Phellopsis obcordata (Kirby)
|
publication ID |
https://doi.org/ 10.5281/zenodo.180605 |
|
DOI |
https://doi.org/10.5281/zenodo.6235454 |
|
persistent identifier |
https://treatment.plazi.org/id/753687D8-FFB6-FFA1-1DC1-7AA1FF78FEEF |
|
treatment provided by |
Plazi |
|
scientific name |
Phellopsis obcordata (Kirby) |
| status |
|
( Figs. 1A View FIGURE 1. A , 2 View FIGURE 2 A, 2C, 8A)
Bolitophagus obcordatus Kirby, 1837: 236 .
Boletophagus obcordatus LeConte, 1853: 235 . LeConte, 1862: 216. LeConte and Horn, 1883: 365. Casey, 1907b: 470. Ś lipiński and Lawrence, 1999: 23. (lapsus calami)
Nosoderma obcordatum: Heyden, 1885: 307 . LeConte, 1853: 235.
Phellopsis obcordata: Horn, 1870: 273 . Hubbard and Schwarz, 1878: 640. Henshaw, 1881: 255 [in part]. Lewis, 1887: 219. Champion, 1894: 114. Hamilton, 1895: 341. Leng, 1920: 223. Leonard, 1928: 401. Böving and Craighead, 1931: pl. 52. Chagnon, 1935: 278. Gebien, 1936: 668. Brimley, 1938: 190. Triplehorn, 1952: 1 –3. Peterson, 1960: 180, fig. C48. Dillon and Dillon, 1961: 464, pl. xlvi. Chagnon and Robert, 1962: 330. Boddy, 1965: 78. Pielou, 1966: 1235. Pielou and Verma, 1968: 1184. Keleinikova and Mamaev, 1971: 125. Arnett, 1983: 17. Papp, 1984: 162. Lawrence, 1991: 518–519. Campbell, 1991: 252 [in part]. Steiner, 1992: 25 –30. Lawrence, 1994: 341. Downie and Arnett, 1996: 1080. Ś lipiński and Lawrence, 1999: 21. Steiner, 1999: 138 –139. Ivie, 2002: 458–460[in part]. Triplehorn and Johnson, 2005: 435.
Diagnosis: Distinguished from the other North American species, P. porcata , by the lack of intertuberculate setae on the hypomeron ( Fig. 2 View FIGURE 2 C). Other useful characters include the presence of large distinct round punctures on the elytra, 10–13 between the apical edge of the scutellary striole and subapical nodule; the ridge in the 3rd elytral interval very weak or absent medially and more strongly arcuate around the scutellum; the strongly bisinuate lateral margin of the pronotum; the smooth basal connecting ridge between the 1st and 3rd elytral interval which lacks a depression; and the lateral subapical nodule of the declivity strongly directed away from the plane of the body. This species can be confused with P. amurensis , but has the tuberculation on the apical half of the pronotum stronger, and the lateral elytral margin smooth rather than serrate.
Description (male): Length 11–16 mm. Reddish brown to dark brown; dorsal setose vestiture sporadic; most elytral punctures large and clearly visible; vestiture consisting of short, slightly thickened setae, lacking significant intertubercle setation on prothoracic and elytral surfaces. Head on dorsal surface with weak indistinct tubercles between frons; outer margin of suprantennal ridges concave; lateral margin of epistoma anterior to suprantennal ridge short (0.12–0.17 mm); ventral surface of head with distinct and regular tubercles; gula wide; subgenal ridge rounded, with slight depression medially; subgenal ridge longer, extending below eye; eye set below genal surface. Post-occipital suture deep and narrowly divided. Last antennomere with micro-setose patch of sensilla oval; preapical patches oblong. Ligula shallowly emarginate.
Pronotum evenly tuberculate; paired elevations on apical margin of pronotum broadly and weakly divided at midline; lateral margin of pronotum strongly bisinuate; hypomeron without intertuberculate setae, cuticular surface between tubercles with smooth microsculpture.
Dorsal elytral surfaces with small, often obscure tubercles; humerus slightly flattened; scutellum oval, shallowly set below elytral ridges; scutellary striole distinct; 10–13 large, rounded elytral punctures along midline between scutellary striole and large nodule at start of apical declivity; median subapical elytral nodule usually noticeably larger than lateral nodule; lateral nodule of apical declivity usually strongly projected away from body plane at approximately 45° angle; paired nodules considerably larger than single nodule near apex; ridges in 3rd and 5th elytral intervals, overlapping at most only at base and apex but not medially; nodule in 3rd interval never connected to ridge; area around elytral suture weakly elevated and flattened, never with large tubercles. Metasternum with small, relatively uniform tubercles; ventrite tuberculation reduced medially, uniformly spaced laterally. Tarsal setation more dense on ventral surface, setae relatively uniform in thickness.
Aedeagus ( Fig. 8 View FIGURE 8 A), relatively short and broad; apex of parameres almost straight; apical margin of basal stop with moderate concave depression medially, and lightly setose.
FEMALE: Same as male except lacking setose pit on submentum.
LARVA: (modified from Peterson 1951 and Lawrence 1991). Body elongate, 15–25 mm when extended, subcylindrical, white or creamy in color; head, pronotum, asperities, and legs lightly sclerotized; tarsal claws, urogomphi, and mouthparts heavily sclerotized.
Head subquadrate, lateral margins rounded, dorsoventrally flattened, prognathus, with sparse patches of short setae, 5 stemmata on each side, frontal arms lyriform. Antennae 3-segmented, anterolaterally inserted. Mandibles large, prognathus, apical tip bidentate. Maxilla thick and fleshy, with apical setae; cardo bifid.
Prothorax enlarged, partly enclosing head when retracted. Meso-and metathorax with arcuate rows of asperities, followed by sparse patches of asperities; spiracles annular-biforous.
Abdominal segments subequal in size, segments 1–6 with dorsal rows and patches of asperities, 9 with sclerotized granulate basal spots and well-developed recurved urogomphi; ventral surface of 2–8 with progressively strong patches of asperities, 8 with dense patch. Legs short, 5-segmented; coxae large and transverse, short setae concentrated on apical surfaces, claw elongate hook.
Variation: The dorsal elytral sculpture is less variable than in P. porcata . Southern Appalachian specimens (North Carolina, Tennessee, West Virginia, and Georgia) frequently have the humeri more flattened and pointed apically.
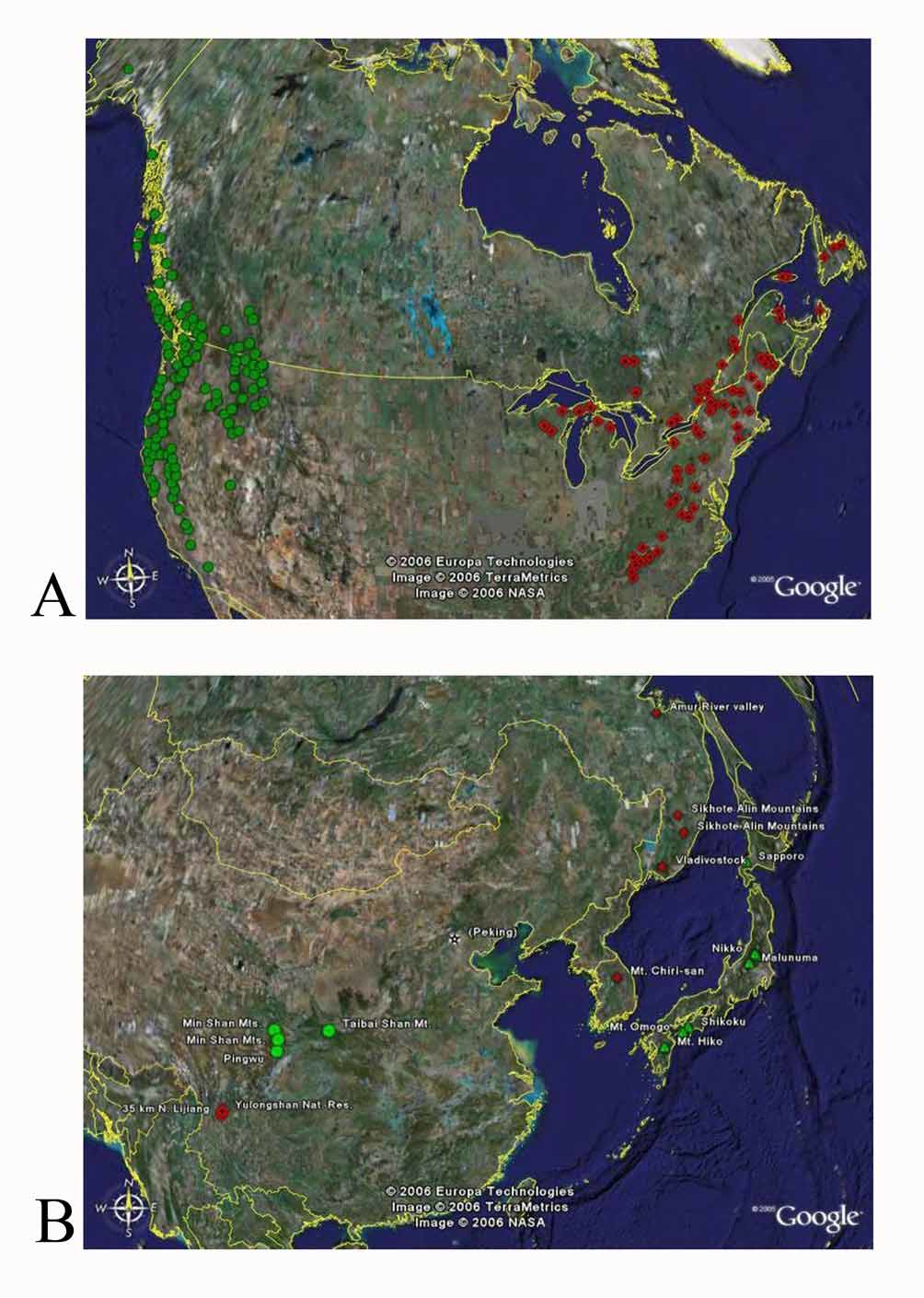
Distribution ( Fig. 1A View FIGURE 1. A ): Restricted to boreal forests of eastern portions of North America, widespread but uncommon from Newfoundland south to Northern Georgia and west to northern Wisconsin and the Upper Peninsula of Michigan. In more southern locales the species appears to be confined to higher elevations of the Appalachian Mountains.
Recorded distribution: A summary of the distribution from the 752 specimens examined is presented here as COUNTRY: PROVINCE or STATE: county (when available). For complete label data see Foley (2006). CANADA: NEW BRUNSWICK, NEWFOUNDLAND, NOVA SCOTIA, ONTARIO, QUEBEC. UNITED STATES: CONNECTICUT: Litchfield. GEORGIA: Rabun. MASSACHUSETTS: Worcester. MARYLAND: Garrett. MAINE: Cumberland, Hancock, Kennebec, Knox, Lincoln, Penobscot, Piscataquis, Washington. MICHIGAN: Cheboygan, Emmet, Marquette, Schoolcraft. NORTH CAROLINA: Avery, Buncombe, Burke, Haywood, Macon, Watauga. NEW HAMPSHIRE: Coos, Grafton, Rock, Carr. NEW JERSEY: Hudson ( Steiner 1992) NEW YORK: Erie, Essex, Franklin, Herkimer, St. Lawrence, Tompkins, Ulster, Wayne. PENNSYLVANIA: Dauphin, Forest, Huntington, Monroe, Westmoreland. TENNESSEE: Blount, Carter, Sevier, Tusculum. VIRGINIA: Albemarle, Giles, Highland, Lee, Madison, Page, Washington. VER- MONT: Addison, Bennington, Lamoille. WISCONSIN: Florence, Forest. WEST VIRGINIA: Greenbrier, Pendleton, Pocahontas, Preston.
Types: LECTOTYPE, here designated: Specimen of undetermined sex in the BMNH. Circle label with “N. Scotia ” on underside; “5969, B” on front/ Round red-ringed “ Type ” label/ Bolitoph obcordatus ; N. Scotia 5969; Rev. W. Kirby/ white card “ Lectotype ” underlined in red, Bolitophagus obcordatus ; Kirby 1873; designated by M.A. Ivie 2005.
Notes: The name P. obcordata has recently been applied to an assumed trans-North American species ( Campbell 1991, Ivie 2002), but is here restricted to the species occurring in Eastern North America. The historical discussion of the North American species names has been tumultuous and has included very little diagnostic support. A summary of this history is provided in the taxonomic history of the genus above. The difficulty in separating the two North American species was frequently acknowledged by ambiguous characterizations of the relationship ( LeConte 1853, Horn 1870, LeConte and Horn 1883), or stated as very clearly defined ( Casey 1907a). We have found that 100% of specimens can be assigned to one of the 2 North American species recognized here using the characters in the key and diagnosis. These characters are correlated with a geographic division across the North American Great Plains. This division is well supported based on the glaciations and subsequent drying out of the North American Great Plains ( Kavanaugh 1988, Marek and Kavanaugh 2005). Hopefully this taxonomic confusion has now been resolved.
Roughly 40% of specimens examined were collected pre-1940, and represented a more geographically broad distribution than the post-1940 specimens. This may be an indication of a negative correlation between human disturbance in Eastern North America and habitat availability. The post-World War II distribution of this species appears to be restricted to highly fragmented old growth remnants of a historically widespread forested habitat and has drawn some interest as a biological indicator of old growth forest habitats ( Steiner 1992).
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |
Phellopsis obcordata (Kirby)
| Foley, Ian A. & Ivie, Michael A. 2008 |
Nosoderma obcordatum:
| Heyden 1885: 307 |
| LeConte 1853: 235 |
Phellopsis obcordata:
| Triplehorn 2005: 435 |
| Steiner 1999: 138 |
| Downie 1996: 1080 |
| Lawrence 1994: 341 |
| Steiner 1992: 25 |
| Campbell 1991: 252 |
| Papp 1984: 162 |
| Arnett 1983: 17 |
| Keleinikova 1971: 125 |
| Pielou 1968: 1184 |
| Pielou 1966: 1235 |
| Boddy 1965: 78 |
| Chagnon 1962: 330 |
| Dillon 1961: 464 |
| Triplehorn 1952: 1 |
| Brimley 1938: 190 |
| Gebien 1936: 668 |
| Chagnon 1935: 278 |
| Leonard 1928: 401 |
| Leng 1920: 223 |
| Hamilton 1895: 341 |
| Champion 1894: 114 |
| Lewis 1887: 219 |
| Henshaw 1881: 255 |
| Hubbard 1878: 640 |
| Horn 1870: 273 |
Boletophagus obcordatus
| Casey 1907: 470 |
| LeConte 1883: 365 |
| LeConte 1862: 216 |
| LeConte 1853: 235 |
Bolitophagus obcordatus
| Kirby 1837: 236 |