Paguritherium manggagaway, Detorre & Williams & Boyko, 2023
|
publication ID |
https://doi.org/ 10.11646/zootaxa.5249.1.2 |
|
publication LSID |
lsid:zoobank.org:pub:2253400C-B886-4DD9-951B-CDF232813BAA |
|
DOI |
https://doi.org/10.5281/zenodo.7688304 |
|
persistent identifier |
https://treatment.plazi.org/id/6DF83F59-1CE5-4874-BFAF-529E5388F874 |
|
taxon LSID |
lsid:zoobank.org:act:6DF83F59-1CE5-4874-BFAF-529E5388F874 |
|
treatment provided by |
Plazi |
|
scientific name |
Paguritherium manggagaway |
| status |
sp. nov. |
Paguritherium manggagaway View in CoL n. sp.
Figures 7–11 View FIGURE 7 View FIGURE 8 View FIGURE 9 View FIGURE 10 View FIGURE 11
unidentified entoniscid Williams et al. 2019: 85, 101.
Paguritherium sp. DeTorre 2022: 4 , 12, 17–22, 24, figures, 7 – 12.
Material examined: Philippines. Ovigerous female holotype (9.9 mm; USNM 1522338 ), ovigerous female paratype (8.1 mm; USNM 1522339 ), male allotype (0.8 mm; USNM 1522340 from paratype female), infesting male Calcinus gaimardii (3.6 mm SL), inhabiting unknown shell, Lalaguna Beach , Puerto Galera , 13°31′32″ N, 120°58′8″ E (type locality), coll. J.D. Williams, 31 July 1997. GoogleMaps Ovigerous female paratype (10.5 mm) ( USNM 1522341 ), infesting male C. gaimardii (5.5 mm SL), inhabiting unknown shell, Lalaguna Beach , Puerto Galera , 13°31′32″ N, 120°58′8″ E, coll. J.D. Williams, 28 July 1997. GoogleMaps Two ovigerous female paratypes (9.0 mm and 7.5 mm) ( USNM 1522342 ), infesting male C. gaimardii (4.1 mm SL), inhabiting unknown shell; ovigerous female paratype (15.9 mm) ( USNM 1522343 ), infesting female C. latens (3.0 mm SL), inhabiting unknown shell, Lalaguna Beach, Puerto Galera , 13°31′32″ N, 120°58′8″ E, coll. J.D. Williams, 31 July 1997. GoogleMaps Ovigerous female paratype (9.8 mm) ( USNM 1522344 ), infesting female Calcinus latens (5.2 mm SL), inhabiting unknown shell, Sombrero Island, Puerto Galera, 13°41′53″ N, 120°49′47″ E, coll. J.D. Williams, 5 July 1997; GoogleMaps ovigerous female paratype (6.5 mm) ( USNM 1522345 ), infesting female C. latens (3.7 mm SL), inhabiting unknown shell, Sombrero Island, Puerto Galera , 13°41′53″ N, 120°49′47″ E, coll. J.D. Williams, 5 July 1997. GoogleMaps Female paratype (7.5 mm) and multiple larvae on SEM stub ( USNM 1522340 ), infesting male C. gaimardii (5.4 mm SL), inhabiting unknown shell, Lalaguna Beach , Puerto Galera , 13°31′32″ N, 120°58′08″ E, coll. J.D. Williams, 3 March 1999. GoogleMaps Female paratype (broken, not measured; USNM 1522347 ), infesting male C. gaimardii (4.0 mm SL), inhabiting unknown shell, Batangas, Anilao , 13°42′14.5″ N, 120°52′45.3″ E, coll. J.D. Williams, 13 February 1999. GoogleMaps Female paratype (broken, not measured) and male paratype (2.4 mm) ( ZRC 2022.0963 ), infesting female C. gaimardii (4.2 mm SL), inhabiting unknown shell, Batangas, Anilao, 13°42′14.5″ N, 120°52′45.3″ E, coll. J.D. Williams, 13 February 1999. GoogleMaps Female (8.5 mm) and male (0.8 mm) ( ZRC 2022.0964 ), infesting unidentified hermit crab (damaged), Sombrero Island, Puerto Galera , 13°41′53″ N, 120°49′47″ E, coll. J.D. Williams, 10 June 2000. GoogleMaps Female paratype (8.0 mm) and multiple larvae on SEM stub ( ZRC 2022.0965 ), female infesting C. gaimardii (3.1 mm SL), inhabiting Drupella cornus (Röding, 1798) , host also with bopyrid parasite in right branchial chamber ( Bopyrissa marami ; ZRC 2018.0820 ), Lalaguna Beach, Puerto Galera , 13°31′32″ N, 120°58′08″ E, coll. J.D. Williams, 18 June 2000. GoogleMaps
Etymology: The specific name is from the Tagalog deity Manggagaway, one of the goddesses of Kasamaan (underworld) opposed to Bathala (the supreme being, maker of all things) and blamed as the cause of disease ( Piscos 2019).
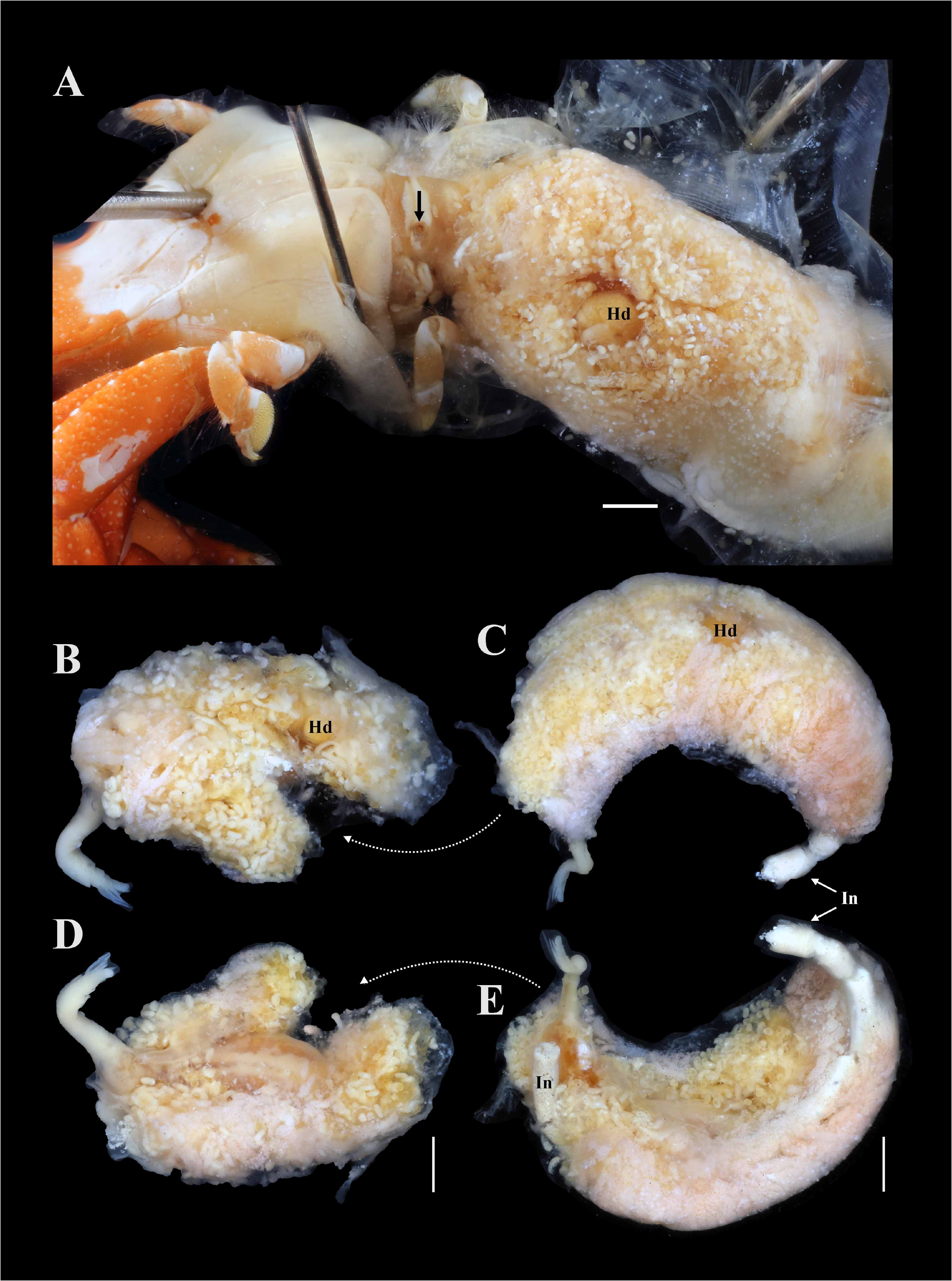
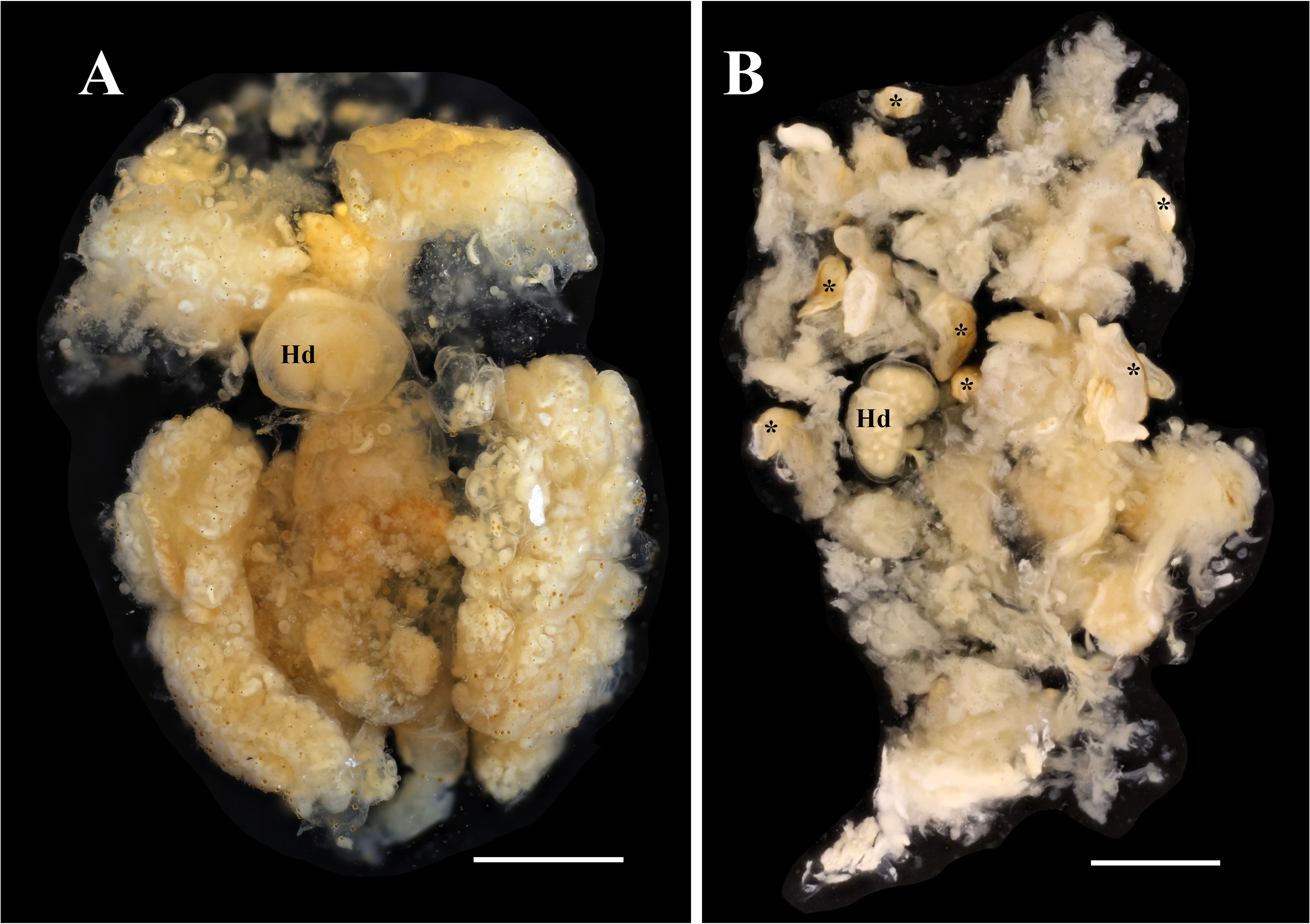
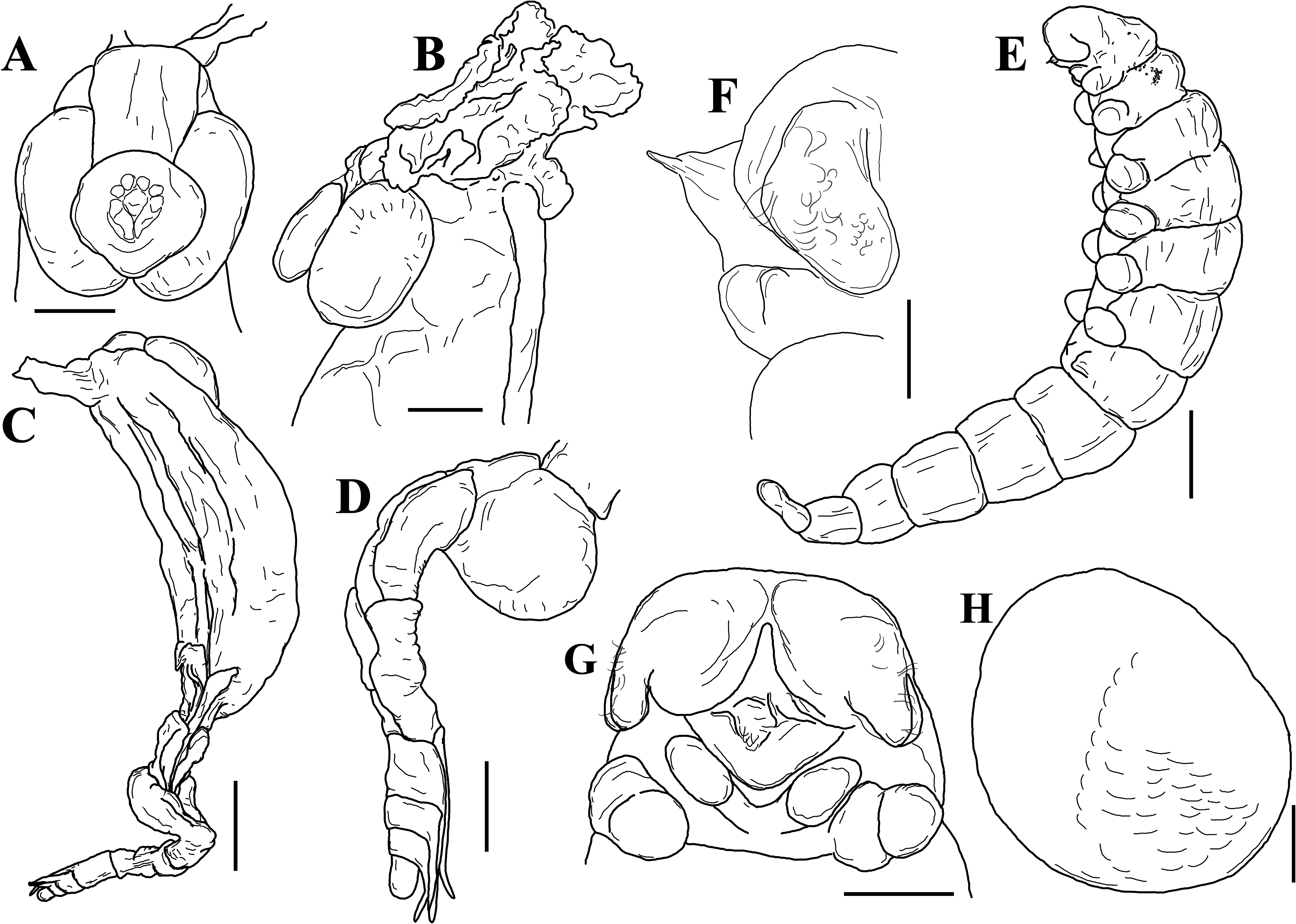
Description of female (based on holotype, USNM 1522338, and paratypes): Female in abdomen of host surrounded by host-induced sheath. Female positioned with anterior end at posterior end of host and ventral surface of parasite directed to dorsal surface of host (fig. 7A). Pleon extended into thoracic cavity of host and continuing to exit pore in middle of fifth pereomere (fig. 7A), or more anteriorly, including to branchial chamber, mouthparts, or at base of host eye stalk. Head bilobed ( Figs. 8 View FIGURE 8 , 9A, B View FIGURE 9 ), with approximately spherical lobes. Antennules and antennae as fused pads anterior to head (fig. 9A, B). Pair of small maxillipeds present. Five pairs of oostegites present; oostegite 1 largest, ascendant lobe extending anterior to head and smaller recurrent lobe ( Figs. 7B–E View FIGURE 7 , 8A View FIGURE 8 ). Pleon slender, stalk-like, of five pleomeres plus pleotelson (fig. 9C, D); pleomeres subequal in size, tapering slightly posteriorly; channel formed between five pairs of long pleopods, at least last two pairs of pleopods extending beyond pleotelson (fig. 9C, D). Uropods absent. Rounded heart in pleomere 1 ( Figs. 9D View FIGURE 9 ). Brood development asynchronous; multiple stages of development observed in single female ( Figs. 7 View FIGURE 7 , 8A View FIGURE 8 ).
Description of male (based on allotype, USNM 1522340, and paratypes): Male found within brood chamber. In present samples, only one male accompanying female (when present). Body curved ventrally, pale in color with few spots of pigmentation (fig. 9E). Short blunt head fused with pereomere 1; antennulae large, lobelike, extending posterolaterally with 16–18 setae. Oral cone large; rounded maxillipeds present (fig. 9G). Pereon of seven pereomeres, six pairs of small rounded pereopods on pereomeres 1–6; increasing slightly in size from pereopod 1 to 5, sixth pereopod subequal in size to fourth; pereopods unsegmented and distally covered in scales (fig. 9E, H), pereopods absent or each reduced to small nodule on pereomere 7 (fig. 9E). Pleon of five segments plus pleotelson; pleomeres gradually smaller in size posteriorly, without appendages, pleotelson undivided; uropods absent (fig. 9E).
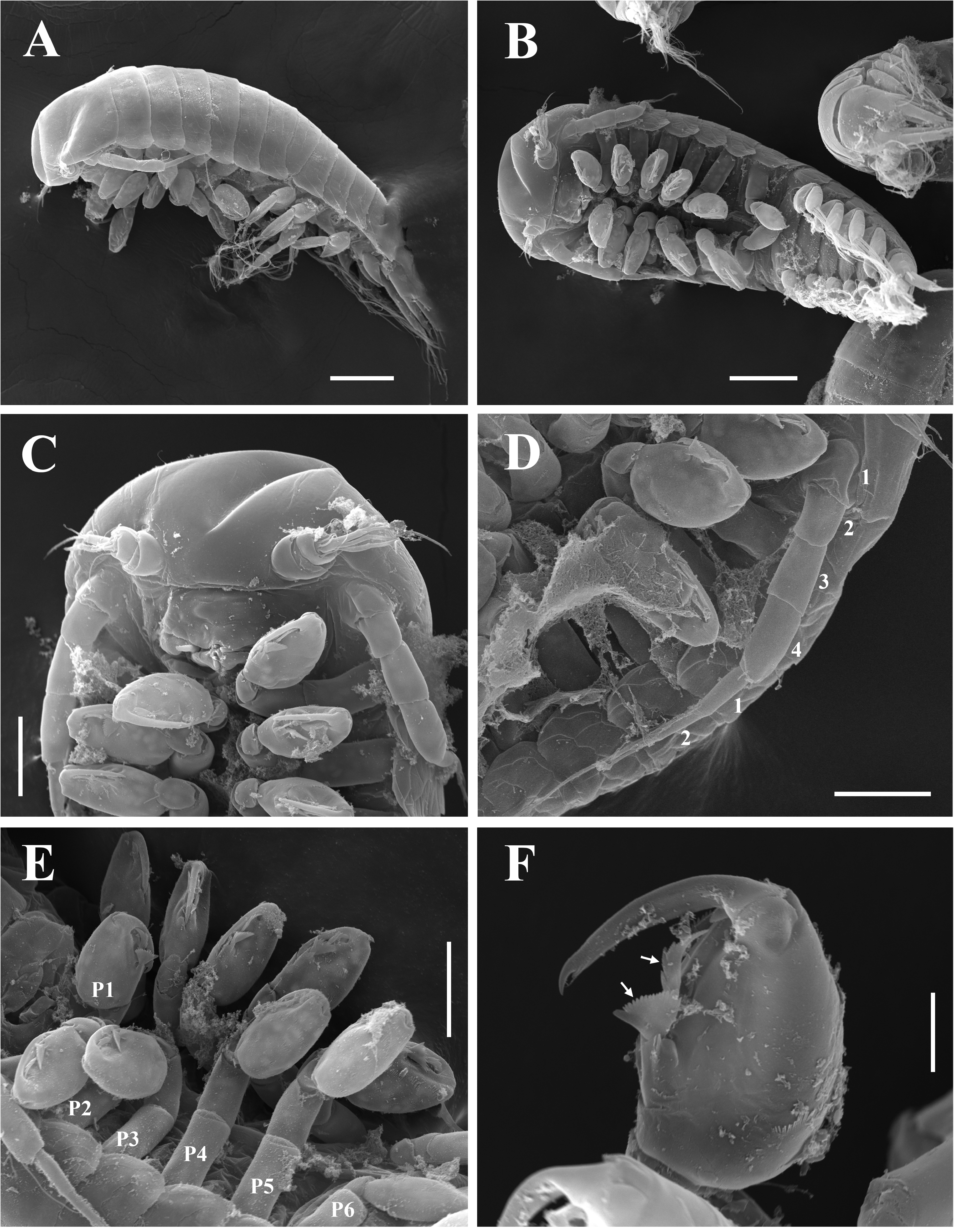
Description of epicaridium larva (based on USNM 1522340 and ZRC 2022.0965): Approximately 373 µm in length (fig. 10A). Body teardrop shaped, dorsally convex; anterior margin of head rounded (fig. 10A, B). Oral cone with pair of maxillipeds present on ventral side (fig. 10C). Antennulae of three short, rounded segments subequal in length; basal segments wider than terminal segment; terminal segment with three long setae and several short setae (fig. 10C, D). Antenna of six segments, four peduncular and two flagellar; three short setae and one longer stout seta at end of first flagellar segment, one long serrated seta with annulation at base at end of second flagellar segment; antenna approximately half total body length when fully extended (fig. 10A, D). Pair of pereopods present on pereomeres 1–6; five anterior pairs equal in size (fig. 10A, B); pereopods 1–5 subchelate, each with ovate propodus with few short setae and long curved dactylus; dactylus approximately half length of propodus (fig. 10E, F); one broad serrated fan-like seta and one more elongate denticulate seta on propodus opposite dactylus of first five pereopods (fig. 10F). Pereopod 6 of four segments, slightly shorter than preceding pereopods; merus short, carpus and propodus fused, lanceolate in shape with few short setae and scales; dactylus reduced to small triangular extension (fig. 11A–C). Pleon with five pairs of biramous pleopods, pleopods 1–4 subequal in size, with three long setae on truncate exopod and one long setae on pointed endopod (fig. 11A, D); pleopod 5 reduced, without setae (fig. 11E). Uropods biramous; exopod slightly larger than endopod, with one long seta and one short seta, endopod with one long seta (fig. 11F).
Remarks: Paguritherium was previously a monotypic genus, with P. alatum as the type species ( Reinhard 1945; Adkison 1990). Paguritherium manggagaway n. sp. matches the diagnostic features of the genus (e.g., female body is slender and lacking ovarian processes; four pairs of well-developed oostegites, fifth pair rudimentary; male antennulae tuberculiform and pereopods stump-like) ( Reinhard 1945). The females of P. manggagaway n. sp. bear a strong resemblance to those of P. alatum but differ in the length of the pleopods ( P. manggagaway n. sp. has much longer pleopods relative to body length than P. alatum ). Ovigerous females of P. manggagaway n. sp. could be detected in fixed host hermit crab specimens prior to dissection in some cases. The head of the entoniscid was sometimes visible through the host abdominal cuticle and the abdomen of these hosts was more distended than those of uninfested individuals; in addition, the eggs and larvae of the entoniscid were visible through the host abdominal cuticle in some cases (fig. 7). Reinhard (1945) and Adkison (1990) also indicated that eggs and larvae of P. alatum could be seen through the host abdomen. No developing juvenile females (stages 3–9 sensu Kuris et al. 1980) of P. manggagaway n. sp. were found, even in those host specimens systematically dissected; these stages may be relatively short in duration. Reinhard (1945) also found no juvenile females in his samples of P. alatum .
As in P. alatum , the pleon of P. manggagaway n. sp. can extend to an exit pore near the anterior end of the host, including the host eyestalks, but can also be found terminating near the mouthparts, branchial chamber or pereomeres. Although the pleon of most female entoniscids end in the branchial chamber, several species of entoniscids have a larval exit pore on or around the host eye stalk ( Shiino 1942; Adkison 1990). In one of the doubly infested hermit crabs, the pleon of the posterior female paratype extended to the base of the pleon of the anterior female holotype (fig. 7 B–E); it appears that the host derived sheath connected the two females, such that the epicaridium larvae of both females would be released through the exit pore of the more anterior female.
Two female paratypes (USNM 1522341 and 1522347) were found with calcified parts (fig. 8B) and the bodies in poor condition. This could be due to host response to parasitism ( Kuris et al. 1980). Other studies have noted the presence of dead female entoniscids encapsulated or degenerating within host crabs ( Miyashita 1941; Shiino 1942) but did not comment further upon parasite mortality. In some cases, the entoniscids can be rendered nearly unrecognizable due to encapsulation and calcification by the host ( Kuris et al. 1980).
The male of P. manggagaway n. sp. is very similar to that of P. alatum , but with more pronounced antennulae. Regarding the epicaridium larvae, P. manggagaway n. sp. can be distinguished from P. alatum based on the presence of specialized fan-like seta on the propodus of each of the first five pereopods. This feature was not found in P. alatum by Adkison (1990) or in the present samples of that species. Although it is possible that they were overlooked, these setae are relatively large and have been documented in other entoniscids. For example, such setae were described from Entoniscus japonicus Shiino, 1942 infesting the anomuran host Petrolisthes japonicus (De Haan, 1849) native to the Indo-West Pacific ( Shiino 1942). These setae were described as curved, forked spines or serrated disks on the margin facing the dactylus on the propodus of each of the first five pereopods ( Shiino 1942). The annulation of the terminal setae on the antennae (see Watling 1989) of P. manggagaway n. sp. has not been reported before in entoniscids, but it may have been previously overlooked in studies which did not use SEM. Unfortunately, the description of entoniscid larvae (epicaridium, microniscus and cryptoniscus) is lacking for many species, requiring new collections of entoniscids and ideally examination of these stages with SEM. The annulation of the distal seta makes it appear to be a third flagellar segment, but this is unlikely because nearly all other entoniscid epicaridium larvae have four peduncular and two flagellar antennal segments. There are likely other differences in the antennulae, antennae, and mouth parts of the epicaridium larvae between the two species of Paguritherium ; future analyses using SEM should be completed on epicaridium and cryptoniscus larvae of P. alatum from the east coast of the United States and P. manggagaway n. sp.
| USNM |
USA, Washington D.C., National Museum of Natural History, [formerly, United States National Museum] |
| ZRC |
Singapore, National University of Singapore, Raffles Museum of Biodiversity Research, Zoological Reference Collection |
| USNM |
Smithsonian Institution, National Museum of Natural History |
| ZRC |
Zoological Reference Collection, National University of Singapore |
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
SubOrder |
Epicaridea |
|
SuperFamily |
Bopyroidea |
|
Family |
|
|
SubFamily |
Entoniscinae |
|
Genus |
Paguritherium manggagaway
| Detorre, Marissa, Williams, Jason D. & Boyko, Christopher B. 2023 |
Paguritherium sp. DeTorre 2022: 4
| DeTorre, M. 2022: 4 |