Sechelleptus arborivagus, Vandenspiegel & Henrard & Mathys, 2021
|
publication ID |
https://doi.org/ 10.5852/ejt.2021.755.1395 |
|
publication LSID |
lsid:zoobank.org:pub:95C519A7-D485-4762-A876-C069EA4A3231 |
|
DOI |
https://doi.org/10.5281/zenodo.4966005 |
|
persistent identifier |
https://treatment.plazi.org/id/0F7B3368-17F8-4CAD-8B11-C34B564FE8DE |
|
taxon LSID |
lsid:zoobank.org:act:0F7B3368-17F8-4CAD-8B11-C34B564FE8DE |
|
treatment provided by |
Felipe |
|
scientific name |
Sechelleptus arborivagus |
| status |
sp. nov. |
Sechelleptus arborivagus View in CoL sp. nov.
urn:lsid:zoobank.org:act:0F7B3368-17F8-4CAD-8B11-C34B564FE8DE
Figs 3–8 View Fig View Fig View Fig View Fig View Fig View Fig
Diagnosis
A medium-sized arboreal millipede with relatively long legs, particularly similar to S. variabilis by sharing the structure of the male first leg and rather simple gonopods with the metaplica widened and a little higher than proplica, the latter without lateral cone. The two species differ by the gonotelopodite being apically divided in two branches in S. arborivagus sp. nov. and simple in S. variabilis .
Etymology
Referring to the ecology of the species, which has always been observed climbing trees.
Material examined
Holotype
FRANCE – Department of Mayotte (Comoros archipelago) • ♂; Mt. Tchaourembo ; 12°52′14″ S, 045°08′44″ E; 540–550 m a.s.l.; 25 Nov. 2019; D. VandenSpiegel and A. Mathys leg.; on tree; by hand; GenBank accession numbers: MW168813 View Materials (COI), MW148622 View Materials (16S rRNA); BE_RMCA_MYR. Dip.22874 . GoogleMaps
Paratypes
FRANCE – Department of Mayotte (Comoros archipelago) • 1 ♂, 1 ♀; same collection data as for holotype; GenBank accession numbers: MW168814 View Materials (COI), MW148623 View Materials (16S rRNA); BE_RMCA_ MYR.Dip.22875 GoogleMaps • 9 ♀♀; same collection data as for holotype; GenBank accession numbers: MW168815 View Materials (COI), MW148624 View Materials (16S rRNA); BE_RMCA_MYR.Dip.22876 GoogleMaps .
Additional material
FRANCE – Department of Mayotte (Comoros archipelago) • 1 ♀; Mt. Benara ; 12°52′ S, 045°11′ E; 23 Jan. 1999; R. Jocqué and G. De Smet leg.; forest; by hand; GenBank accession numbers: MW148621 View Materials (16S rRNA); BE_RMCA_MYR.Dip.17917 GoogleMaps .
Description
Holotype
With 57 body rings (plus telson, no apodous rings); ca 100 mm long, 7 mm wide.
LIVE COLORATION ( Fig. 3 View Fig ). Head, collum, antennae, telson, anal valves and legs uniformly light brownish to dark brownish. Metazonae light brown to red-brown. Posterior margin of metazonites dark brown.
HEAD. Smooth. Each eye patch with circa 60 ommatidia arranged in seven horizontal rows ( Fig. 4A View Fig ), Labrum with three smoothly rounded teeth and a single row of 21 short labral setae ( Fig. 4G View Fig ). Clypeus with four supra-labral setae, two on each side ( Fig. 4G View Fig ). Antennae moderately long ( Fig. 3 View Fig ), protruding back to ring 2. Relative length of antennomeres: 1>2>3=4=5>6. Terminal antennomere (disc) with four large sensory cones located together inside a membranous area. Each of antennomeres 5 and 6 apicolaterally with a field of narrow and long sensilla basiconica ( Fig. 4B View Fig ). Gnathochilarium, usual for spirostreptideans ( Fig. 4D View Fig ). Prementum (pm) smooth and straight, not depressed. Mentum (me) smooth. Lamellae linguales each with two strong apical setae, one equally strong seta behind these, plus, basally, an oblique line of four setae. Stipites with a basal longitudinal field of setae, lateral margin in distal half with a row of setae; one isolated, subapical, stout seta or sensillum; cardo small, kidney-shaped. Mandibles ( Fig. 4E, F View Fig ) with stipes devoid of differentiation. Odontomere (od) long, moveable. Sectile edge (se) of psectromere (ps) with four lobes; eight pectinate lamellae (pl). One wide molar furrow (mf).
COLLUM. Smooth, ventrally with six longitudinal furrows, anteroventral angle 80–90°.
BODY RINGS. Prozonae smooth. Metazonae with longitudinal striae ventrally from ca ⅔ ring length below ozopore. Ozopores located on metazonae, starting with ring 6, located close to, but not touching the suture between pro- and metazonae. Limbus simple ( Fig. 4H View Fig ). Defensive glands well-developed ( Fig. 4I View Fig ).
TELSON. Preanal ring with a shallow submarginal depression. Anal valves smooth, without submarginal depression. Hypoproct, small, widely triangular.
LEGS. Length 0.45–0.5 × body diameter, postfemoral and tibial pads ( Fig. 4J View Fig ) from third male leg-pair until beyond midbody, pads decreasing in size posteriorly; claw large, curved ( Fig. 4K View Fig ). First pair of male legs with a well-developed prefemoral process ending in an inward curved tip ( Fig. 5A View Fig ). Coxosternum with a laterobasal field of four strong setae on anterior side.
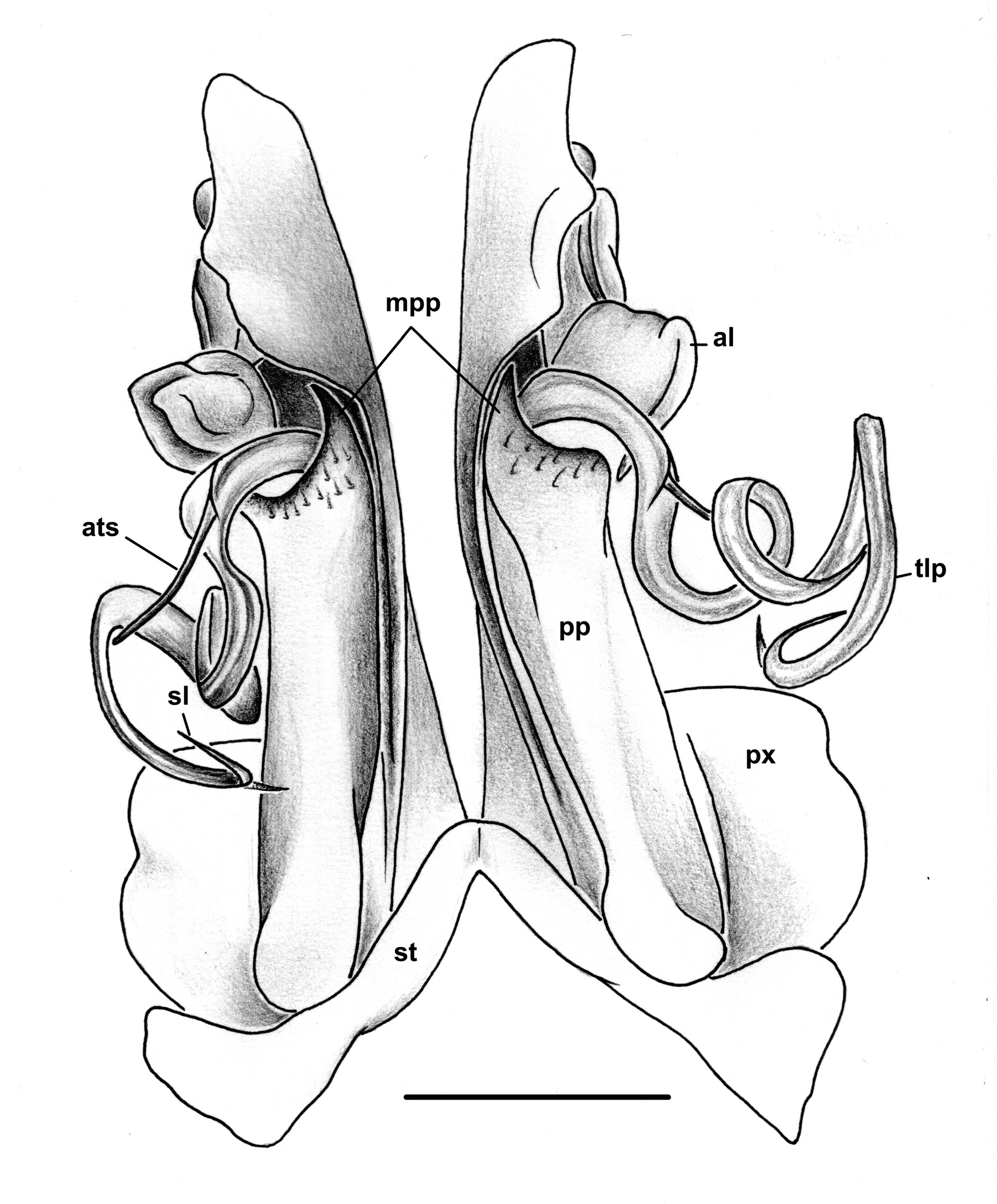
GONOPODS ( Figs 5B–E View Fig , 6 View Fig ). Sternum (st) triangular, not reaching as far distad as paracoxite (px). Metaplica (mp) higher than proplica, rounded apically ( Fig. 5C View Fig ; mp). Proplica with straight sides, in apical part with scattered short setae, ending apically in a more or less spiniform mesapical projection ( Fig. 5C View Fig ; mpp) and a well-developed, apicolateral, lamellose lobe ( Fig. 6C View Fig ; al); telopodite ( Fig. 5B, E View Fig ; tlp) long and slender, without a distinct demarcation between femoral and postfemoral parts, femorite with a small and pointed antetorsal process ( Fig. 5E View Fig ; ats), postfemorite spiralled, ribbon-shaped, broad and long, with a divided tip, the longer branch carrying the terminal opening of the solenomere ( Fig. 5B View Fig ; sl).
Paratypes
Male similar to holotype.
Female coloration as in male, but generally larger in size than male (up to 120 mm long 9 mm wide (58–61 body rings plus telson, no apodous rings). Vulvae located in membranous pouches attached to coxae 2 and 3 and to the inner lateral margin of ring 1, simple, consisting of two simple, subequallysized, moderately sclerotized valves, the aboral valve with an apical cluster of setae; ridge between valves covered with a lateral longitudinal operculum ( Fig. 7 View Fig ).
Distribution
The species seems endemic to Mayotte ( Fig. 8 View Fig ).
Affinities
On the basis of the gonopod structure having the telopodite with a spine arising well distad of the knee, a ribbon-shaped distal part, and a small free solenomerite arising just near the apex, the new species is manifestly a new member of the large genus Sechelleptus . Following the key published by Jeekel in 1999, arborivagus keys out close to sulcicollis and macilentus . Indeed the three species have a rather simple gonocoxite with a distally widened metaplica without a strong lateral cone but the new species do not show the small lateral uncus present on the metaplica of sulcicolis and possess a more or less spiniform mesapical projection on the proplica which is not present in sulcicolis neither in macilentus . By the overall shape of the male first leg and gonocoxite, the new species seems to be especially close to S. variabilis , also from the Comoros, but it differs strikingly by the structure of the gonotelopodite (in S. variabilis the gonotelopodite has a simple and pointed tip carrying the terminal opening of the seminal groove whereas in the new species the gonotelopodite has a divided tip, the longer branch carrying the terminal opening of the seminal groove) as well as by the larger body size and the longer and curved claws ( Fig. 4K View Fig vs Fig. 2C View Fig ). Other important differences concern the defensives glands, large in S. arborivagus sp. nov. ( Fig. 4I View Fig ) (vs inconspicuous in S. variabilis ( Fig. 2A View Fig )), and the size of eyes: in the new specie the eyes are larger and include 60 ± 5 ommatidia (n = 10) arranged in 12 rows; whereas in S. variabilis the eyes, smaller, include 34 ± 3 (n = 10) ommatidia arranged in 9 rows.
Natural history
Most of the specimens belonging to the new species were collected on Mt Tchaourembo (see Fig. 8 View Fig ) in a forest fragment at 500–550 m a.s.l. All specimens were seen in trees and never in pairs, the males being rare (sex ratio> 1/6). The species possesses enlarged ommatidia, relatively long legs with strongly curved tarsal claws, as well as a tendency for specimens to secrete extremely copiously from their defensive glands when irritated. Such modifications are considered by several authors as an adaptation to tree climbing and to arboreal life ( Enghoff & Enghoff 1976; Hoffman & Howell 1983; VandenSpiegel 2001).
Discussion
Millipede systematics is mainly based on male gonopods because they use to be species-specific ( Bond et al. 2003). However, studies based on DNA have demonstrated that molecular divergence in different millipede groups may not reflect divergence in morphology-based identifications and may hide considerable variation ( Bond & Sierwald 2002; Bond et al. 2003; Adams et al. 2009; Mwabvu et al. 2013, 2015; Tinago et al. 2017). Although our relatively small taxon sampling, the phylogenetic analysis strongly recovers Sechelleptus as monophyletic and discriminates at least two or three different groups. Furthermore, the mean inter-specific distance values (14.9% for COI and 5.1% for 16S) were remarkably similar to previous studies that reported the presence of high genetic divergence among population of different spirostreptid species ( Mwabvu et al. 2013, 2015), suggesting the existence of more than one species in those taxa. It is argued that high level of divergence between identified spirostreptid species may indicate that changes in genital morphology occur rather slowly relative to the high rate of substitution in mitochondrial sequences (especially for COI), and may underestimate species diversity. This also appears to be the case among the different forms of Mayottan Sechelleptus , which also share strongly similar gonopods. At the first glance, the new species of Sechelleptus seems to be a giant form of S. variabilis . However, although only subtle morphological differences are observed within the gonopods, the comparatively large body size and the behavior of S. arborivagus sp. nov. are remarkable. These observations finally corroborate our molecular analyses that clearly show sufficient genetic difference between the different Sechelleptus species collected on Mayotte (22.6% for COI and 6.6% for 16S between S. arborivagus sp. nov. and S. variabilis ).
The genetic analyses also suggest the presence of another different species, i.e., DU1, although its phylogenetic position remains unresolved. This unique specimen found at Mont Combani is a sub-adult female that could not allow a formal identification, but, judging from its general appearance, appears to be an intermediate from between the two Sechelleptus species collected on Mayotte. The genetic divergences, along with adaptations to arboreal life observed in the novel species, may indicate an “adaptive micro-radiation” on Mayotte Island or even the Comoros. However, the inclusion of more specimens, including adult males, in phylogenetic analyses is needed to test this hypothesis and evaluate the status of that putative new species.
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
SubOrder |
Spirostreptidea |
|
Family |
|
|
Genus |