Nennalpheus sibogae ( De Man, 1910 )
|
publication ID |
https://doi.org/ 10.11646/zootaxa.4646.1.5 |
|
publication LSID |
lsid:zoobank.org:pub:9DBCFAB6-5D6A-40E8-AC85-617E02879679 |
|
persistent identifier |
https://treatment.plazi.org/id/B074274D-FFB9-FFF4-FF31-FA6DFC54045D |
|
treatment provided by |
Plazi |
|
scientific name |
Nennalpheus sibogae ( De Man, 1910 ) |
| status |
|
Nennalpheus sibogae ( De Man, 1910) View in CoL
Figures 6–8 View FIGURE 6 View FIGURE 7 View FIGURE 8
Alpheopsis Sibogae De Man 1910: 307 View in CoL ; De Man 1911: 181, (1915) pl. 5, fig. 18. Nennalpheus sibogae View in CoL — A.H. & D.M. Banner 1981: 219, fig. 1s; A.H. & D.M. Banner 1984: 44; D.M. & A.H. Banner 1985: 36; Chace 1988: 70 (key only); Holthuis 1993: 207, fig. 201 (from De Man 1911); Hayashi 1996: 385, figs. 310, 311; Nomura et al. 1996: 12; Nomura & Asakura 1998: 28; Anker et al. 2006: 2517, fig. 5e; Minemizu 2013: 106, colour photograph. Alpheus sp.— Allen 1996: 33, colour photograph.
Material examined. Papua New Guinea: 1 female (cl 6.3 mm), MNHN-IU-2014-6057, Madang, sta.PR97, 05°12.4’S 145°49.0’E, depth: less than 10 m, leg. A. Anker et al., 24.11.2012 GoogleMaps [fcn PR97-PZD-365]; 1 ovigerous female (cl 8.2 mm), MNHN-IU-2014-6058, Madang, Tab Island , sta. PR162, 05°10.1’S 145°50.2’E, inner slope, depth: 1–4 m, leg. A. Anker et al., 03.12.2012 GoogleMaps [fcn PR162-PZD-515B]; 1 male (cl 6.7 mm), MNHN-IU-2014-6059, Madang, same collection data as for previous specimen [fcn PR162-PZD-515L] GoogleMaps ; 1 male (cl 4.8 mm), MNHN-IU-2014-6016, Madang, south of Kranket Island , sta. PR64, 05° 12.19.1988 ’’S 145°48.45.6048’’E, coral reef, leg. MNHN team, 19.11.2012 [fcn PR64-PZD-275]; 1 male (cl 5.3 mm), 1 ovigerous female (cl 5.1 mm), OUMNH. ZC. 2003.11.0017, Hansa Bay, Laing Island , outer reef, depth: 5 m, dead corals, leg. S. De Grave & H. Wilkins, 06.10.1993 . Philip- pines: 1 specimen, not sexed (cl not measured), USNM 222982 About USNM , Palawan, Ulugan Bay, west of Camungyan Island , Smithsonian Philippine Expedition , sta. SP-32-2, 10.1544N 118.7640E, R/ V Sting Ray V, depth: 9–15 m, collector unknown, 10.07.1978 GoogleMaps . Vietnam: 1 female (cl 7.3 mm), OUMNH. ZC. 2014.02.0020, Nha Trang Bay, Mung Island , under rocks, depth: 8–10 m, leg. I.N. Marin, 16.05.2004 . Hawaii : 1 female (carapace damaged, cl not measured), QM W21829, Oahu, leg. R. Holcom, date unknown. Scattered Islands (Îles Éparses) : 1 male (cl 6.0 mm), FLMNH UF 20912 , Glorioso Islands (Îles Glorieuses), off Grande Glorieuse , 11°33’16.2”S 47°17’40.6”E, reef slope, depth: 5–20 m, leg. M. Malay et al., 05.05.2009 GoogleMaps [fcn MEPA-01113]. Mayotte: 1 ovigerous female (cl 5.7 mm), FLMNH UF 13629 , Passe en S ( Longogori ), gentle coral reef slope, corals and rocks, under large flat pieces of coral rubble on sand, depth: 7 m, leg. A. Anker & F. Michonneau, 02.06.2008 [fcn MAY 08 -228]; 1 ovigerous female (cl 7.7 mm), FLMNH UF 13671 , same collection data as for previous specimen [fcn MAY 08 -237] .
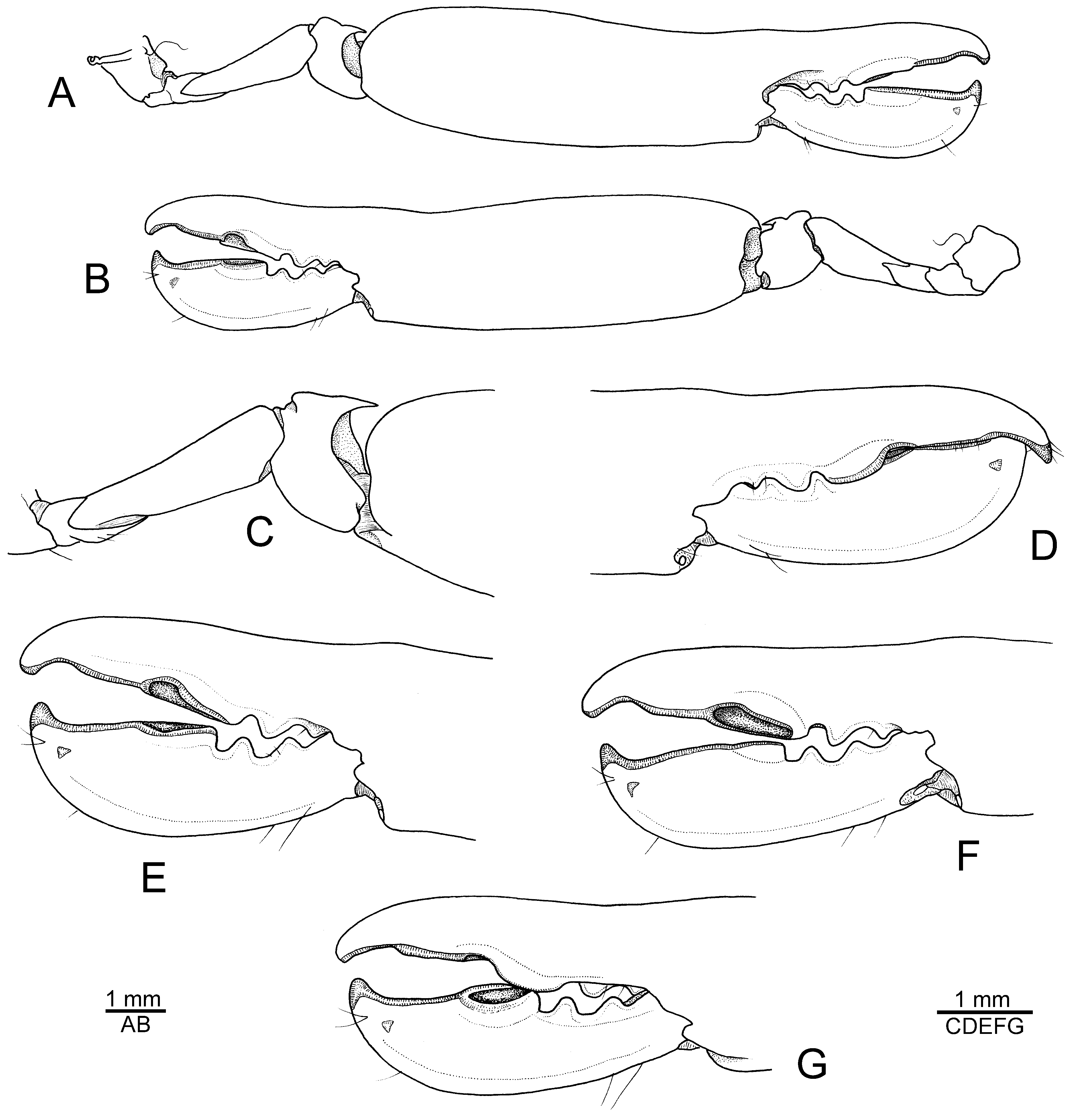
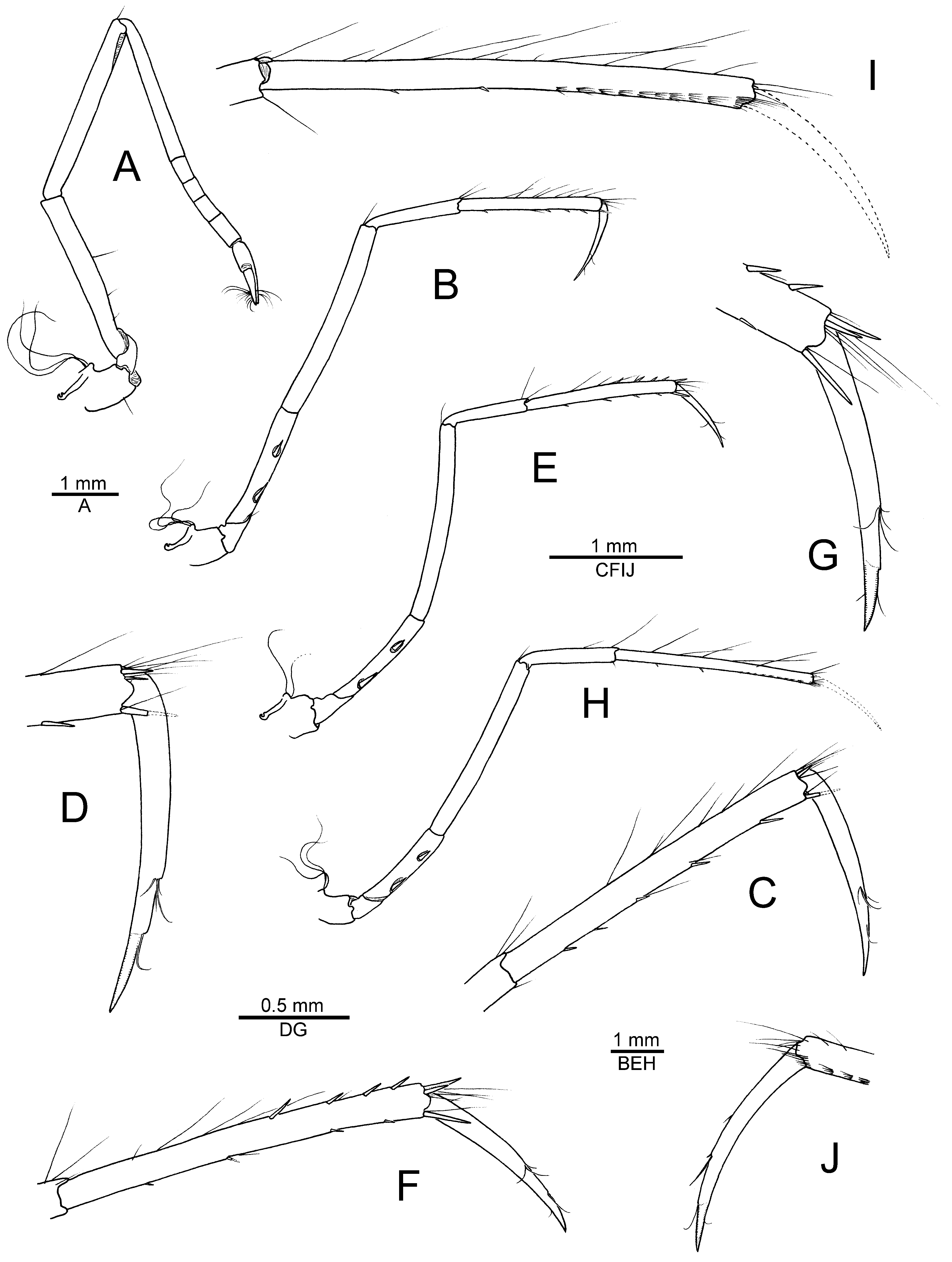
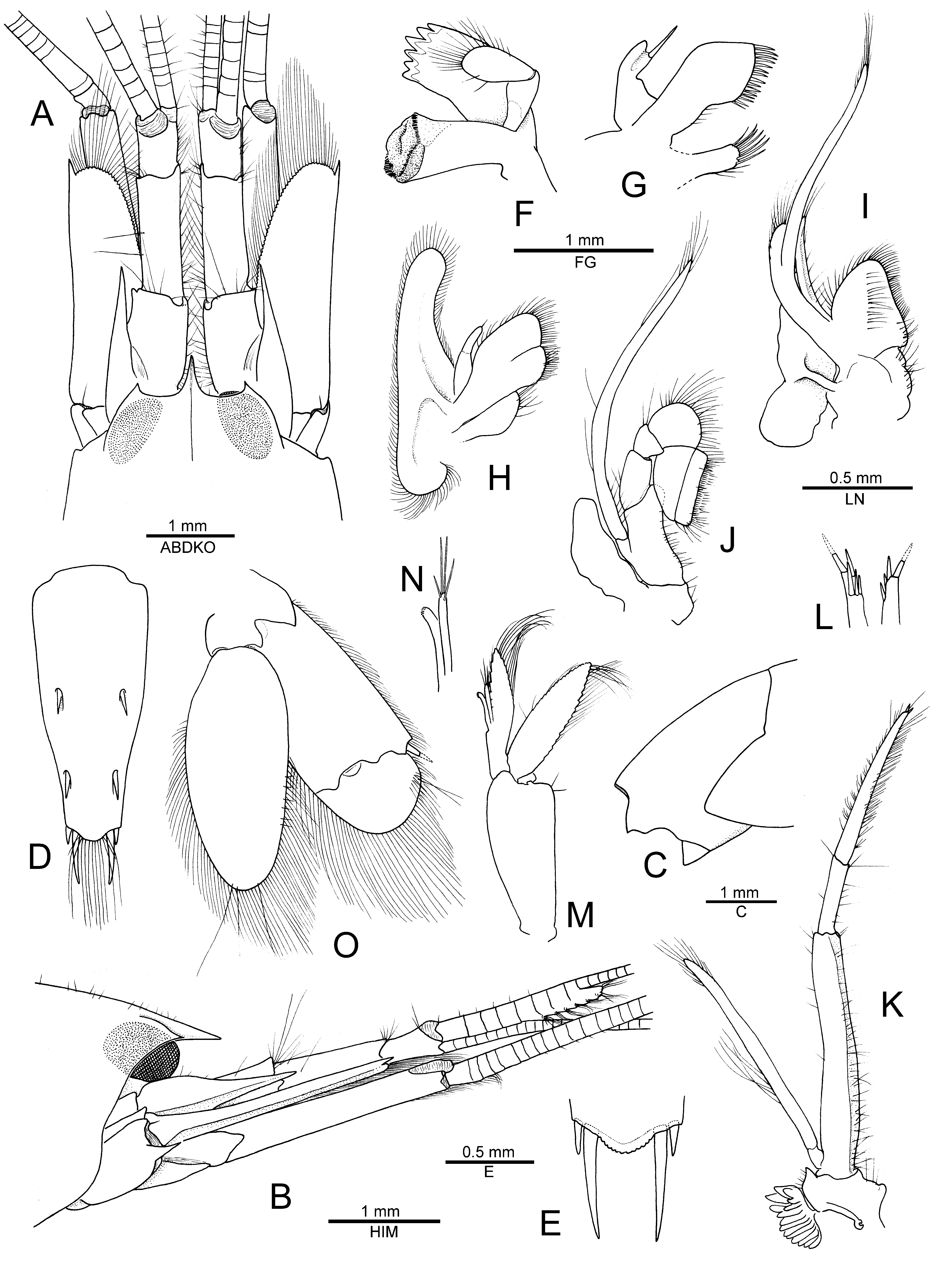
Description. See De Man (1910, 1911) for a detailed account of morphological characters and illustrations of the type specimen from Indonesia. Hayashi (1996) provided additional figures of the Japanese material, while Anker et al. (2006) illustrated some interesting features of the cheliped. To complement these accounts, the most important morphological features (including mouthparts) of a male specimen from Madang, Papua New Guinea, are illustrated in Figs. 6 View FIGURE 6 , 7 View FIGURE 7 of the present study and briefly discussed below.
Distribution. Indo-West Pacific: Indonesia: Sapeh Straits, Lombok Straits ( De Man 1910, 1911), Sulawesi (present study, photographic record, Fig. 8D, E View FIGURE 8 ); Philippines: Palawan; Vietnam: Nha Trang; Papua New Guinea: Hansa Bay, Madang (present study); Vanuatu: Efate Islands ( Banner & Banner 1984); Hawaii: Oahu (present study); Japan: Kii Peninsula (Kushimoto) and Ryukyu Islands (Kerama group) ( Hayashi 1996; Nomura et al. 1996; Nomura & Asakura 1998); Comoro Archipelago: Mayotte Island (present study); Scattered Islands: Glorioso Islands (present study).
Remarks. Nennalpheus sibogae has been relatively well described and illustrated by De Man (1910, 1911), with a few additional illustrations provided later by Hayashi (1996: fig. 310, 311) and Anker et al. (2006: fig. 5e). In addition, in situ colour photographs were published in some popular guides, e.g. Allen (1996, as Alpheus sp.), Allen & Steene (1998, same photograph, as? Nennalpheus sp.) and Minemizu (2013). The new material extends rather considerably the previously recorded distribution range of the species from the western Pacific ( Japan to Vanuatu) to the southwestern Indian Ocean ( Mayotte and Glorioso Islands) and central Pacific (Hawaiian Archipelago).
The mouthparts of N. sibogae ( Fig. 6 View FIGURE 6 F–J) were briefly described by Banner & Banner (1981) as “similar to those found in Alpheus ”, but never illustrated before. They appear to be of a generic alpheid type (see Coutière 1899; Anker 2001), with the mandible bearing a two-jointed palp, a well-developed incisor process with six teeth, and a large blunt molar process ( Fig. 6F View FIGURE 6 ); the maxillule with only one robust seta on the lower lobe of the palp ( Fig. 6G View FIGURE 6 ); the maxilla with a deep cleft on the dorsal endite and small non-subdivided endopod ( Fig. 6H View FIGURE 6 ); the first maxilliped with moderately expanded caridean lobe and non-subdivided endopod ( Fig. 6I View FIGURE 6 ); and the second maxilliped very typical to alpheids, with small oval-shaped epipod ( Fig. 6J View FIGURE 6 ). The third maxilliped is generally long and slender, with a distinct acutely produced lateral plate on the coxa ( Fig. 6K View FIGURE 6 ) and some stout spiniform setae on the apex of the ultimate article ( Fig. 6L View FIGURE 6 ).
The chelipeds ( Fig. 7 View FIGURE 7 A–E) of N. sibogae are equal in size and symmetrical in shape, and are generally very similar to those illustrated by De Man (1911), Hayashi (1996) and Anker et al. (2006). The cutting edge of the dactylus possesses a distinct thickening or bulge, which can be lodged in a shallow depression or fossa on the opposed margin of the pollex ( Fig. 7C, E View FIGURE 7 , see discussion below). This feature immediately distinguishes N. sibogae and N. inarticulatus from the above-described new species, in which the finger armature is quite different ( Fig. 3 View FIGURE 3 , see above). The fourth pereiopod ( Fig. 7I View FIGURE 7 ) is lacking a row of stout spiniform setae on the dorsal surface of the propodus, which appears to be a characteristic feature of N. gabonensis sp. nov. ( Fig. 4F, G View FIGURE 4 ). Finally, in N. sibogae , the sternal plates of the pleon are unarmed (not illustrated), whereas the frontal margin of the carapace possesses two acute orbital teeth ( Fig. 6A, B View FIGURE 6 ), two other important distinguishing features between N. sibogae and the new eastern Atlantic species (see above).
New high-quality in vitro and in situ colour photographs ( Fig. 8 View FIGURE 8 ) show living specimens of N. sibogae from Papua New Guinea, Mayotte and Indonesia. The colouration of N. sibogae is generally similar to that of N. gabonensis sp. nov., with the red chromatophores being able to contract or extend depending on the individual’s condition (stress, exposure to light, day or night time), resulting in a more or less intense general red colour ( Fig. 8 View FIGURE 8 ). Both new and previously published in situ photographs show that the shrimps carry both chelipeds extended forward and tightly appressed to each other, almost giving impression of a single structure (e.g. Minemizu 2013; see also Fig. 8 View FIGURE 8 C–E). In sedated shrimps, they can be slightly dislocated, but essentially remain in the same position ( Fig. 8A, B View FIGURE 8 ). While cautiously manipulating captured specimens, the author was able to make some interesting observations on its defensive snapping behaviour. The shrimps are able to produce a clearly audible snapping sound with both of their chelipeds. The sound is produced by the rapid closure of the dactylus, with its bulging part expelling water from the shallow fossa on the opposed margin of the pollex, although it remains presently unknown whether any cavitation phenomena are involved in this type of snapping.
So far, snapping behaviour has been reported only in five alpheid genera, viz. Alpheus Fabricius, 1798 , Synalpheus Spence Bate, 1888 , Racilius Paul’son, 1875, Metalpheus Coutière, 1908 and Pomagnathus Chace, 1937 , although in the latter genus, the snapping ability was rather suspected due to the great similarity of the major claw to that of Metalpheus (Anker et al. 2006) . In a recent study, Kaji et al. (2018) also observed rapid closures of the chelae in other alpheid genera, most notably in Salmoneus Holthuis, 1955 , although without a loud or at least audible snapping sound. Clear and audible snapping has also been observed in some members of Leptalpheus Williams, 1965 and Alpheopsis , interestingly, not involving a tooth-fossa system (A. Anker, unpublished data). On the other hand, snapping ability is also suspected in some other alpheid genera that possess a tooth-fossa (or bulge-groove) system on their cheliped fingers, such as Amphibetaeus Coutière, 1897 , Richalpheus Anker & Jeng, 2006 and Bannereus Bruce, 1988 . For the same reasons, it is also highly likely that the two other species of Nennalpheus , viz. N. inarticulatus and N. gabonensis sp. nov., are able to produce snapping sounds with their chelipeds. Alternative snapping mechanisms in the Alpheidae will be discussed in more detail elsewhere.
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
InfraOrder |
Caridea |
|
Family |
|
|
Genus |
Nennalpheus sibogae ( De Man, 1910 )
| Anker, Arthur 2019 |
Alpheopsis Sibogae De Man 1910: 307
| Banner, A. H. & Banner, D. M. 1985: 36 |
| Banner, A. H. & Banner, D. M. 1984: 44 |
| Banner, A. H. & Banner, D. M. 1981: 219 |
| De Man, J. G. 1911: 181 |
| De Man, J. G. 1910: 307 |