Phareicranaus tizana, Colmenares, Pío A. & Tourinho, Ana Lúcia, 2014
|
publication ID |
https://doi.org/ 10.11646/zootaxa.3768.1.4 |
|
publication LSID |
lsid:zoobank.org:pub:702D1957-4C7A-4CC8-BE71-7EF45FCDE609 |
|
DOI |
https://doi.org/10.5281/zenodo.6126967 |
|
persistent identifier |
https://treatment.plazi.org/id/F74B87B2-FFBE-FF82-B5CA-FE2322E54BC2 |
|
treatment provided by |
Plazi |
|
scientific name |
Phareicranaus tizana |
| status |
sp. nov. |
Phareicranaus tizana View in CoL sp. nov.
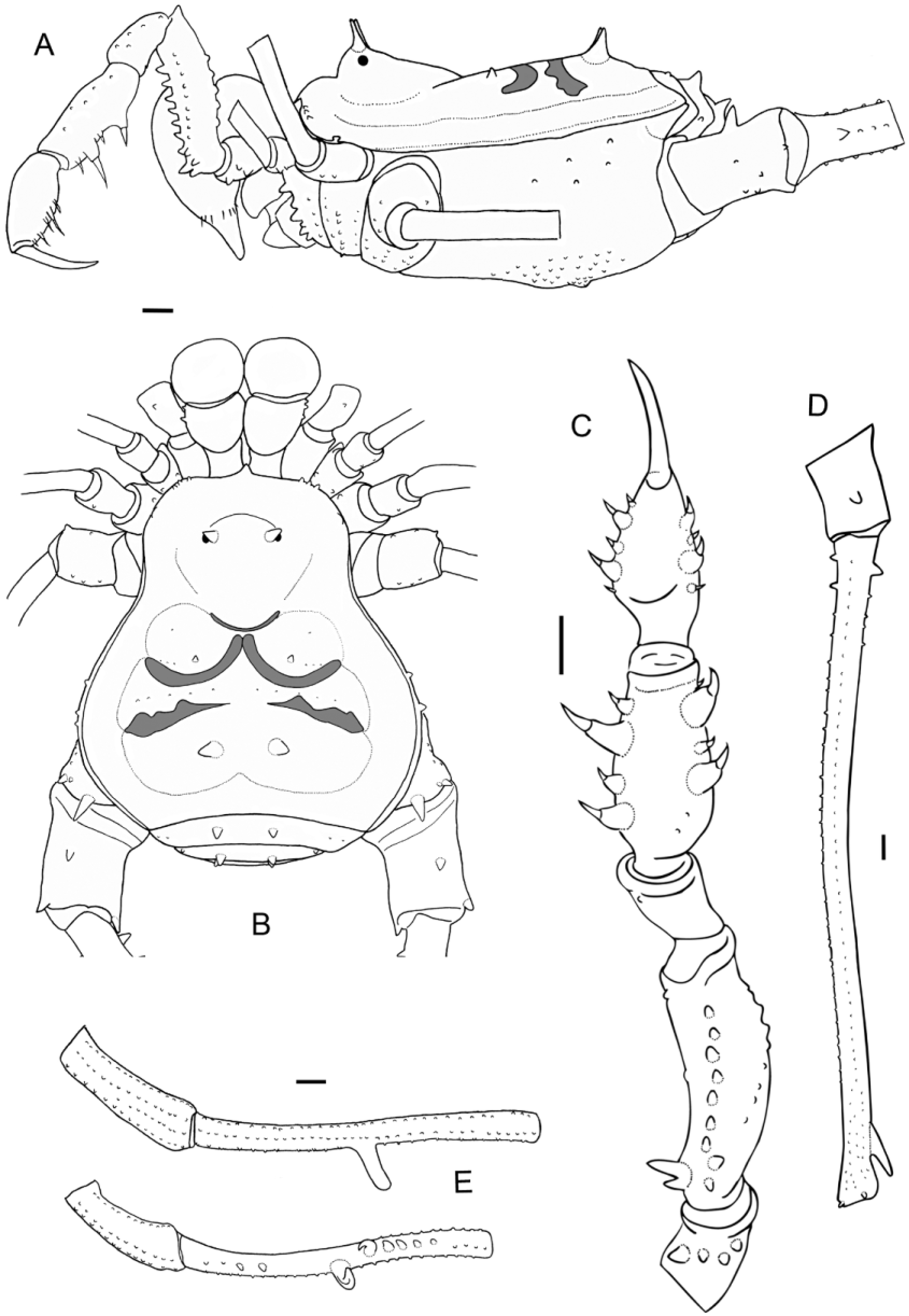
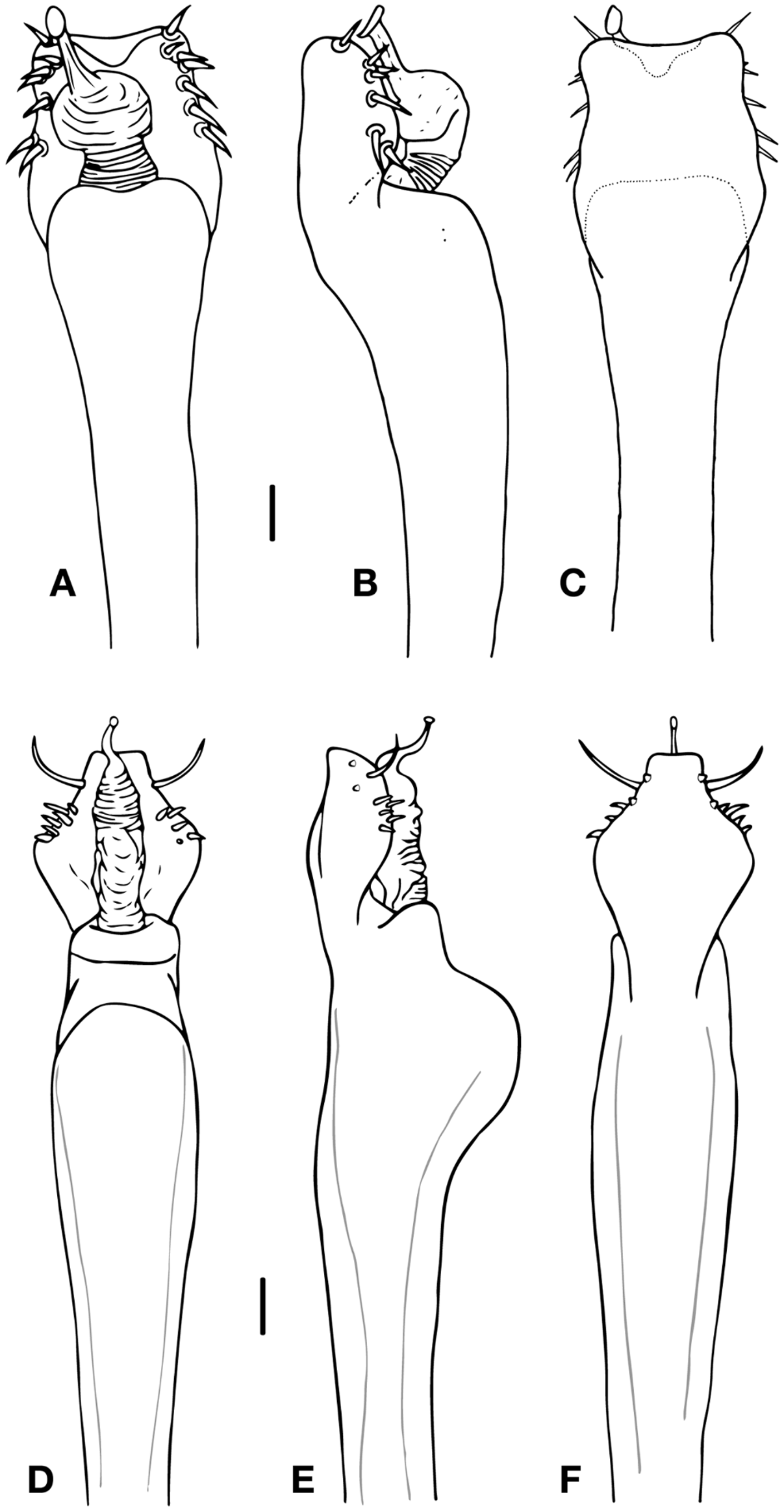
( Figs. 2 View FIGURE 2 A–D; 3 D–F; 4 A; 6)
Type material. Male Holotype: (MIZA-0016292) Ayajpaina, Municipio Machiques de Perijá, Sierra de Perijá, Zulia, Venezuela (10°03´00”N, 72°45¨58”W), 1200 msnm. 07/X/2007. P. Colmenares col. Paratypes: 1 female (MIZA-0016293) and 2 inmature (MIZA-0016294), with same data as holotype; 1 male and 1 female (INPA-OP- 2075), 3 females and 1 immature (MIZA-0016295), Ayajpaina, Municipio machiques de Perijá, Sierra de Perijá, Zulia, Venezuela (10°03´00”N, 72°45¨58”W), 1150 msnm. 07/i/2008. P. Colmenares col.
Etymology. Tizana is a traditional and popular Venezuelan drink made of orange juice and granadina, mixed with several pieces of different fruits. It is more characteristic in the Zulia state, where it is part of the local folklore. Noun in apposition.
Diagnosis. Femur IV slightly curved ( Fig. 2 View FIGURE 2 D), with a large ventro-distal tubercle. Tibia IV with two strong ventral tubercles in the middle, curved towards each other ( Fig. 2 View FIGURE 2 E). Penis with five latero-basal lanceolate setae in two rows (2+3), one sinous latero-distal larger setae, and two small ventral setae on each side ( Fig. 3 View FIGURE 3 D–F).
Distribution. Known only from the type locality ( Fig. 6 View FIGURE 6 ).
Description. Male holotype. Measurements: Dorsal scutum length 12; width 12.25; prosomal length 5.75; width 7.41; pedipalpal femur 5.75; femur IV 28.33; leg I 30.91; II 64.83; III 47.83; IV 70.41.
Dorsal scutum ( Fig. 2 View FIGURE 2 A, B). Anterior border with a median projection between the chelicerae and a lateral row of 3–4 small tubercles on each side. Eye mound with two spiniform tubercles, each one with a small basal tubercle behind. Carapace smooth. Area I with two small paramedian spiniform tubercles and a posterior row of three small tubercles; II with a posterior row of 5–6 small tubercles; III with two spiniform tubercles, two lateral and two posterior small tubercles. Free tergite I with a pair of larger paramedian spiniform tubercles and 2–3 smaller tubercles on each side, II with a pair of larger paramedian spiniform tubercles and a smaller tubercle on each side, III with only a paramedian pair of spiniform tubercles. Anal operculum with some small tubercles without arrangement.
Venter. Coxa I with a median row of five tubercles, three anterior, five posterior and three apical (anterior larger than the others); II with a median row of 11 small tubercles, four anterior, four posterior and four apical; III with a median row of 9–10 median tubercles, four anterior, four-five posterior and four apical; IV with a median row of 10–12 tubercles, several tubercles without arrangement over the surface and a pair of very low and tuberculated processes close to the spiracles.
Chelicerae. Basichelicerite with seven tubercles on bulla; hand with several small frontal tubercles; fixed finger with three teeth; movable finger with four teeth.
Pedipalps ( Fig. 2 View FIGURE 2 C). Coxa with two ventral tubercles. Trochanter with two tubercles over a dorsal hump and 4–5 ventral tubercles (mesal larger than the others). Femur with three ventro-basal tubercles (larger mesal bifid and two ectal), a median row of six subequal ventral strong tubercles, a retrolateral row of 7–9 very low tubercles and a dorsal row of 7–8 tubercles (the apical larger). Patella granular, with 11–14 dorsal tubercles unequally distributed and one prolateral apical tubercle. Tibia dorsally granular, ventrally with four ectal (IiIi) and four mesal spines (IiIi). Tarsus dorsally granular, ventrally with five ectal (iIiIi) and five mesal spines (IiIi).
Legs ( Fig. 2 View FIGURE 2 D, E). Coxa: I with an anterior and a posterior dorsal tubercle; II with an anterior dorsal tubercle; III smooth; IV with 4–5 latero-anterior tubercles and three apical tubercles (the posterior larger and sharp). Trochanter: I dorsally granulated and with three ventral tubercles; II dorsally granulated, with two retrolateral tubercles and four ventro-apical tubercles; III dorsally granulated, with three prolateral tubercles, four retrolateral, one median and four ventro-apical tubercles; IV with one dorsal tubercle, six prolateral (the apical larger), five retrolateral (the apical larger), and 8–10 small ventral tubercles. Femora: I–IV with row of small tubercles; III with one basal retrolateral tubercle and two dorso-apical tubercles; IV slightly curved, with one prolateral and two retrolateral basal tubercles followed by a row of tubercles decreasing in size until the first half, one larger and curved ventral spiniform sub-apical tubercle and two dorso-apical tubercles. Patella I–IV tuberculated. Tibia: I–III granulated; IV slightly s-curved, ventrally with a row of four tubercles in the first half, two tubercles in the middle (the proximal larger and procurved, followed by another slightly smaller and retrocurved) and a row of 4–5 tubercles decreasing in size in the second half ( Fig. 2 View FIGURE 2 E). Tarsal formula: 9(3)/20(3–4)/9–10/12.
Penis ( Fig. 3 View FIGURE 3 D–F). Ventral plate with slightly concave distal border, concave lateral borders and ventrally with two small setae on each side. Distal corners smooth. Two groups of setae: five laterobasal lanceolate setae in two rows (2+3) and one larger, sinuous latero-distal setae. Glans with small dorsal process. Stylus arising from glans. Apex not bent nor swollen.
Color (in alcohol). Body and legs dark brown. Dorsal scutum dark brown. Anterior part of the prosoma and eye mound, with small reticle that extends until the posterior border, wich possess a larger and transversal light reticle. Pedipalps reticulated. Groove I with a very thin and short white stripe. Groove II with two white stripes almost joined in the middle. Groove III with two well separated white stripes. A longitudinal, soft brown, discontinuous thin stripe that goes from groove I to the posterior margin, where it fuses with another similar, but tranversal, soft brown stripe. A thin white stripe between the two paramedian tubercles of free tergite III. Cheliceral fingers light brown to dark brown. Tarsus light brown. A view of a live specimen in figure 4 A.
Female paratype. Pedipalp with tubercles slightly smaller than the male. Spiniform tubercles of area III and free tergites proportionally larger than the male. Without the ventral process near the stigmata. Body generally darker than the male.
Natural history and conservation. This species occurs in highly humid evergreen montane forest, between 1100 and 1950 MASL in the northern part of the Andes in Venezuela. They are found near small streams, generally over the vegetation and over tree trunks close to the water. This species shares habitat with other large harvestmen, such as cosmetids Cosmetus sp. and Cynorta sp.
Phareicranaus tizana sp.nov. and the other harvestmen species found in the Sierra de Perijá are under risk of local extinction because of the extensive cultivation of "Malanga" (Xanthsoma sagittifolium Araceae ) using fire; this type of cultivation is used by the indigenous Yukpa people and some minor farmers in the mountains and foothills, the negative impact of the fire to the fauna and flora has been also well documented by the local media. Other threat is the mineral national program which is extracting carbon from the northern foothills of the Sierra de Perijá mountains in the last twenty years.
Phareicranaus manauara (Pinto-da-Rocha, 1994) ( Figs. 4 View FIGURE 4. A B, 5 A–B)
Santinezia manauara Pinto-da-Rocha, 1994: 29 [Type Locality: Brazil, Amazonas, Manaus, Reserva Ducke.]; Pinto-da-Rocha & Kury 2003: 190; Kury 2003: 98.
Phareicranaus manauara View in CoL : Pinto-da-Rocha & Bonaldo, 2011: 20.
Social behavior. We checked site one during the day and nights, but specimens of P. manauara View in CoL were only active and seen on vegetation and three trunks during night observations (8– 11 p.m.), during the day they were aggregating within a sheltered area in loose aggregation. During the first day we only made night observations at site one, we counted 10 individuals, three alpha males and four females on vegetation, and one group of three individuals in loose aggregation, two females and a male. A couple on the three trunk were disturbed by our presence and dispersed after ten minutes of observation; they moved closer to the bush where the group was aggregating. In day two, during the day, they were in loose aggregation inside a tubular-like cave shelters formed by rotten logs partially covered by large fallen palm leaves. At night we observed 15 adults forming small groups on top of the leaves of one single median sized herb bush (about 140 cm tall) standing on the left side of the shelter. Nearly each stem of the plant had at least a couple of P. manauara View in CoL ; just one single stem had one aggregation of four individuals, two stems with aggregations of three individuals ( Fig. 5 View FIGURE 5 A), and two stems with one couple each ( Fig. 5 View FIGURE 5 B).
Males were with their bodies in opposed direction, however, it seemed that the distribution of individuals was spatially organized. Adults of both genders resting in the leaves of lower vegetation were only detected alone; they were distancing about 10–20 cm and never sharing the same stem or leaf. Most of the individuals were resting in the leaves of the higher bushes of the herb, some were resting on top of the leaves, and others were upside down, resting on the inferior face of the leaves; some males were even upside down hanging only with three posterior legs (Legs IV and the right or the left leg III) ( Fig. 5 View FIGURE 5 B), holding onto the plant stem. In the loose aggregations the males touched the female with legs II and sometimes also touched other males in the bush. Males frequently were positioned standing on top of the resting females covering them with their bodies, legs I and III directed anteriorly and legs IV posteriorly. We did not observed them mating. The fourth day of observation occurred about 20 days later. The individuals were still together in the same bushes in site one, but more spread out in the vegetation, occupying an area of no more than 9 m 2. Other observations gregarious behavior in P. manauara View in CoL were reported for the Experimental Farm of the Federal University of Amazonas/UFAM close to the city of Manaus, AM, Brazil ( Lança 2011); 57 individuals were sampled and were always seen in small or median bushes and small palm leaves forming groups of several immovable individuals.
Maternal care. We observed one adult female guarding 29 nymphs in different stages of development at site two ( Fig. 4 View FIGURE 4. A B); both female and nymphs were resting on a tree trunk at the main access trail of Reserva Ducke in Manaus, AM, Brazil. The female was resting close to the nymphs keeping the typical posture observed in the other cases of maternal care ( Villarreal & Machado 2011; Machado & Macías-Ordóñez 2007a). We made the observations for two days; we only observed them briefly because after ~5 minutes of observation the nymphs dispersed and quickly found shelter in a tunnel formed by fallen palm leaves covering the tabular roots of a large tree. The early staged nymphs dispersed first; the adult female first followed the nymphs as they were dispersing, then guarded the entrance of the shelter while the nymphs aggregated within. In the first day of observation the female stayed immovable for at least four minutes, then she moved to the left side of the nymph group and touched one of them with the right second leg, and afterwards entered the shelter.
As in the case of Phareicranaus calcariferus ( Townsend et al. 2009) , the younger instars were found in large numbers and they were standing slightly apart from the older instars; the last ones were resting under and on top of an Araceae View in CoL leaf attached to the tree trunk where both instars and adult female were. During the first night we observed two individuals, older instars, resting on the inferior face of the leaf and other two resting on the plant stem, while all the younger instars were forming an aggregation on the right side of the plant. On the second day, two older instar were resting under the Araceae View in CoL leaf and two were on top of the inferior face of the same leaf. One male nymph was observed close to the younger instar aggregation, but it maintained the distance of about 3–4 cm. During the second day of observation, when disturbed, the nymphs dispersed and the female moved to the top of the shelter, above the aggregation, but kept guarding the remaining nymphs. On the fourth day we found only one female at night in the trunk of site two, but no nymphs, which had apparently dispersed during the period between visits; the shelters were inspected and no harvestmen were found. In site one a female was observed in the bush, but between site one and two we found two alpha males, one beta male and eight more females foraging on the palm bushes and stems; they were distancing about 1 m, likely dispersing.
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
SubOrder |
Laniatores |
|
Family |
|
|
Genus |
Phareicranaus tizana
| Colmenares, Pío A. & Tourinho, Ana Lúcia 2014 |
Santinezia manauara
| Kury 2003: 190 |
| Kury 2003: 98 |