 (listed); Haimovici et al., 2003: 38, 39 (cited); Meneses & Paesch, 2003: 8, 25, 45 (cited); Bernades et al., 2005: 49, 70 (cited); Compagno et al., 2005: 74 (description; global); Hazin et al., 2006 (cited); Jablonski et al., 2006: 110, 177, 178 (cited); Louro & Rossi-Wongtschowski, 2007: 18, 27–30, 49, figs. 15, 16 (cited); Carrier et al., 2010: 44 (cited); Viana, 2011: 92–129, figs. 35–56 (revision; Brazil); Rosa & Gadig, 2014: 92 (listed; Brazil).
Squalus cubensis View in CoL (not Howell-Rivero): Figueiredo, 1977 (in part): 8 (description; Southern Brazil); Lucena & Lucena, 1981: 4 (listed; Brazil); Myagkov & Kondyurin, 1986: 1–20, fig. 1 (A, E, F, H) (revision; Atlantic); Lessa et al., 1999: 14, 61, 150 (cited, listed; Northeast Brazil); Compagno, 2002: 384 (description; Northern and Southern Brazil, Argentina, Uruguay); Compagno et al., 2005: 75, pl. 3 (description; Southwest Atlantic Ocean); Last et al., 2007: 21–22, figs.10A, 11A (cited; Southeast Brazil); Nunan & Senna, 2007: 169, 170 (in part) (cited; Brazil); Viana, 2011 (in part): 130–162, figs. 57–77 (revision; Brazil).
Squalus View in CoL sp. of the megalops-acutipinnis-cubensis group Cadenat & Blache, 1981: 51, 52, fig. 31 (F, G) (revision; Mediterranean Sea); Figueiredo, 1981: 17 (listed; Brazil).
Squalus View in CoL sp. of the megalops View in CoL group: Marques, 1994 (cited; Brazil); Gomes et al., 1997: 95, 98–109 (description; Brazil); Marques, 1999 (cited; Brazil).
Squalus View in CoL sp.: Gomes et al., 1997: 98 (listed; Brazil); Tomás et al., 2010 (cited; Southeast Brazil).
Squalus View in CoL of the megalops / cubensis group: Gadig, 2001: 29, 36, 54, 58–60 (listed; Brazil).
Squalus View in CoL sp. A: Soto, 2001: 95, 96 (listed; Brazil); Soto & Mincarone, 2004: 74 –79 (listed; Brazil).
Squalus View in CoL sp. 2: Gomes et al. 2010: 44 –46 (cited; Brazil).
Holotype. MNRJ 30188, adult male, 525 mm TL, north coast of Espírito Santo state, Brazil, 19°42'54"S, 39°25'57"W, 195 m. Collected on 30 June 2000, Station 0 531, Thalassa cruise, Revizee program.
Paratypes (11 specimens). MNRJ 30173, adult female, 590 mm TL, south coast of Bahia state, between Itacaré and Ilhéus, Brazil, 14°28'58"S, 38°54'0"W, 278 m; MNRJ 30174, adult female, 580 mm TL, south coast of Bahia state, between Itacaré and Ilhéus, Brazil, 14°28'58"S,3 8°54'0"W, 278 m; MNRJ 30175, adult female, 560 mm TL, south coast of Bahia state, between Itacaré and Ilhéus, Brazil, 14°28'58"S, 38°54'0"W, 278 m; MNRJ 30176, adult female, 540 mm TL, south coast of Bahia state, between Itacaré and Ilhéus, Brazil, 14°28'58"S, 38°54'0"W, 278 m; MNRJ 30177, adult male, 450 mm TL, Morro de São Paulo, Bahia, Brazil, 13°21'51"S, 38°40'49"W, 421 m; MNRJ 30181, adult male, 482 mm TL, Canavieiras, Bahia, Brazil, 15°42'41"S, 38°37'18"W, 251 m; MNRJ 30183, juvenile female, 425 mm TL, Salvador, Bahia, Brazil, 13°8'54"S, 38°28'41"W, 334 m; MNRJ 30184, adult male, 480 mm TL, Salvador, Bahia, Brazil, 13°8'54"S, 38°28'41"W, 334 m; MNRJ 30185, adult male, 440 mm TL, Salvador, Bahia, Brazil, 13°8'54"S, 38°28'41"W, 334 m; MNRJ 30186, adult female, 590 mm TL, north coast of Espírito Santo state, Brazil, 19°42'54"S, 39°25'54"W, 202 m; MNRJ 30187, adult female, 610 mm TL, north coast of Espírito Santo state, Brazil, 19°42'54"S, 39°25'57"W, 195 m.
Non-type material (9 specimens). MZUFBA uncatalogued, juvenile female, 420 mm TL, adult male, 502 mm TL, Praia do Forte, Mata de São João, Bahia, Brazil; NUPEC 96, adult male, 460 mm TL, South of Barra de Santos, São Paulo, Brazil; NUPEC 1354, neonate female, 285 mm TL, juvenile female, 355 mm TL, Ilha Vitória, São Paulo, Brazil; TAMAR 10.38, adult male, 490 mm TL, locality same as MZUFBA; TAMAR 10.39, juvenile female, 459 mm TL, locality same as MZUFBA; TAMAR 10.41, juvenile female, 445 mm TL, locality same as MZUFBA; TAMAR 10.42, juvenile female, 410 mm TL, locality same as MZUFBA.
Diagnosis. Squalus albicaudus sp. nov. can be distinguished from its congeners by the following combination of characters: caudal fin with a mostly white ventral caudal lobe, dorsal caudal margin white at midline, and postventral caudal margins broadly white; pectoral-fin posterior margin broadly white; first dorsal fin with anterior margin also conspicuously white on its anterior half. Squalus albicaudus sp. nov. differs from all species of the S. mitsukurii group by: snout short vs. snout large; pectoral-fin free rear tips pointed vs. pectoral-fin free rear tips rounded; dermal denticles lanceolate and unicuspid vs. dermal denticles rhomboid and tricuspid. Squalus albicaudus sp. nov. is clearly distinct from S. cubensis by: snout strongly pointed (vs. snout somewhat rounded); second dorsal-fin spine not reaching second dorsal-fin apex (vs. spine reaching second dorsal-fin apex); first dorsal fin with dark apex, but not as a black blotch (vs. conspicuous black blotch on both dorsal fins); pectoral fins with posterior margin broadly white (vs. narrowly white). These two species also differ in external morphometrics such as: shorter first dorsal fin (anterior margin length 10.9%, 9.7%–11.2% TL vs. 11.6%, 11.6%–12.7% TL in S. cubensis ); shorter second dorsal fin (anterior margin length 9.2%, 8.8%–10.8% TL vs. 12.3%, 11.2%–11.6% TL in S. cubensis ; inner margin length 5.0%, 4.1%–5.2% TL vs. 5.6%, 5.5%–6.0% TL in S. cubensis ); more slender second dorsal-fin spine (width at base 0.9%, 0.6%–0.9% TL vs. 1.0%, 1.0%–1.2% TL in S. cubensis ); and clasper much more elongated in (inner margin length 7.1%, 6.9%–7.7% TL vs. 8.0%, 3.3%–3.8% TL in S. cubensis ).
Description. External morphology. Measurements and counts are summarized in Tables 10–11 View TABLE 10 View TABLE 11 . Body fusiform and thin throughout, slightly arched dorsally, equally deep from head to trunk (head height 1.0, 0.8–1.0 times trunk height and abdomen height), and broader at head than at trunk (head width 1.2, 1.0–1.4 times trunk height, and 1.4, 1.2–1.4 times abdomen height). Head very short and narrow (length 21.8%, 18.7%–24.7% TL; its greatest width at mouth (11.1%, 10.9%–12.0% TL). Snout pointed at tip, conspicuously short (length 7.1%, 6.8%– 7.8% TL); anterior margin of nostrils bifurcate and wide, equally near snout tip and mouth; prenarial length 1.0, 0.8–1.0 times distance from nostrils to upper labial furrow, and 0.5, 0.4–0.5 times preoral length; internarial space 1.1, 0.8–1.0 times eye length. Eyes oval and markedly large (length 4.0%, 4.2%–5.3% TL, and 1.5, 1.8–2.1 times their height); anterior margin of eyes convex and posterior margin notched; interorbital space 8.4%, 8.2%–8.9% TL. Prespiracular length 12.5%, 11.7%–13.8% TL, and 0.5 (0.6–0.7) times prepectoral length and 1.7 (1.7–1.9) times preorbital length. Spiracles crescent-shaped and wide, their length 1.5% (1.3%–2.0% TL), and 0.4, 0.3–0.4 times eye length. Prebranchial length 1.5 (1.3–1.5) times prespiracular length. Gill slits vertical and low, with fifth gill slit 1.1 (1.0–1.4) higher than first gill slit.
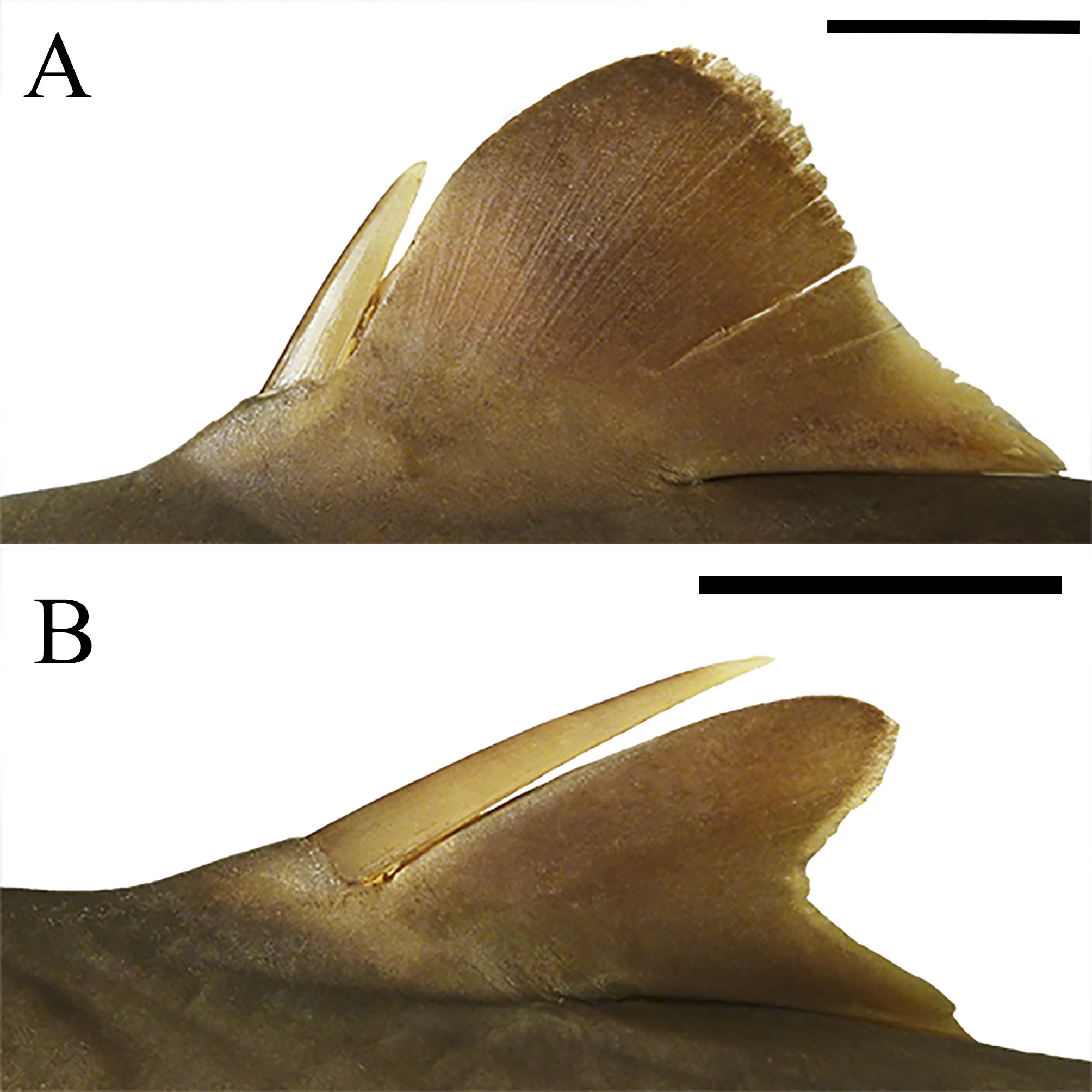
Preoral length 9.8%, 9.2%–11.2% TL and equal to 1.2, 1.3–1.5 times mouth width. Mouth arched and strongly broad, its width 1.5, 1.5–1.8 times prenarial length and 1.9, 1.9–2.0 times internarial space; upper labial furrow with thin fold, very large (length 2.3%, 2.1%–2.5% TL); lower labial furrow also large, although without a fold. Teeth unicuspid and similar in both jaws, wide and flattened labial-lingually at crown, imbricate laterally; upper teeth much smaller than lower teeth; cusp oblique, somewhat elongate and heavy, directed laterally; mesial heel slightly notched; distal heel rounded; mesial cutting edge somewhat straight; apron thick and rather elongate. Three series of teeth in upper jaw and two series of teeth in lower jaw in holotype (paratypes 2–3 series in both jaws); tooth rows ranging from 12–12 (13–14 paratypes) in upper jaw and 11–11 (11–12 paratypes) in lower jaw ( Fig. 43 View FIGURE 43 ). Pre-first dorsal fin length 1.1 (1.2–1.8) times prepectoral length. Origin of first dorsal fin anterior to pectoral free rear tips. First dorsal fin with anterior margin markedly convex and posterior margin straight, although concave anteriorly; first dorsal-fin apex rather rounded; first dorsal-fin free rear tip rounded ( Fig. 44 View FIGURE 44 ); first dorsal-fin inner margin very short, its length 5.6% (4.9%–6.1% TL); first dorsal fin short (length 1.7, 1.6–2.0 times its height) and low (height 7.9%, 6.8%–8.0% TL and 1.4, 1.2–1.6 times first dorsal-fin inner margin length, and 1.1, 0.9–1.1 times preorbital length). First dorsal-fin spine slender, thick (its base width 1.0%, 0.7%–1.1% TL) and elongate (its length 4.2%, 2.3%–4.1% TL), reaching one-half of first dorsal-fin height (0.3–0.5 times first dorsal-fin height in paratypes). First dorsal-fin length 1.3 (1.0–1.3) times larger than second dorsal-fin length. Interdorsal space 1.1 (1.0–1.3) times prepectoral length and 2.2 (1.7–2.6) times dorsal-caudal space. Pre-second dorsal length 2.8, 2.5– 3.1 times prepectoral length and 3.2, 2.8–3.3 times dorsal-caudal margin length. Second dorsal fin also short (length 2.0, 1.9–2.3 times its height) and somewhat tall (height 5.4%, 4.9%–6.2% TL, and 1.1, 0.9–1.3 times second dorsal-fin inner margin length); first dorsal-fin anterior margin convex and posterior margin markedly concave; first dorsal-fin apex rounded and pointed at free rear tip ( Fig. 44 View FIGURE 44 ); second dorsal-fin inner margin short, its length 5.0% (4.1%–5.2% TL). Second dorsal-fin spine conspicuously thin and large, almost reaching second dorsal-fin height, its length 5.1% (3.4%–5.0% TL), and 0.9 (0.7–0.9) times second dorsal-fin height, and 1.2 (1.0– 1.8) times first dorsal-fin spine length.
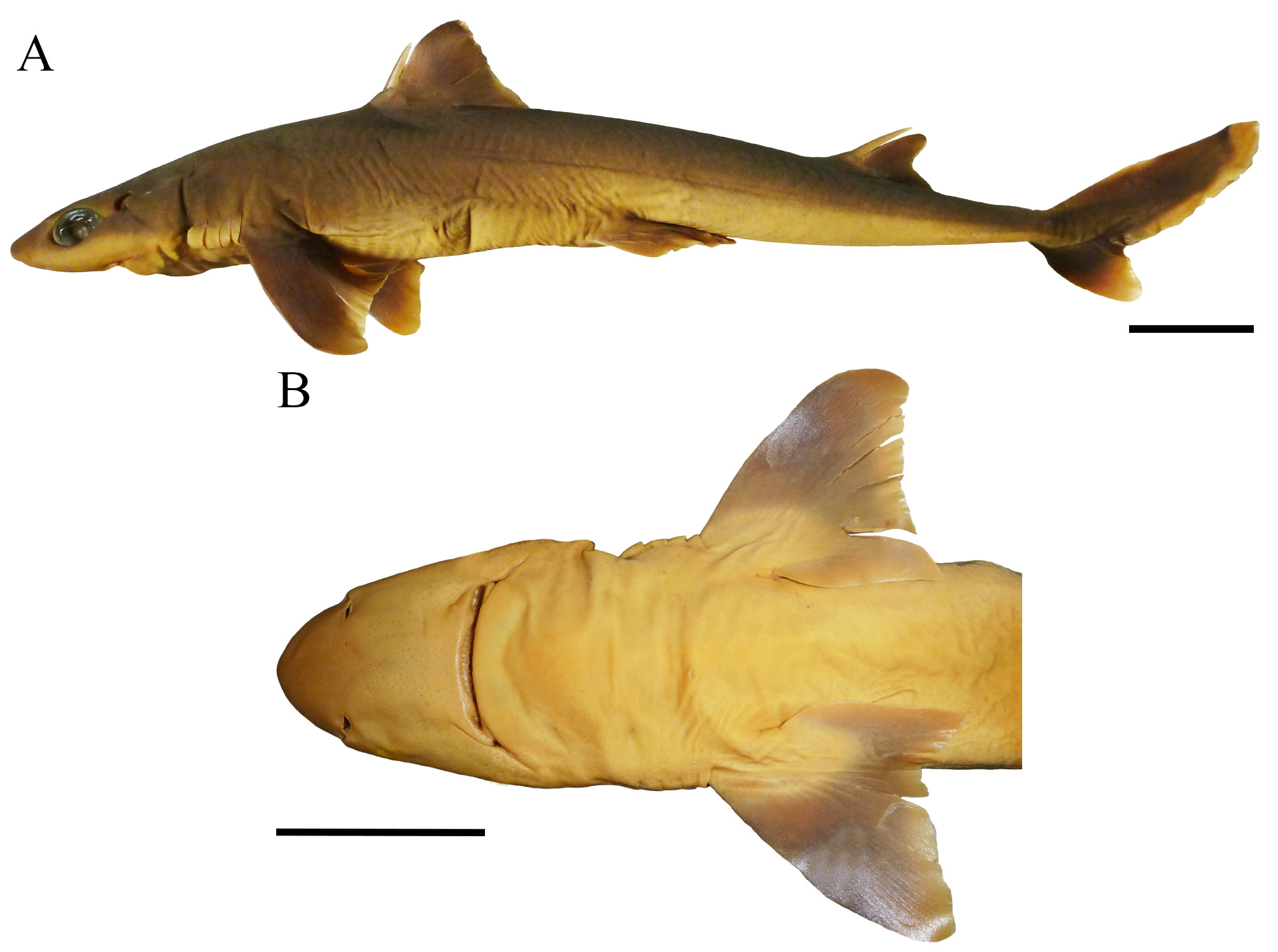
Pectoral fins small but also broad with anterior margin length 13.4% (11.8%–14.4% TL), 1.2 (1.2–1.4) times posterior margin length, and 1.5 (1.3–1.5) times inner margin length; pectoral-fin posterior margin 10.7% (9.7%– 11.8% TL); pectoral-fin anterior margin straight, inner margin convex and posterior margin concave; pectoral-fin apex rounded and lobe-like ( Fig. 42 View FIGURE 42 ); pectoral-fin free rear tips markedly pointed, extending beyond horizontal line at pectoral apex; posterior margin length 1.1 (0.8–1.0) times trunk height.
Pectoral-pelvic space 0.7 (0.5–0.9) times pelvic-caudal space. Pelvic fins nearer first dorsal fin than second dorsal fin in holotype and young paratypes (or nearest second dorsal fin in adult paratypes). Pelvic-fin anterior margin convex and posterior margin straight; pelvic-fin apex rounded and narrow; pelvic-fin free rear tips rounded and lobe-like; pelvic fins elongate, their inner margin length 6.3% (4.2%–6.3% TL). Claspers cylindrical, thin, and flattened dorsoventrally; claspers short, slightly extending beyond pelvic-fin free rear tips, their inner length 7.1% (6.9%–7.7% TL) and 1.1 (1.2–1.4) times larger than pelvic-fin inner margin length; clasper groove longitudinal and small, located dorsally; apopyle narrow, placed in proximal end of clasper groove; hypopyle constricted at distal end of clasper groove; rhipidion narrow and short, blade-like and somewhat thick, positioned medially at distal end of clasper ( Fig. 45 View FIGURE 45 ).
Caudal peduncle slender with soft caudal keel on each side; upper and lower caudal furrows markedly deep. Caudal fin slender and short, its dorsal caudal margin length 19.8% (19.3%–21.2% TL), and 0.9 (0.8–1.1) times head length and 1.7 (1.7–1.9) times preventral caudal margin; dorsal caudal margin straight, post-ventral caudal margin (upper and lower) convex ( Fig. 46 View FIGURE 46 ); posterior tip pointed; caudal fork between lobes with conspicuous concavity and very narrow (width 6.5%, 6.2%–7.2% TL); ventral tip rounded; preventral caudal margin also convex and short, its length 11.4% (10.5%–11.9% TL) and 1.8 (1.7–2.6) times pelvic-fin inner margin length.
Dermal denticles ( Fig. 47 View FIGURE 47 ). Denticles unicuspid, lanceolate and markedly slender, their length much greater than width; dermal denticles moderately asymmetrical and near each other but not imbricate; median and lateral ridges prominent, bifurcate anteriorly; crown conspicuously expanded anteriorly and rather expanded laterally; cusp rounded and elongate posteriorly.
Coloration ( Fig. 42 View FIGURE 42 ). Body brownish gray dorsally and white ventrally, gray laterally from between the pectoral and pelvic fins, turning whitish at caudal fin origin. First dorsal fin brownish, darker at fin web to apex; anterior margin broadly white on anterior half and dark brown at apex; posterior margin slightly white on anterior half and brown at apex; first dorsal-fin spine gray, darker anteriorly, white laterally and attip. Second dorsal fin brown throughout (few paratypes with white apex); second dorsal-fin posterior margin white; second dorsal-fin spine also gray, darker anteriorly and white at tip. Pectoral fins brown dorsally and ventrally (except at base) with posterior and inner margins conspicuously white, including apex and free rear tips. Pelvic fins light brown dorsally and white ventrally; pelvic posterior margin broadly white. Caudal fin mostly dark brown with dorsal caudal margin markedly white at midline; upper caudal blotch dark gray distally at dorsal caudal margin; post-ventral caudal margins conspicuously white, except for light gray caudal fork (caudal bar); posterior caudal tip also broadly white; preventral caudal margin dark gray anteriorly; ventral caudal lobe conspicuously white, including ventral caudal tip; caudal stripe blackish, thick and short.
Skeletal morphology. Measurements and counts are summarized in Tables 3 View TABLE 3 , 11 View TABLE 11 .
Neurocranium ( Fig. 48 View FIGURE 48 ). Neurocranium greatest width at level of postorbital processes (width 57.1% CL) and narrower at interorbital region (28.8% CL) and between opisthotic processes in the otic region (36.5% CL). Rostrum spoon-like, comprised by precerebral fossa profound and short (its length 40.1% CL), narrower proximal and distally than at its median portion; lateral rostral cartilages slightly cylindrical, flattened laterally; two lateral rostral appendices anteroventrally, hook-like; rostral prominence small, placed medially at tip of rostrum; rostral keel conspicuous and elongate, its length 25.0% CL, slightly transcending anterior margin of nasal capsules; prefrontal fontanelle constricted and rounded, anterior to brain at rostrum base. Nasal capsules rounded and markedly wide (width across nasal capsules 52.8% CL), located lateral to rostrum; nasal apertures ventral; subnasal fossa oval and large, located at each side of rostral keel.
Squa'us a'bicaudus sp. nov. Squa'us cubensis
Measurements Paratypes Paratypes Gu1f οf Mexicο Caribbean Sea
Hο1οtype Hο1οtype
Cranial roof arched medially, strongly concave laterally at interorbital region, and elongate (postcerebral length 58.5% CL); supraorbital crest with deep longitudinal sulcus, with a series of 10 foramina for the superficial ophthalmic branch of the trigeminal (V) and facial (VII) nerves; preorbital canal markedly broad, located anterior to a series of foramina for superficial ophthalmic branches (V, VII); canal for the ophthalmicus profundus nerve also clearly wide, placed just anterior to preorbital canal for the ophthalmicus nerve; preorbital processes inconspicuous laterally (width across preorbital processes 48.5% CL); postorbital processes prominent, triangular and elongate (its length 10.2% CL).
Supraethmoidal processes conspicuous, cylindrical and paired, placed medioanteriorly in ethmoidal region; epiphyseal pit prominent and rounded, located posterior to supraethmoidal processes; ethmoideal region somewhat depressed ventrally with ectethmoid process wide, pointed and bifurcated distally; subethmoidean chamber constricted until anterior to basal angle; subethmoidal ridge slender and very elongate, almost reaching the basitrabecular process of basal plate; two small ethmoidal canals, located far anteriorly in the cranial roof at base of each nasal capsule.
Otic region very narrow, comprised by a single and small otic capsule on each side; otic capsule delimited by anterior semicircular canal anteriorly, posterior semicircular canal posteriorly and sphenopterotic ridge laterally; lateral semicircular canal inconspicuous, located ventrally to sphenopterotic ridge; endolymphatic fossa rounded and shallow, placed between otic capsules; two small endolymphatic foramina, located anteriorly in fossa; two posterior perilymphatic foramina wide and rounded; otic crest slightly prominent mediodistally in the otic region; opisthotic processes somewhat pointed posterolaterally at each side of otic region (width across processes 36.5% CL); hyomandibular facet shallow, placed lateroventrally and below lateral semicircular canal; lateral auditory groove profound, positioned anterior to hyomandibular facet; prootic process thick and conspicuous, located posterior to hyomandibular facet (width across hyomandibular facets 41.5% CL).
Orbital region with preorbital wall concave; orbitonasal canal ventral at preorbital wall; interorbital wall deep and large with optic foramen (II) broad, placed midventrally; trochlear foramen (IV) small, placed dorsally near supraorbital crest; eye-stalk prominent, located more posteriorly in interorbital wall and anterior to prooticum foramen; oculomotor foramen (III) and abducens foramen (VI) small, placed, respectively, dorsally and ventrally to eye-stalk; foramen prooticum for the trigeminal (V) and facial (VII) nerves markedly broad, positioned ventroposteriorly in interorbital wall; foramen for efferent of pseudobranchial artery very small, positioned over basal angle; transbasal canal also small, located anteriorly to foramen prooticum.
Basal plate flattened and elongate (its length 38.6% CL), narrower anteriorly at basitrabecular processes (width across processes 16.1% CL) than posteriorly; basitrabecular process bean-shaped and prominent anteriorly; lateral prominence sinuous on each side of basal plate, posterior to basitrabecular processes; single cartilaginous process prominent laterally on each side, somewhat cylindrical (width across processes 30.3% CL); foramen for carotid artery rounded and small, located anteromedially in basal plate; foramen for orbital artery small, located at base of each cartilaginous process.
Occipital region with two triangular occipital condyles, placed ventrally; foramen magnum very wide (its width 9.2% CL); foramen for vagus nerve (X) narrow; glossopharyngeal base markedly thick and subtriangular, placed posterolaterally on each side of occipital region, with foramen for glossopharyngeal nerve (IX) strongly broad and oval.
Pelvic girdle and fin ( Fig. 49 View FIGURE 49 ). Puboischiadic bar transverse, short, slim, and thicker laterally, convex medially and concave more laterally at its anterior portion, posterior portion completely straight; lateral prepelvic process evident on each side, slightly rounded; two expansions lateroposteriorly articulating pubosichiadic bar to pelvic fin; two foramina at each side of puboischiadic bar. Basipterygium vertical, short and straight; anterior pelvic basal element subrectangular, elongate, its length corresponding to one-half length of basipterygium, and associated to three series of small irregular radials; pelvic radials elongate and slender, segmented into proximal and distal elements, the later shorter than the former; 13 total pelvic radials.
Claspers ( Fig. 49 View FIGURE 49 ). Intermediate segment barrel-shaped and short, placed between the pelvic fin basipterygium and axial cartilage; beta cartilage rod-like, cylindrical, and large, its length corresponding to more than one-third length of dorsal terminal cartilage, located dorsally for attaching pelvic fin basipterygium to axial cartilage. Axial cartilage slender and somewhat sinuous with a deep groove evident lateroventrally; end-style very short, not reaching half length of dorsal terminal cartilage (or claw), located medially at end of axial cartilage; dorsal marginal cartilage inconspicuous, laterally over the axial cartilage; dorsal terminal cartilage (or claw) short, its length corresponding to one-third the axial cartilage length, slightly concave and pointed distally, connected anteriorly to dorsal marginal cartilage; dorsal terminal 2 cartilage leaf-like, very soft and small, attached anteriorly to dorsal marginal cartilage and medially to dorsal terminal cartilage (or claw), externally supporting the rhipidion. Ventral marginal cartilage conspicuous and thick, bowl-shape, broader posteriorly than anteriorly, with a prominent folded plate laterally; ventral terminal cartilage spatula-like, small, its length less than one-half of axial cartilage length, attached proximally to ventral marginal cartilage and axial cartilage; accessory terminal 3 cartilage (or spur) thick and short, its length corresponding to one-half axial cartilage length, inserted into folded plate of ventral marginal cartilage, slightly distally pointed with two vertical grooves, one medially and the second ventrally.
Vertebral counts ( Table 11 View TABLE 11 ). Monospondylous vertebrae 39 in holotype (40–41 in paratypes); precaudal vertebrae 82 (81–89); total vertebrae 110 (110–116).
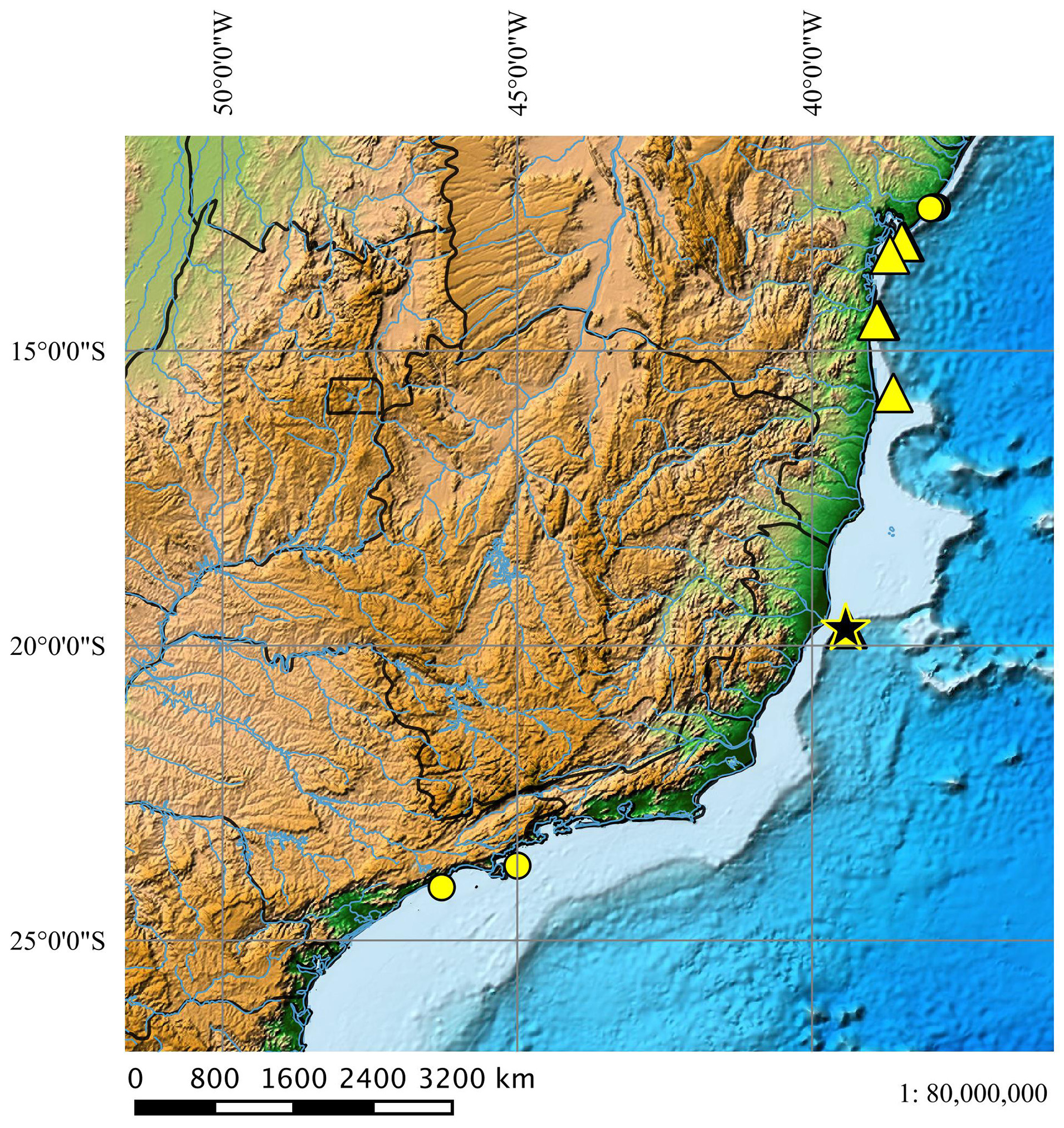
Geographical distribution ( Fig. 50 View FIGURE 50 ). Squalus albicaudus occurs in warm tropical waters between the northeastern and southeastern Brazilian coast and is often registered between the states of Bahia and Espírito Santo. Its distribution to southern Brazil is unknown.
Etymology. From the Latin albus, white, and cauda, tail, in reference to the white ventral lobe of its caudal fin.
Remarks. Squalus cubensis is a valid species described originally from Havana, Cuba and occurs in the Caribbean Sea ( Howell-Rivero, 1936b; Cervigón & Alcal, 1999; Compagno et al., 2005). Squalus cubensis is very similar to S. albicaudus in general morphology, sharing the following characters: dermal denticles unicuspid, slender and lanceolate, their length much greater than width; caudal fin with postventral margins conspicuously white; snout very short; and pectoral fins short but wide. One of the main characters employed to distinguish S. cubensis from its congeners has been the shape of the pectoral fins, with a strongly concave posterior margin and markedly pointed free rear tips (e.g. Bigelow & Schroeder, 1957; Garrick, 1960; Bass et al., 1976; Muñoz-Chápuli & Ramos, 1989). The same pattern of pectoral fins, however, is observed in other species, such as S. brevirostris , S.
blainvillei , S. megalops (including S. cf. megalops from South Africa), S. crassispinus , and S. albicaudus , indicating that it is not useful to differentiate S. cubensis from other species of the genus, including S. albicaudus from the SWAO. These characters have contributed to the taxonomic confusion regarding the occurrence of S. cubensis in the SWAO.
Bigelow & Schroeder (1948, 1957) pointed out that S. cubensis possibly occurs in South America based solely on the description of the pectoral fins of S. blainvillei by Miranda Ribeiro (1907, 1923) from Rio de Janeiro. Morphological characters provided by Miranda Ribeiro (1907, 1923), however, do not apply exclusively to a single species of Squalus , indicating that his observations were based on different species including S. albicaudus and S. acanthias . We verified this when we compared specimens studied by A. de Miranda Ribeiro in the MNRJ from Ilha Rasa (Rio de Janeiro, Brazil) collected by the vessel “Annie. Subsequent authors (e.g. Ledoux, 1970; Figueiredo, 1977; Sadowsky & Moreira, 1981; Myagkov & Kondyurin, 1986) also recognized the Cuban dogfish in southeastern Brazil. Figueiredo (1977) provided an illustration based probably on a juvenile male from Uruguay, but which does not represent S. cubensis or S. albicaudus due to differences in shape of the pectoral fins and coloration. Figueiredo (1981) also stated that two other species of Squalus may occur between Uruguay and southern Brazil in addition to S. cubensis . Squalus albicaudus has not yet been recorded in this area, however, indicating that Figueiredo (1981) was probably referring to another species of Squalus that has a more southern distribution. Large variation in vertebral counts reported in Sadowsky & Moreira (1981) and Myagkov & Kondyurin (1986) also support the current hypothesis that more than a single species of Squalus distinct from S. cubensis occurs in the Southwestern Atlantic Ocean, and that the Cuban species does not occur farther southward in the Atlantic Ocean.
Morphometric differences between S. albicaudus and the Cuban dogfish were not found, however, when nontype specimens of S. cubensis were taken into account ( Table 10 View TABLE 10 ). No differences in vertebral counts were found between S. albicaudus and S. cubensis (see also Muñoz-Chápuli & Ramos, 1989) because S. cubensis always presents a broad range of values. However, S. albicaudus differs slightly from S. cubensis in caudal vertebrae (28, 26–29 vs. 29–30, respectively) as indicated by Springer & Garrick (1964). Specimens of S. cubensis exhibit great range of external measurements that together with vertebral count variations may indicate the existence of more than one morphological group, similar to S. cubensis , in the Western Central Atlantic Ocean and Caribbean Sea. Further investigation on the morphology of S. cubensis as well as molecular analyses are needed in order to better characterize this species.
According to Howell-Rivero (1936b), snout length and first dorsal-fin spine length are smaller in juveniles than in adults in S. cubensis , but no ontogenetic differences in external measurements were found in S. albicaudus by us, supporting its differentiation from S. cubensis . Ledoux (1970) considered S. cubensis to be an extreme subspecies of the Mediterranean S. blainvillei based on overlapping of external measurements. This author overlooked external morphological characters that distinguish these two species, characters typically used in the identification of Squalus species worldwide (e.g. Bass et al., 1976; Last et al., 2007), such as body coloration, length of dorsal spines, and shape of dermal denticles. Many authors (e.g. Bigelow & Schroeder, 1957) have reported overlap in external measurements among specimens of S. cubensis , S. megalops and S. blainvillei as well as in other characters (e.g. dermal denticles, teeth, coloration), impeding easy identification. The same difficulty is noticed for S. albicaudus and S. cubensis , although external morphology and morphometrics have significant taxonomic value in distinguishing them. Muñoz-Chápuli & Ramos (1981) mentioned that S. cubensis differs in clasper morphology from S. megalops from the Eastern Atlantic and Indo-Pacific oceans by possessing a less curved claw (dorsal terminal 2 cartilage) and a massive spur (accessory terminal cartilage). These differences are not apparent among species from the SWAO, although clasper morphology helps recognize groups of species within the genus.
Our current results support for the first time that S. cubensis does not occur further south in the Southwestern Atlantic Ocean. More material from north of northeastern Brazil is urgently needed to understand the northern limits of S. albicaudus , however. Additional comparisons with S. cubensis from the Caribbean Sea are welcome, but the characters provided above (see Diagnosis) allow for the distinction of S. albicaudus and S. cubensis . Despite the lack of the original coloration in the holotype of S. cubensis , it is possible to note a large black blotch at the apex of both dorsal fins. Juvenile specimens from the Western Central Atlantic and Caribbean Sea have large black blotches on the first dorsal fin and only adults have it on both dorsal fins. Squalus albicaudus has the apex of the first dorsal fin darker, but not as a black blotch as it is in S. cubensis , and has the second dorsal fin with a whitish apex. In neonates and small juveniles of S. albicaudus , the black blotch is conspicuous on the first dorsal fin, located farther below on the fin web, while its apex is white, indicating an additional character for separating both species. We also noted that the pelvic fins are closer to the second dorsal fin in S. albicaudus but which changes ontogenetically, as previously pointed out by Sadowsky & Moreira (1981) for specimens Squalus from southeastern Brazil and S. megalops from Australia ( Garrick, 1960). Squalus cubensis has pelvic fins closer to the midline between the origins of the two dorsal fins during all stages of maturity (as observed by Howell-Rivero, 1936b and Bigelow & Schroeder, 1948), which is distinct from S. albicaudus (as noticed by Figueiredo, 1981). However, this character is observed in different species of the genus in which intraspecific variation may also occur, suggesting it to be taxonomically unreliable.
Squalus albicaudus can be further distinguished from S. megalops (data for comparisons below from Last et al., 2007) by: 39 (41–44) monospondylous vertebrae (vs. 37–40 in S. megalops ); 82 (81–89) precaudal vertebrae (vs. 78–84 in S. megalops ); 110 (110–116) total vertebrae (vs. 102–110 in S. megalops ) ( Table 12 View TABLE 12 ). Squalus albicaudus is separate from S. crassispinus by having more slender dorsal-fin spines (first dorsal-fin spine width at base 1.0%, 0.7%–1.1% TL vs. 1.3%, 1.2%–1.3% TL in S. crassispinus ; second dorsal-fin spine width at base 0.9%, 0.6%–0.9% TL vs. 1.5%, 1.3%–1.4% TL in S. crassispinus ). It can be distinguished from S. bucephalus by having a narrower head (width at mouth 11.1%, 10.9%–12.0% TL vs. 13.0%, 12.1%–13.5% TL in S. bucephalus ). Squalus albicaudus is distinct from S. bucephalus and S. notocaudatus by having a lower first dorsal fin (height 7.9%, 6.8%–8.0% TL vs. 8.5%, 8.1%–8.4% TL in S. bucephalus and 8.2%, 8.3%–9.4% TL in S. notocaudatus ). Squalus albicaudus is distinct from S. raoulensis by having smaller pectoral fins (pectoral-fin anterior margin length 13.4%, 11.8%–14.4% TL vs. 15.3%, 15.0–16.9% TL in S. raoulensis ). Squalus albicaudus differs from S. albifrons and S. altipinnis by its shorter first dorsal-fin spine (height 4.2%, 2.3%–4.1% TL vs. 4.8%, 4.4–5.4% TL in S. albifrons and 4.9%, 5.3% TL in S. altipinnis ).
Comparative material. Squalus cubensis (84 specimens): Western North Atlantic, Gulf of Mexico and Caribbean Sea (76 specimens): AMNH 12306, juvenile female, 445 mm TL, Cuba; AMNH 33453, juvenile female, 490 mm TL, Yucatán, Mexico; AMNH 33454, adult male, 535 mm TL, Louisiana, U.S.A.; AMNH 33457, juvenile male, 375 mm TL, Mexico; AMNH 33458, juvenile female, 334 mm TL, Mexico; AMNH 33459, neonate male, 278 mm TL, Mexico; CAS 60863, adult male, 510 mm TL, Puerto Rico; CAS 61162, juvenile male, 400 mm TL, Puerto Rico; CAS 230367, two juvenile males, 395–415 mm TL, South of Pensacola, Florida, U.S.A.; MCZ 1458-S (holotype of S. cubensis ), adult male, 531 mm TL, Havana, Cuba; MCZ 1459-S (paratype of S. cubensis ), neonate male, 210 mm TL, Havana, Cuba; MCZ 1460-S (paratype of S. cubensis ), neonate female, 297 mm TL, Havana, Cuba; MCZ 1461-S (paratype of S. cubensis ), adult female, 690 mm TL, Havana, Cuba; MCZ 1462-S (paratype of S. cubensis ), neonate male, 277 mm TL, Havana, Cuba; MCZ 37398, two juvenile females, 376–420 mm TL, Gulf of Mexico; MCZ 40138, adult male, 522 mm TL, Florida, U.S.A.; MCZ 40681, neonate female, 217 mm TL, Puerto Rico; UF 28449, juvenile female, 460 mm TL, Campeche Bank, Mexico; USNM 157843, neonate female, 205 mm TL, two neonate males, 210–215 mm TL, Alabama, U.S.A., 22º91’N, 79º45’W; USNM 157846, adult male, 495 mm TL, Cuba, 22º91'N, 79º26'W; USNM 157853, neonate female, 290 mm TL, Cuba, 22º91’N, 79º45’W; USNM 158589, juvenile female, 445 mm TL, Florida, U.S.A., 28º82’N, 85º75’W; USNM 160847, juvenile male, 410 mm TL, Gulf of Mexico; USNM 164247, adult female, 595 mm TL, Dominican Republic; USNM 187686, neonate male, 217 mm TL, Jamaica, 16º75’N, 81º45’W; USNM 187689, neonate female, 286 mm TL, Panama, 09º30'N, 80º36'W; USNM 187691, neonate male, 247 mm TL, Honduras, 16º63'N, 86º56'W; USNM 187700, juvenile female, 460 mm TL, Panama, 09º07'N, 81º42'W; USNM 187711, juvenile male, 405 mm TL, Nicaragua, 16º43'N, 81º58'W; USNM 187715, adult male, 460 mm TL, Nicaragua, 12º41’N, 82º25’W; USNM 187716, juvenile male, 403 mm TL, adult male, 510 mm TL, Nicaragua, 16º75’N, 81º45’W; USNM 187717, adult male, 510 mm TL, Nicaragua, 16º75’N, 81º45’W; USNM 187726, neonate male, 188 mm TL, Panama, 09º05'N, 81º37'W; USNM 187933, neonate female, 200 mm TL, neonate male, 192 mm TL; three juvenile males, 405–410 mm TL, Mississippi, U.S.A., 29º18’N, 88º10’W; USNM 187934, adult female, 532 mm TL, Cuba, 23º50'N, 79º45'W; USNM 187935, neonate male, 225 mm TL, neonate female, 306 mm TL, Cuba, 23º86'N, 79º38'W; USNM 187936, neonate female, 255 mm TL, Puerto Rico, 18º52’N, 66º83’W; USNM 187937, juvenile male, 425 mm TL, Cuba, 23º66’N, 79º30’W; USNM 188026, two neonate males, 210–260 mm TL, juvenile male, 422 mm TL, adult female, 400 mm TL, Cuba, 23º50'N, 79º45’W; USNM 188027, juvenile male, 400 mm TL, Cuba, 23º98'N, 79º28’W; USNM 188079, juvenile male, 415 mm TL, neonate female, 230 mm TL, Mississippi, U.S.A., 29º19’N, 88º19’W; USNM 188080, juvenile male, 400 mm TL, Mississippi, U.S.A., 29º21’N, 87º97’W; USNM 188081, two juvenile males, 410–430 mm TL, Mississippi, U.S.A., 29º18’N, 88º11’W; USNM 196544, adult male, 440 mm TL, Cuba, 22º98’N, 79º28’W; USNM 205325, adult male, 610 mm TL, adult female, 650 mm TL, Barbados; USNM 205587, two neonate males, 273–290 mm TL, juvenile male, 410 mm TL, Louisiana, U.S.A., 28º18’N, 90º13’W; USNM 206057, adult male, 540 mm TL, Saint Lucia Island, 13º68’N, 60º88’W; USNM 206058, adult female, 610 mm TL, Haiti, 20º72’N, 73º48’W; USNM 220519, neonate male, 215 mm TL, neonate female, 265 mm TL, Florida, U.S.A., 29º10’N, 88º43’W; USNM 220520, juvenile male, 383 mm TL, Texas, U.S.A., 26º52’N, 96º30’W; USNM 220521, neonate female, 275 mm TL, Texas, U.S.A., 26º50’N, 96º27’W; USNM 220522, juvenile male, 390 mm TL, juvenile male, 410 mm TL, Alabama, U.S.A., 29º17’N, 88º08’W; USNM 220584, neonate female, 235 mm TL, Colombia, 11º40’N, 73º78’W; USNM 220586, juvenile female, 395 mm TL, Texas, U.S.A., 27º80’N, 94º61’W; USNM 220587, juvenile male, 380 mm TL, Texas, U.S.A., 27º70’N, 94º43’W; USNM 220599, juvenile female, 400 mm TL, neonate male, 270 mm TL, two juvenile males, 355–450 mm TL, Texas, U.S.A., 27º43’N, 96º23’W; USNM 220600, neonate female, 267 mm TL, Mississippi, U.S.A., 28º98N, 88º80’W; USNM 220603, two juvenile males, 330–360 mm TL, juvenile female, 350 mm TL, Gulf of Maracaibo, Venezuela, 12º28’N, 72º51’W; USNM 220864, adult female, 460 mm TL, Louisiana, U.S.A., 27º87’N, 92º48’W. Squalus megalops : Southwestern Pacific Ocean (6 specimens): AMS I 16255-001 (holotype of S. megalops ), adult female, 565 mm TL, Port Jackson, New South Wales, Australia; MCZ 38619-S, four juvenile female, 370–535 mm TL, New South Wales, Australia; MCZ 38620-S, juvenile female, 520 mm TL, Kangaroo Island, St. Stevens Bay, Australia. Southeastern Atlantic Ocean (2 specimens): SU 31545, neonate male, 280 mm TL, Table Bay, Cape of Good Hope, South Africa; UF 42102, adult male, 570 mm TL, Marrocos.
Discussion
Our study incorporates a variety of morphological characters to separate S. acanthias , S. lobularis , S. quasimodo , S. bahiensis , and S. albicaudus from their congeners, especially external characters of the body including shape of dorsal, pectoral and caudal fins, as well as width and length of dermal denticles, and color pattern. The external morphology of species of Squalus was always thought to be problematic for distinguishing species due to overlap of various characters used to define them, which led authors to separate species into species-groups of similar external morphology (e.g. Bigelow & Schroeder, 1948; Garrick, 1960; Compagno et al., 2005). When the new species described in this study had overlapping external characters, coloration, morphometrics and vertebral counts were valuable for separating them in agreement with the findings of Last et al. (2007) for Australian species. First dorsal-fin height, first dorsal-fin spine length, pectoral-fin anterior margin length, dorsal-caudal space, and interdorsal space are external measurements that proved diagnostic at species level in our study. It is important to note, however, that continuous morphometric ranges without any discontinuity are often observed when species are compared. Squalus albifrons , S. altipinnis , S. chloroculus , and S. montalbani are examples of species described in Last et al. (2007) in which external morphometric characters used in their diagnoses completely overlapped with congeners. However, continuous morphometric ranges serve as additional morphological information for species separation if ranges are elevated and have a minimum overlap between species. External proportions, such as prenarial length and distance from nostrils to upper labial furrow, as proposed by Bass et al. (1976) and followed by Chen et al. (1979), Compagno (1984), and Marouani et al. (2012), are not useful for differentiating species regionally or in a broader context, but they allow to identify species as part of a group of species with similar external morphology as a first approximation, as noticed by Gomes et al. (2010).
General shape of dermal denticles and pectoral fins, length of snout, and color pattern of caudal fin by themselves were not distinctive for species of Squalus nor for groups of species within the genus. These characters overlap among species from different morphological groups and are still known, however rarely, to be variable in conspecific specimens when different stages of maturity are compared. Last et al. (2007) noticed that S. hemipinnis from Indonesian waters share characters of two different groups of species, the S. megalops and S. mitsukurii groups, indicating that morphological overlapping can potentially occur among species-groups as well.
Squalus lobularis View in CoL , S. bahiensis View in CoL , and S. albicaudus View in CoL share a concave pectoral-fin posterior margin, which should be exclusive to species of the S. megalops View in CoL group as proposed by Bigelow & Schroeder (1948, 1957). Squalus albicaudus View in CoL and S. acanthias View in CoL share unicuspid dermal denticles, a character of the S. megalops View in CoL group according to Bigelow & Schroeder (1948, 1957), even though the former species has lanceolate dermal denticles while the latter has bat-shaped denticles. Our findings disagree with Bigelow & Schroeder (1948, 1957) and Bass et al. (1976) who pointed out that S. acanthias View in CoL has tricuspid dermal denticles. However, S. acanthias View in CoL has a prenarial length equal or slightly greater than the inner nostril-labial furrow space, a character that should be restricted to species of the S. mitsukurii View in CoL group as suggested by Bass et al. (1976). Thus, we prefer not to classify the new species of Squalus View in CoL described here in any of the species-groups proposed. These new species can be identified by characters in combination but which are clearly diagnostic, although not autapomorphic. In this way we avoid compounding the current confusion regarding the identity and composition of species-groups of Squalus View in CoL .
Muñoz-Chápuli & Ramos (1989) provided complementary characters of neurocranial morphology to distinguish S. blainvillei and S. megalops from the Eastern Atlantic Ocean and Mediterranean Sea, as the former species has a single prominent cartilaginous process in the basal plate while the latter species has two. All species of Squalus examined herein have only one cartilaginous process, in agreement with the observations of Muñoz- Chápuli & Ramos (1989) and Marouani et al. (2012) for S. blainvillei . Muñoz-Chápuli & Ramos (1989) also defined cranial measurements that are useful for separating species, representing the first such attempt within Squaliformes , which was followed by Marouani et al. (2012) and the present study, with some modifications (see Material and Methods). Our results indicate some variation in these measurements between species of Squalus from the Southwestern Atlantic Ocean; for instance, width across nasal capsules, width across preorbital and postorbital processes, length of postorbital process, distance between orbital processes, distance across opisthotic processes and hyomandibular facets, and subethmoidean width. A larger number of samples are required to evaluate more accurately variation in cranial measurements and whether they may be useful as diagnostic characters for species of the genus.
Muñoz-Chápuli & Ramos (1989) and Marouani et al. (2012) also noticed differences in clasper morphology between S. acanthias , S. blainvillei , S. acutirostris , S. cubensis and S. megalops , in which, according to them, the dorsal terminal (claw) and accessory terminal 3 (spur) cartilages vary in length and shape in mature males. Squalus acanthias , S. lobularis and S. bahiensis have a very elongate and slender spur, and a claw that is large and markedly concave and hook-like at the tip, while S. albicaudus has a very short and heavy spur, and a claw that is short and almost straight at the tip, as is shown by Muñoz-Chápuli & Ramos (1989) for S. acanthias and S. blainvillei , and for S. acutirostris , S. cubensis and S. megalops , respectively. Similarity in these terminal cartilages, however, support that clasper morphology is not useful for separating species of Squalus but only into species-groups with similar external morphology. A broader range of species is required for analyzing comparatively whether clasper morphology is species-specific.
The new species of Squalus described in this study indicate that the diversity of the genus in the Southwestern Atlantic Ocean is greater than was previously thought, supporting the assumptions of Figueiredo (1981), Gomes et al. (1997), Gadig & Gomes (2003), and Rosa & Gadig (2014). There is a clear global tendency to increase the number of recognized species in the genus over the past decade or so, as seen for the Southwestern Pacific (e.g. Last et al., 2007), North Pacific (e.g. White & Iglésias, 2011), and Indian Oceans (e.g. Baranes, 2003), with recent systematic studies based on molecular analysis (e.g. Naylor et al., 2012) pointing out that some undescribed species may still occur in some regions. A worldwide investigation of the taxonomy of the family Squalidae based solely on morphological characters (Viana & Carvalho, in prep.) is being finalized in order to elucidate in detail the morphological and systematic complexity that still remains within Squalus and Cirrhigaleus .
TABLE 11. Meristic data fοr Squa ̸ us a ̸ bicaudus sp. nοv. va 1 ues fοr S. cubensis are a 1 sο prοvided fοr cοmparisοns.
| Squa'us a'bicaudus | Squa'us cubensis | ||
|---|---|---|---|
| Character | Paratypes Hο1οtype N Range | Hο1οtype Mοde N | Paratypes Nοn-type specimens Range Mοde N Range Mοde |
| precauda1 vertebrae | 82 6 81 - 89 | 83 85 3 | 87 - 89 - 11 83 - 92 84 |
| cauda1 vertebrae | 28 10 26 - 29 | 27 30 3 | 27 - 29 - 11 26 - 31 29 |
| tοta1 vertebrae | 110 6 110 - 116 | 111 115 3 | 114 - 117 117 11 111 - 118 114 |
| mοnοspοndy1οus vertebrae | 39 10 40 - 41 | 40 43 3 | 41 - 44 - 11 39 - 43 41 |
| dip1οspοndy1οus vertebrae | 71 6 69 - 75 | 71 72 3 | 70 - 76 - 11 70 - 75 71 |
| upper tοοth rοws (right) | 12 11 13 - 14 | 13 14 4 | 10 - 13 13 12 11 - 14 13 |
| upper tοοth rοws (1eft) | 12 10 13 - 14 | 14 14 4 | 11 - 13 13 11 12 - 14 13 |
| 1οwer tοοth rοws (right) | 11 11 10 - 13 | 11 11 4 | 10 - 13 - 12 11 - 14 12 |
| 1οwer tοοth rοws (1eft) | 11 10 11 - 13 | 12 12 4 | 10 - 12 10 12 11 - 14 13 |
| upper tοοth series | 3 11 2 - 3 | 2 2 4 | 2 - 2 2 12 2 - 2 2 |
| 1οwer tοοth series | 2 11 2 - 3 | 2 2 4 | 2 - 2 2 12 2 - 2 2 |
| prοpterygium radia1s | - 2 1 - 1 | 1 - - | - - 12 1 - 2 1 |
| mesοpterygium radia1s | - 2 9 - 9 | 9 - - | - - 12 8 - 10 9 |
| metapterygium radia1s | - 2 8 - 8 | 8 - - | - - 12 6 - 8 7 |
| tοta1 pectοra1 radia1s | - 2 18 - 18 | 18 - - | - - 12 16 - 20 18 |
| tοta1 pe1vic radia1s | - 5 12 - 13 | 12 - - | - - 9 12 - 15 13 |
TABLE 10. Externa 1 measurements expressed as percentage οf tοta 1 1 ength (% ΤL) fοr Squa ̸ us a ̸ bicaudus sp. nοv. va 1 ues fοr S. cubensis are a 1 sο prοvided fοr cοmparisοns. N ∶ number οf specimens; x ∶ mean; SD ∶ standard deviatiοn.
| N | Range | x | SD | N | Range | x | SD | N | Range | x | SD | N | Range | x | SD | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ΤL (mm) | 525.0 | 11 | 425.0 - 610.0 | 522.5 | 68.4 | 525.0 | 4 | 210.0 - 690.0 | 368.5 | 217.5 | 12 | 275.0 - 532.0 | 415.3 | 77.9 | 14 | 205.0 - 650.0 | 418.2 | 161.9 |
| PCL | 81.5 | 11 | 78.1 - 82.5 | 80.0 | 1.3 | 79.0 | 4 | 74.1 - 79.7 | 76.9 | 2.4 | 12 | 77.5 - 81.6 | 79.3 | 1.3 | 14 | 76.6 - 81.5 | 79.4 | 1.4 |
| PD2 PD1 | 62.9 25.1 | 11 11 | 57.8 - 64.4 27.1 - 39.9 | 61.0 29.7 | 2.2 3.5 | 61.9 30.5 | 4 4 | 58.9 - 63.8 28.9 - 31.0 | 60.4 30.0 | 2.2 0.9 | 12 12 | 60.2 - 64.6 30.3 - 43.4 | 62.3 33.2 | 1.3 3.5 | 14 14 | 58.9 - 66.3 29.4 - 34.4 | 62.1 31.1 | 2.3 1.4 |
| SvL | 30.5 | 11 | 42.5 - 55.9 | 46.4 | 3.5 | 48.6 | 4 | 45.2 - 50.7 | 47.9 | 2.4 | 12 | 28.9 - 50.8 | 46.5 | 5.7 | 14 | 41.9 - 52.5 | 46.7 | 3.1 |
| PP2 | 27.6 | 11 | 39.3 - 54.2 | 43.7 | 3.9 | 45.7 | 4 | 42.9 - 47.8 | 45.7 | 2.2 | 12 | 43.1 - 48.9 | 45.2 | 1.7 | 14 | 40.7 - 50.8 | 44.2 | 3.1 |
| PP1 | 22.9 | 11 | 20.2 - 24.0 | 21.6 | 1.0 | 22.9 | 4 | 21.7 - 24.1 | 22.8 | 1.3 | 12 | 21.4 - 44.7 | 25.0 | 6.4 | 14 | 19.5 - 23.4 | 22.4 | 1.0 |
| HDL | 21.8 | 11 | 18.7 - 24.7 | 21.7 | 1.4 | 23.6 | 4 | 22.8 - 24.6 | 23.7 | 0.8 | 12 | 20.4 - 25.9 | 23.0 | 1.4 | 14 | 19.9 - 24.0 | 22.9 | 1.0 |
| PG1 | 18.1 | 11 | 17.4 - 19.6 | 18.3 | 0.6 | 20.0 | 4 | 19.4 - 20.5 | 20.1 | 0.5 | 12 | 17.4 - 21.6 | 19.5 | 1.0 | 14 | 17.1 - 20.5 | 19.5 | 1.1 |
| PSP | 12.5 | 11 | 11.7 - 13.8 | 12.7 | 0.6 | 13.0 | 4 | 12.6 - 14.2 | 13.8 | 0.8 | 12 | 11.7 - 14.1 | 13.1 | 0.6 | 14 | 10.7 - 14.8 | 13.1 | 1.0 |
| PΟB PRN | 7.1 5.2 | 11 11 | 6.8 - 7.8 4.3 - 5.0 | 7.5 4.6 | 0.3 0.2 | 7.3 4.3 | 4 4 | 7.5 - 8.1 4.0 - 4.9 | 7.7 4.7 | 0.3 0.4 | 12 12 | 6.4 - 8.1 3.9 - 5.0 | 7.3 4.5 | 0.5 0.4 | 14 14 | 6.8 - 8.2 4.3 - 5.4 | 7.5 4.7 | 0.4 0.3 |
| PΟR | 9.8 | 11 | 9.2 - 11.2 | 10.0 | 0.5 | 9.4 | 4 | 9.6 - 11.4 | 10.8 | 0.8 | 12 | 8.8 - 11.1 | 10.1 | 0.7 | 14 | 8.7 - 11.8 | 10.4 | 0.8 |
| INLF | 5.2 | 11 | 4.6 - 5.5 | 5.1 | 0.3 | 5.2 | 4 | 5.1 - 6.0 | 5.7 | 0.4 | 12 | 4.7 - 5.8 | 5.3 | 0.3 | 14 | 3.8 - 5.9 | 5.2 | 0.5 |
| MΟW | 7.9 | 11 | 6.9 - 7.7 | 7.4 | 0.2 | 7.5 | 4 | 7.8 - 8.5 | 8.0 | 0.3 | 12 | 7.0 - 9.7 | 8.4 | 0.7 | 14 | 7.2 - 9.4 | 8.0 | 0.6 |
| ULA | 2.3 | 11 | 2.1 - 2.5 | 2.3 | 0.1 | 2.4 | 4 | 2.1 - 2.9 | 2.6 | 0.3 | 12 | 2.2 - 4.3 | 2.7 | 0.6 | 14 | 2.3 - 2.8 | 2.5 | 0.2 |
| INW | 4.3 | 11 | 3.6 - 4.3 | 4.0 | 0.2 | 3.6 | 4 | 2.7 - 4.4 | 3.6 | 0.7 | 12 | 3.9 - 4.5 | 4.2 | 0.2 | 14 | 3.6 - 4.8 | 4.3 | 0.3 |
| INΟ | 8.4 | 11 | 8.2 - 8.9 | 8.5 | 0.2 | 8.5 | 4 | 8.5 - 10.1 | 9.3 | 0.7 | 12 | 7.5 - 9.7 | 9.0 | 0.6 | 14 | 7.8 - 11.1 | 9.0 | 0.8 |
| EΥL | 4.0 | 11 | 4.2 - 5.3 | 4.8 | 0.4 | 4.2 | 4 | 3.7 - 4.9 | 4.3 | 0.6 | 12 | 3.9 - 5.1 | 4.6 | 0.4 | 14 | 3.2 - 6.0 | 4.4 | 0.7 |
| EΥH SPL | 2.6 1.5 | 11 11 | 2.2 - 2.8 1.3 - 2.0 | 2.4 1.5 | 0.2 0.2 | 1.8 1.6 | 4 4 | 1.3 - 2.9 1.2 - 1.8 | 2.1 1.5 | 0.6 0.2 | 12 12 | 1.6 - 2.9 1.3 - 2.0 | 2.3 1.7 | 0.4 0.3 | 14 14 | 1.0 - 2.7 1.0 - 2.1 | 2.0 1.6 | 0.4 0.3 |
| GS1 | 2.2 | 11 | 1.9 - 2.3 | 2.1 | 0.2 | 1.4 | 4 | 1.5 - 1.9 | 1.7 | 0.2 | 12 | 1.2 - 2.2 | 1.7 | 0.3 | 14 | 1.2 - 2.4 | 1.8 | 0.3 |
| GS5 | 2.4 | 11 | 2.0 - 2.8 | 2.4 | 0.3 | 2.3 | 4 | 1.9 - 2.5 | 2.2 | 0.3 | 12 | 1.7 - 2.5 | 2.1 | 0.2 | 14 | 1.9 - 2.7 | 2.3 | 0.3 |
| IDS | 25.9 | 11 | 22.8 - 27.5 | 25.0 | 1.2 | 23.8 | 4 | 22.2 - 26.1 | 23.6 | 1.7 | 12 | 22.4 - 28.4 | 25.1 | 1.8 | 14 | 20.0 - 27.7 | 24.1 | 2.4 |
| DCS | 11.7 | 11 | 10.7 - 14.1 | 11.6 | 1.0 | 11.5 | 4 | 9.8 - 11.6 | 10.9 | 0.7 | 12 | 9.7 - 12.3 | 11.2 | 0.9 | 14 | 10.3 - 12.0 | 11.0 | 0.5 |
| PPS | 19.0 | 11 | 15.4 - 25.4 | 18.8 | 2.6 | 19.0 | 4 | 17.9 - 23.6 | 20.6 | 2.6 | 12 | 14.6 - 22.9 | 19.2 | 2.7 | 14 | 14.9 - 22.7 | 17.7 | 2.5 |
| PCA | 29.0 | 11 | 25.4 - 29.4 | 27.3 | 1.0 | 26.7 | 4 | 22.6 - 27.6 | 25.0 | 2.2 | 12 | 24.8 - 30.6 | 27.1 | 1.9 | 14 | 26.2 - 29.2 | 27.7 | 0.9 |
| D1L D1A | 13.6 10.9 | 11 11 | 12.4 - 14.5 9.7 - 11.2 | 13.1 10.6 | 0.6 0.5 | 14.7 11.6 | 4 4 | 12.5 - 14.6 11.6 - 12.7 | 13.8 12.3 | 0.9 0.5 | 12 12 | 12.0 - 13.7 9.1 - 12.0 | 13.0 10.5 | 0.5 0.9 | 14 14 | 12.6 - 14.5 9.5 - 14.2 | 13.6 11.2 | 0.6 1.2 |
| D1B | 8.2 | 11 | 7.0 - 8.7 | 7.6 | 0.5 | 8.8 | 4 | 6.9 - 8.9 | 8.3 | 1.0 | 12 | 6.7 - 7.9 | 7.4 | 0.4 | 14 | 6.9 - 9.3 | 7.8 | 0.6 |
| D1H | 7.9 | 11 | 6.8 - 8.0 | 7.4 | 0.3 | 8.6 | 4 | 7.5 - 9.0 | 8.4 | 0.7 | 12 | 6.3 - 8.4 | 7.3 | 0.8 | 14 | 6.6 - 9.6 | 8.0 | 0.8 |
| D1I | 5.6 | 11 | 4.9 - 6.1 | 5.5 | 0.3 | 6.1 | 4 | 5.8 - 6.9 | 6.3 | 0.5 | 12 | 5.1 - 6.8 | 6.1 | 0.6 | 14 | 5.4 - 6.7 | 5.9 | 0.4 |
| D1P | 8.8 | 11 | 8.0 - 9.4 | 8.8 | 0.4 | 8.4 | 4 | 6.8 - 9.9 | 7.9 | 1.4 | 12 | 7.1 - 9.7 | 8.4 | 0.8 | 14 | 7.5 - 10.1 | 9.2 | 0.8 |
| D1ES | 4.2 | 11 | 2.3 - 4.1 | 3.5 | 0.6 | 4.3 | 4 | 2.1 - 4.7 | 3.2 | 1.1 | 12 | 2.5 - 4.7 | 3.6 | 0.8 | 14 | 2.1 - 4.9 | 3.5 | 0.9 |
| D1BS | 1.0 | 11 | 0.7 - 1.1 | 0.8 | 0.1 | 0.9 | 4 | 0.8 - 1.1 | 1.0 | 0.1 | 12 | 0.6 - 1.1 | 0.9 | 0.2 | 14 | 0.6 - 1.1 | 0.9 | 0.1 |
| D2L D2A | 10.9 9.2 | 11 11 | 10.4 - 12.6 8.8 - 10.8 | 11.4 9.7 | 0.7 0.6 | 13.1 12.3 | 4 4 | 11.6 - 12.4 11.2 - 11.6 | 12.1 11.4 | 0.3 0.2 | 12 12 | 10.6 - 12.8 9.4 - 11.0 | 11.6 10.2 | 0.6 0.5 | 14 14 | 11.0 - 12.7 9.1 - 11.8 | 12.0 10.5 | 0.5 0.8 |
| D2B | 6.3 | 11 | 5.7 - 7.2 | 6.5 | 0.5 | 7.6 | 4 | 6.6 - 6.9 | 6.7 | 0.1 | 12 | 5.5 - 7.9 | 6.6 | 0.7 | 14 | 5.9 - 7.7 | 6.7 | 0.6 |
| D2H | 5.4 | 11 | 4.9 - 6.2 | 5.7 | 0.4 | 6.9 | 4 | 5.9 - 7.5 | 6.7 | 0.7 | 12 | 4.7 - 6.9 | 5.8 | 0.8 | 14 | 5.3 - 7.7 | 6.2 | 0.7 |
| D2I | 5.0 | 11 | 4.1 - 5.2 | 4.9 | 0.3 | 5.6 | 4 | 5.5 - 6.0 | 5.8 | 0.3 | 12 | 4.7 - 6.1 | 5.3 | 0.4 | 14 | 4.8 - 6.4 | 5.5 | 0.5 |
| D2P | 4.9 | 11 | 4.5 - 6.1 | 5.2 | 0.5 | 5.1 | 4 | 2.8 - 5.5 | 4.4 | 1.2 | 12 | 4.3 - 5.8 | 5.0 | 0.4 | 14 | 3.5 - 6.0 | 5.0 | 0.7 |
| D2ES | 5.1 | 10 | 3.4 - 5.0 | 4.4 | 0.5 | 6.0 | 4 | 4.2 - 6.5 | 5.3 | 1.0 | 12 | 4.0 - 6.0 | 5.0 | 0.6 | 14 | 4.2 - 6.3 | 5.1 | 0.6 |
| D2BS | 0.9 | 11 | 0.6 - 0.9 | 0.8 | 0.1 | 1.0 | 4 | 1.0 - 1.2 | 1.1 | 0.1 | 12 | 0.7 - 1.1 | 0.9 | 0.1 | 14 | 0.8 - 1.1 | 1.0 | 0.1 |
| P1A | 13.4 | 11 | 11.8 - 14.4 | 13.6 | 0.7 | 15.2 | 4 | 13.3 - 15.8 | 14.2 | 1.2 | 12 | 13.2 - 14.7 | 13.9 | 0.5 | 14 | 11.3 - 15.1 | 13.6 | 1.2 |
| P1I P1B | 9.0 4.0 | 11 11 | 8.6 - 10.2 3.7 - 4.7 | 9.5 4.2 | 0.5 0.3 | 11.5 3.9 | 4 4 | 8.8 - 11.0 4.0 - 4.4 | 10.1 4.2 | 1.0 0.2 | 12 12 | 4.2 - 12.1 3.7 - 9.8 | 9.8 4.7 | 1.9 1.6 | 14 14 | 3.8 - 11.2 3.5 - 10.4 | 9.7 4.9 | 1.8 1.6 |
| P1P | 10.7 | 11 | 9.7 - 11.8 | 10.6 | 0.6 | 12.1 | 4 | 8.7 - 10.1 | 9.3 | 0.6 | 12 | 9.2 - 11.9 | 10.6 | 1.0 | 14 | 8.1 - 12.6 | 10.4 | 1.2 |
| P2L | 12.1 | 11 | 9.9 - 12.2 | 10.9 | 0.7 | 13.0 | 4 | 9.4 - 11.5 | 10.7 | 1.0 | 12 | 10.0 - 12.3 | 11.1 | 0.8 | 14 | 10.0 - 12.9 | 11.2 | 0.9 |
| P2I | 6.3 | 11 | 4.2 - 6.3 | 5.2 | 0.7 | 6.9 | 4 | 5.6 - 6.7 | 6.1 | 0.6 | 12 | 4.3 - 8.1 | 5.7 | 0.9 | 14 | 4.3 - 7.0 | 5.7 | 0.7 |
| CDM | 19.8 | 11 | 19.3 - 21.2 | 20.1 | 0.8 | 21.6 | 4 | 20.8 - 23.8 | 22.0 | 1.4 | 12 | 20.1 - 22.9 | 21.3 | 0.9 | 14 | 19.5 - 22.9 | 21.5 | 1.0 |
| CPv | 11.4 | 11 | 10.5 - 11.9 | 11.2 | 0.4 | 12.3 | 4 | 10.5 - 13.5 | 12.0 | 1.3 | 12 | 10.5 - 12.0 | 11.1 | 0.5 | 14 | 5.2 - 13.9 | 11.3 | 2.0 |
| CFW | 6.5 | 11 | 6.2 - 7.2 | 6.7 | 0.2 | 6.8 | 4 | 6.6 - 7.0 | 6.8 | 0.2 | 12 | 6.3 - 7.4 | 6.8 | 0.3 | 14 | 5.5 - 7.7 | 6.7 | 0.6 |
| HANW HAMW | 7.1 11.1 | 11 11 | 6.5 - 7.4 10.9 - 12.0 | 7.0 11.3 | 0.3 0.4 | 7.0 10.8 | 4 4 | 6.6 - 9.0 11.2 - 12.3 | 7.6 11.7 | 1.0 0.5 | 12 12 | 6.6 - 7.6 9.8 - 13.0 | 7.1 11.7 | 0.3 0.9 | 14 14 | 6.5 - 10.6 10.1 - 13.8 | 7.3 11.7 | 1.0 0.9 |
| HDW | 11.9 | 11 | 10.9 - 12.9 | 12.1 | 0.5 | 12.5 | 4 | 10.8 - 13.5 | 12.2 | 1.2 | 12 | 10.8 - 13.5 | 12.3 | 0.8 | 14 | 10.2 - 15.0 | 12.4 | 1.3 |
| ΤRW | 9.9 | 11 | 8.0 - 11.9 | 10.5 | 1.1 | 9.8 | 4 | 6.3 - 11.0 | 9.1 | 2.0 | 12 | 7.8 - 10.6 | 9.1 | 0.9 | 14 | 6.7 - 13.9 | 10.0 | 2.0 |
| ABW | 8.2 | 11 | 8.0 - 10.9 | 9.5 | 0.9 | 6.3 | 4 | 5.5 - 8.8 | 7.5 | 1.4 | 12 | 6.2 - 9.5 | 7.5 | 0.9 | 14 | 6.3 - 11.8 | 8.3 | 1.4 |
| HDH | 10.3 | 11 | 9.3 - 11.7 | 10.6 | 0.8 | 8.8 | 4 | 9.3 - 11.1 | 9.9 | 0.8 | 12 | 8.4 - 12.5 | 10.0 | 1.2 | 14 | 8.8 - 11.6 | 10.4 | 0.7 |
| ΤRH | 10.0 | 11 | 10.5 - 12.7 | 11.8 | 0.7 | 8.0 | 4 | 8.0 - 12.7 | 10.6 | 2.3 | 12 | 7.9 - 13.5 | 10.1 | 1.4 | 14 | 8.8 - 12.6 | 10.5 | 0.9 |
| ABH | 10.3 | 11 | 9.6 - 13.2 | 11.7 | 1.1 | 7.9 | 4 | 7.3 - 10.5 | 9.4 | 1.5 | 12 | 7.3 - 11.1 | 8.8 | 1.2 | 14 | 7.6 - 12.1 | 9.8 | 1.4 |
| CLΟ CLI | 4.1 7.1 | 4 4 | 4.0 - 4.8 6.9 - 7.7 | 4.4 7.4 | 0.4 0.4 | 4.4 8.0 | 2 2 | 1.2 - 1.5 3.3 - 3.8 | 1.4 3.5 | 0.2 0.3 | 8 8 | 1.3 - 4.8 2.7 - 7.9 | 3.6 6.2 | 1.3 1.8 | 7 7 | 1.3 - 5.9 1.7 - 7.6 | 3.4 5.1 | 1.8 2.5 |
TABLE 12. Range of vertebral counts of species of Squalus taken from Last et al. (2007) for comparisons.
| Monospondylous vertebrae Precaudal vertebrae | Caudal vertebrae | Total vertebrae | |
|---|---|---|---|
| S. megalops | 37–40 78–84 | – | 102–110 |
| S. crassispinus | 39–42 82–86 | 24–27 | 107–111 |
| S. bucephalus | 45 86–89 | 27–30 | 113–118 |
| S. raoulensis | 41–43 84–85 | 27–28 | 112–113 |
| S. albifrons | 44–46 89–93 | 26–31 | 116–122 |
| S. altipinnis | 42–44 88–92 | 26–28 | 114–120 |
| S. notocaudatus | 47–49 94–97 | 29–30 | 123–127 |
| S. hemipinnis | 35–38 72–76 | 22–26 | 96–100 |
| S. montalbani | 41–47 79–85 | – | 105–114 |
| S. chloroculus | 43–46 84–86 | 27–30 | 111–115 |
| S. edmundsi | 43–44 86–91 | 24–30 | 113–120 |
| S. grahami | 38–42 80–87 | 26–32 | 108–116 |
| S. nasutus | 36–39 78–83 | 23–28 | 103–109 |
| S. griffini | 45–47 86–91 | 26–30 | 113–121 |
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
ParvPhylum |
Chondrichthyes |
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |
Squalus albicaudus
| De, Sarah T., De, Marcelo R. & Gomes, Ulisses L. 2016 |
Squalus
| Gomes 2010: 44 |
Squalus
| Gadig 2001: 29 |
Squalus
| Soto 2004: 74 |
| Soto 2001: 95 |
Squalus
| Gomes 1997: 95 |
Squalus
| Gomes 1997: 98 |
Squalus
| Cadenat 1981: 51 |
| Figueiredo 1981: 17 |