Pelopscreadium aegyptense, Dronen, Norman O., Blend, Charles K., Khalifa, Refaat M. A., Mohamadain, Hoda S. & Karar, Yasser F. M., 2016
|
publication ID |
https://doi.org/ 10.11646/zootaxa.4127.3.9 |
|
publication LSID |
lsid:zoobank.org:pub:F7E466CB-8EF0-468F-AEF6-542522233FB6 |
|
DOI |
https://doi.org/10.5281/zenodo.5612500 |
|
persistent identifier |
https://treatment.plazi.org/id/877112D5-C451-41F4-9A69-994C5A7C90BB |
|
taxon LSID |
lsid:zoobank.org:act:877112D5-C451-41F4-9A69-994C5A7C90BB |
|
treatment provided by |
Plazi |
|
scientific name |
Pelopscreadium aegyptense |
| status |
sp. nov. |
Pelopscreadium aegyptense n. sp.
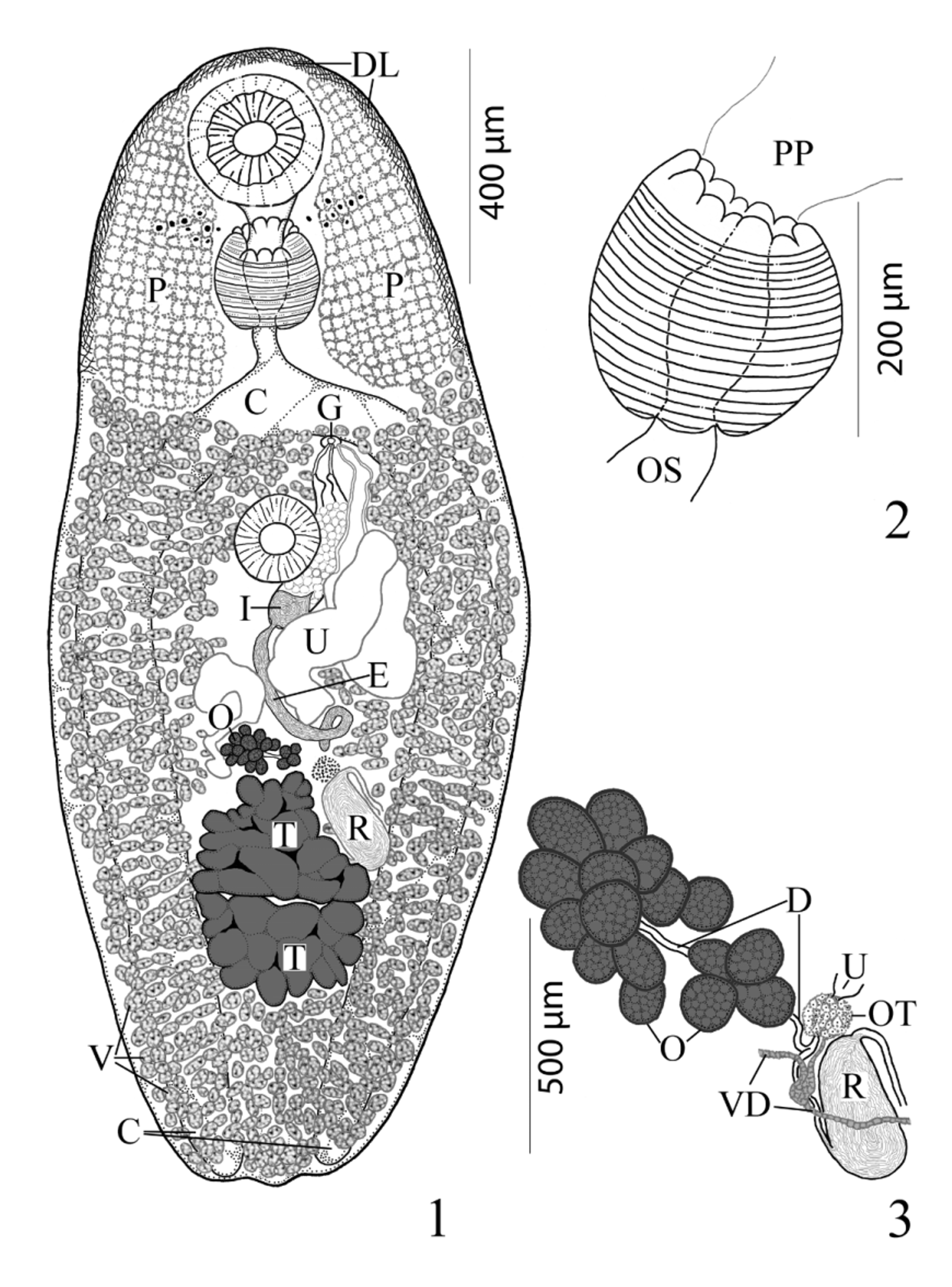
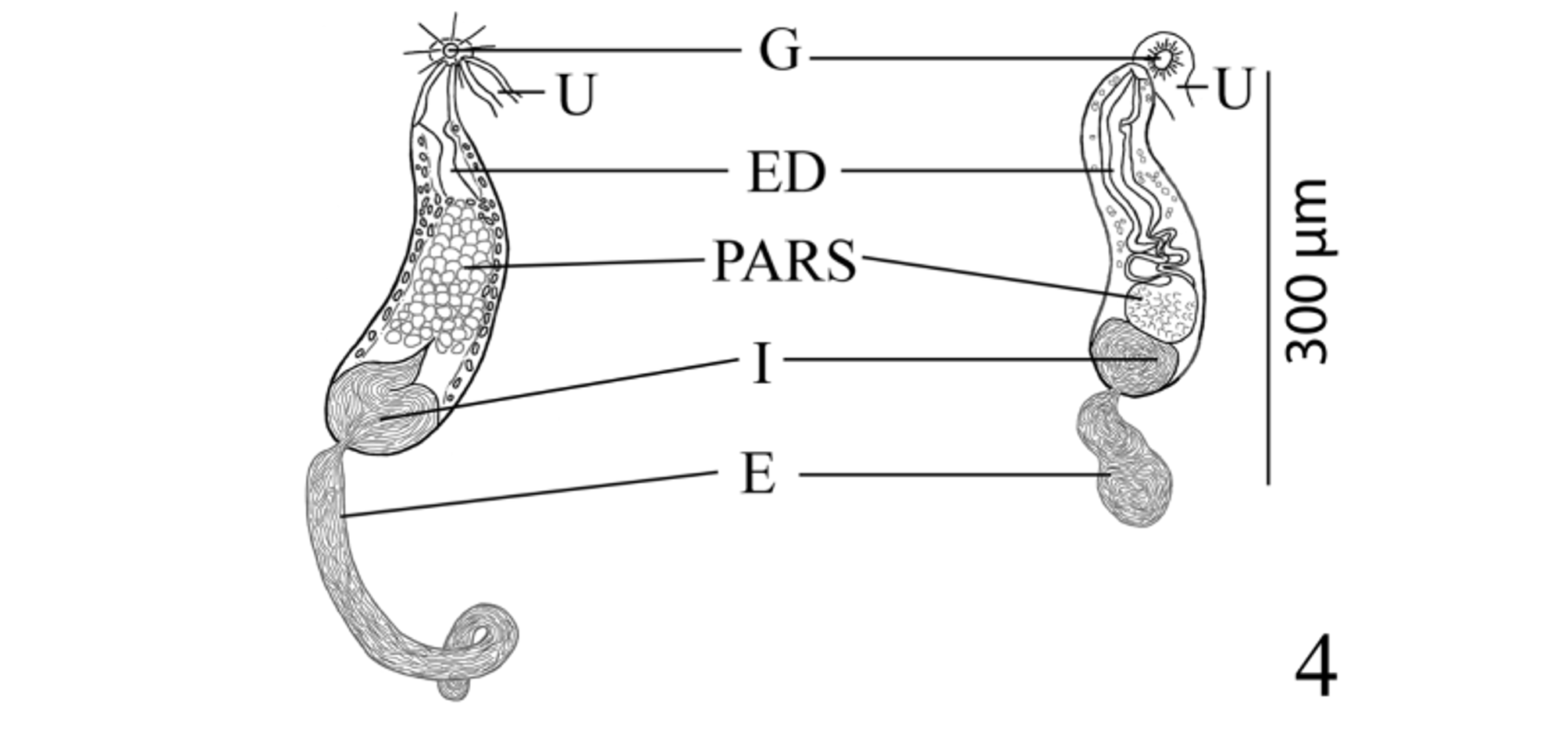
( Figs. 1–4 View FIGURES 1 – 3 View FIGURE 4 )
Type-host. Yellow boxfish, Ostracion cubicus Linnaeus ( Tetraodontiformes : Ostraciidae ).
Type-locality. Northern Red Sea, off Sharm El-Naga, Makadi Bay, Southern Hurghada, Egypt (26°55.16’N, 33°56.05’E – 26°53.59’N, 33°59.49’E, depth = 0.5–2.5 m; 26/May/2012).
Site of infection. Lower intestine.
Type-material. BM(NH) holotype 2016.4.22.1, BM(NH) paratypes 2016.4.22.2, BM(NH) vouchers 2016.4.22.3-10.
Pevalence. 2 of 8 host specimens (25% infected).
Intensity. 13–17 worms/host specimen.
Mean intensity. 15 (30/2).
Relative density/abundance. 3.75 (30/8).
Etymology. The species designation “ aegyptense ” refers to the geographical location where this new digenean was collected, i.e. off Egypt.
Description. [Based on 18 mature specimens. Measurements, morphometric percentages and morphometric ratios are given in Table 1 View TABLE 1 .] Body elongate-oval, with maximum width in middle third of body. Anterior end broadly rounded; posterior end less broadly rounded; pre-oral lobe present. Forebody gradually narrows near anterior end; remnants of cercarial eyespots often obvious in first third of forebody. Hindbody wider than forebody, gradually narrows in posterior third of body. Large internal patches of vacuolated cells form sponge-like pelops (“shoulder pads”) laterally between anterior extremity and level 2/3 to 3/4 length of forebody. Tegument densely spined anteriorly; spines short, generally scale-like, becoming less dense posterior to sponge-like pelops, sparsely distributed from posterior extent of sponge-like pelops to near posterior extremity. Distinct, narrow, welldifferentiated layer (band) of cells immediately inside anterior margin of body extends posteriorly on both sides two-thirds length of forebody. Oral sucker spherical to transversely elongate-oval, distinctly sub-terminal, somewhat embedded in parenchyma, partially covered by tegument, unspecialized with conspicuous central mouth opening. Ventral sucker sessile, spherical to ovoid, immediately pre-equatorial, occasionally equatorial, unspecialized, smaller than oral sucker. Prepharynx short, broad and either entirely outside in 89% [n=16] or retracted within posterior concavity of oral sucker in 11% [n=2] of specimens. Pharynx muscular, subspherical, narrower than oral sucker; 5 dorsal papillae (line of 3 large middle papillate projections with 1 somewhat smaller papillate projection that abuts each end of row on dorsal rim of anterior pharyngeal aperture) and 3 ventral papillate projections on ventral side of margin of aperture (like petals of flower) with 2 extensions of pharyngeal wall inserted between dorsal and ventral rows of papillae. Esophagus distinct, short, straight, longer than prepharynx. Intestinal bifurcation in posterior forebody. Ceca 2, simple, equal in length, widest just posterior to ventral sucker, somewhat arcuate, pass along lateral margins of worm to posterior end, where they abruptly terminate near body wall; ani absent.
Testes 2, transversely elongate, slightly to deeply lobed, median, tandem, contiguous, in posterior third of body. Distance from ventral sucker to anterior testis relatively long. External seminal vesicle long, tubular, sinuous throughout, larger than internal seminal vesicle, reaches from posterior aspect of sinistral rim of ventral sucker or slightly dorsal to it to about level of ovary. “ Opechona - type ” cirrus-sac ( Bray 2005; Bray & Cribb 2012), large, claviform, sinistral to mid-line, extends posteriorly from short distance anterior to ventral sucker to short distance posterior to it, encloses ejaculatory duct, pars prostatica and internal seminal vesicle. Internal seminal vesicle oval, less extensive than pars prostatica, in posterior region of cirrus-sac. Pars prostatica conspicuous, thick-walled, almost straight, elongate-oval, much longer than ejaculatory duct, lined with anuclear cell-like bodies (anuclear blebs). Ejaculatory duct short, distinct. Genital atrium distinct. Genital pore immediately post-bifurcal, ventrosubmedian, sinistral, in forebody, overlaps left cecum or opens just medial to it.
......continued on the next page
......continued on the next page n = Sample size. NG = Not given in original description.
1 Calculated from measurements given in the original description. 2 Entirely within posterior concavity of oral sucker.
3Calculated from Fig. 18 of the original description.
4 Calculated from Fig. 20 of the original description.
5 Based on 8 specimens exclusive of the holotype where the terminal genitalia was clearest.
Ovary immediately pre-testicular, sometimes contiguous with anterior testis, dextral to nearly medial, multilobed, consists of about 9–16 separate sub-globular follicles. Seminal receptacle canalicular, large, elongate-oval, sinistral to ovary, located beside or ventral to sinistral rim of anterior testis, narrows anteriorly to form Laurer’s canal which winds back posteriorly along sinistral side of seminal receptacle and opens dorsally at level of anterior third of seminal receptacle. Uterus pre-testicular, tubular, intercecal, mainly in anterior hindbody, begins at level of ovary and passes antero-sinistrally and laterally to ventral sucker, may overlap anterior margin of ovary and posterior margin of ventral sucker. Metraterm distinct, thick-walled, lined by cells with large nuclei (i.e. “muscular” wall of Bray & Cribb 1998), extends posteriorly about 3/4 length of cirrus-sac, enters genital atrium from left. Vitellarium follicular; fields extend along lateral margins from near level of intestinal bifurcation to posterior extremity, completely confluent in forebody and in post-testicular region, often encroaches into immediate pre-ovarian space. Follicles numerous, subspherical to elongate-oval or irregular in shape, both ventral and lateral to ceca but not dorsal. Eggs relatively numerous, oval, small, operculate, thin-shelled.
Excretory vesicle I-shaped, wide, appears to reach almost to level of intestinal bifurcation. Excretory pore terminal to dorsally subterminal.
Remarks. The new species has an “ Opechona - type ” cirrus-sac and is placed in the Lepocreadiidae based on the reorganization of the Lepocreadioidea by Bray & Cribb (2012). In addition, the presence of a genital pore that is ventral and submedian, two testes, sponge-like lateral patches (pelops) in the anterior portion of the forebody instead of the lateral margins in the anterior portion of the body forming an incomplete scoop (i.e. not joined posteriorly), and ceca that extend close to the posterior body wall place this new species in Pelopscreadium n. gen.; however, there are distinct differences between the new species and the type-species of the new genus, P. spongiosum n. comb. (syn. Bianium spongiosum ).
Pelopscreadium spongiosum resembles P. aegyptense n. sp. in lacking a scoop and instead possessing large, internal patches of voluminous vacuolated cells which form symmetrical, sponge-like, lateral pelops in the forebody of the worm in the region between the anterior margin of the oral sucker and about the level of the intestinal bifurcation ( Bray & Cribb 1998). Along with the sponge-like, lateral pelops both species are also somewhat similar in overall appearance, egg size (60–63 × 40–45 µm in P. spongiosum vs 56–65 × 38–45 µm in P. aegyptense ), sucker ratio (1:0.73–1:0.74 vs 1:0.59–1:0.94), possessing a sinistrally submedian and post-bifurcal genital pore, and both have a similar vitelline distribution ( Table 1 View TABLE 1 ; Bray & Cribb 1998). Pelopscreadium aegyptense and P. spongiosum differ in a number of features, the most striking of which is the extent and appearance of the external seminal vesicle and the distribution of the contents of the cirrus-sac. Within the cirrussac, the pars prostatica of P. spongiosum was described as “oval, vesicular”; whereas, the pars prostatica of P. aegyptense is more elongate with a conspicuously longer longitudinal axis (about 63 vs 89–165 µm) and it generally occupies a greater portion of the cirrus-sac (23% vs 26–41% of the cirrus-sac length, respectively). The external seminal vesicle of P. spongiosum was described by Bray & Cribb (1998, figs. 18 & 20) as “saccular, reaching to about level of ovary”, and it is illustrated as a simple, moderately-wide, oblong pouch (about 150 µm long—see Table 1 View TABLE 1 ). However, the external seminal vesicle of P. aegyptense is tubular, considerably more elongate and sinuous, and longer than the cirrus-sac (485–540 compared to 150 µm). Additionally, the cirrus-sac of P. aegyptense ranges from terminating at the level of the posterior margin of the ventral sucker to surpassing the ventral sucker a relatively short distance into the hindbody; whereas, in P. spongiosum it extends some distance into the hindbody (about half the length of the distance between the ventral sucker and the ovary). Another difference between these two species is the appearance of the testes. In P. aegyptense the testes are consistently irregular in shape and slightly to deeply lobed, but in P. spongiosum they are subglobular and entire (smooth) ( Bray & Cribb 1998, fig. 18). Although any differences in testicular shape (entire vs lobed) may simply reflect the potentially “plastic” nature of this morphological feature within many groups of digeneans (e.g. see Dronen et al. 2014, for a discussion of the difficulties in using testicular shape as a distinguishing taxonomic feature for species within the opecoelid genus Neolebouria Gibson, 1976 View in CoL ) and on its own may not be a “strong enough character” to distinguish species, it is likely that in this particular instance, this feature may be more taxonomically (phylogenetically) informative than in some other digenean groups. We also observed that although the body lengths of P. aegyptense and P. spongiosum overlap ( Table 1 View TABLE 1 ), specimens of P. aegyptense tend to be larger overall (1,352–1,412 × 438–489 vs 1,160–2,642 × 775–1,356 µm). The extent of the metraterm relative to body length differed between these two species (about 75 µm, or 5% the length of the body in P. spongiosum vs 180–335 µm or 7–16% in P. aegyptense ), and while both species parasitize the yellow boxfish, O. cubicus View in CoL , their geographical localities are considerably distant ( Australia vs Red Sea off Egypt).
TABLE 1. Measurements, morphometric percentages and morphometric ratios of Pelopscreadium spongiosum (Bray & Cribb, 1998) n. gen., n. comb. and Pelopscreadium aegyptense n. sp. from the yellow boxfish, Ostracion cubicus Linnaeus, 1758 (Tetraodontiformes: Ostraciidae), with holotype data followed by the ranges and means in parentheses.
| Species/Feature | P. aegyptense n. sp. n=18 | P. spongiosum (Bray & Cribb, 1998) n. gen., n. sp. n=2 |
|---|---|---|
| Locality | Red Sea, Macady Bay, Off Hurghada, Egypt | Off Lizard Island, Queensland, Australia |
| Body length | 1,555 (1,160–2,642; 2,027) | (1,352–1,412) |
| Body width | 1,312 (775–1,356; 1,014) | (438–489) |
| Forebody length | 996 (581–1,054; 808) | (467–487) |
| [Percentage of body length] | 64 [25–64; 39] | [34–35]1 |
| Body spines length | 10–13 (8–13; 11) | (10) |
| Oral sucker length | 304 (133–356; 250) | (134–148) |
| Oral sucker width | 382 (162–385; 279) | (187–193) |
| [Percentage of body length] | 25 [13–25; 18] | [13–14]1 |
| Pre-oral lobe | 69 (43–83; 59) | (19–32) |
| [Percentage of body length] | 4 [2–5; 3] | [0.9–2.3] |
| Prepharynx length | 10 (10–78; 49) | 0 2 |
| [Percentage of body length] | 6 (6–22; 17) | - |
| Pharynx length | 245 (167–256; 210) | (131–135) |
| Pharynx width | 284 (180–348; 270) | (129) |
| Oral sucker/pharynx width ratio | 1.3 (1:0.5–1.4; 1.0) | (1:0.7–1:0.8) |
| [Percentage of body length] | 18 [12–18; 13] | [about 9]1 |
| Esophagus length | 137 (43–151 (100) | (64–71) |
| Ventral sucker length | 206 (135–223; 186) | (129–135) |
| Ventral sucker width | 255 (151–255; 203) | (136–142) |
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
SubClass |
Digenea |
|
Order |
|
|
Family |
|
|
Genus |
Pelopscreadium aegyptense
| Dronen, Norman O., Blend, Charles K., Khalifa, Refaat M. A., Mohamadain, Hoda S. & Karar, Yasser F. M. 2016 |
Neolebouria
| Gibson 1976 |