Chama striata, Koppka, Jens, 2015
|
publication ID |
https://doi.org/ 10.11646/zootaxa.3927.1.1 |
|
publication LSID |
lsid:zoobank.org:pub:42B56D11-9B18-4FCC-B632-30A46AB0205C |
|
DOI |
https://doi.org/10.5281/zenodo.6102700 |
|
persistent identifier |
https://treatment.plazi.org/id/039087D7-C006-4602-FF68-FE74FB9C3497 |
|
treatment provided by |
Plazi |
|
scientific name |
Chama striata |
| status |
|
Nanogyra (Palaeogyra) virgula ( Deshayes, 1831)
Fig. 13.3–4; Pl. 8.3–8; Pl. 9; Pl. 10.1
1801 Gryphaea angustata Lamarck—Lamarck : p. 399 (nomen nudum, no description). 1817 Chama striata n. sp. —Smith: p. 45 (nomen nudum, adequate description). 1819 Gryphaea angustata Lamarck—Lamarck : p. 200 (nomen nudum, short description). 1821 Ostrea virgula —Defrance: p. 26 (nomen nudum, no description).
1830 Exogyra virgula, Voltz. —Thurmann: p. 13.
* 1831 Gryphoea virgula, Def. —Deshayes: p. 90, pl. 5, figs. 12–13 (type, ICZN opinion 310). 1833b Exogyra Virgula nobis—Goldfuss: p. 33, pl. 86, fig. 3a,b.
1836 Exogyra angustata Lamarck—Bronn : p. 325, pl. 18, fig. 15a,b.
1836 Exogyra angustata Lamarck—Bronn : p. 325, pl. 18, fig. 15a,b.
1837 Exogyra virgula, Sow. —Koch & Dunker: p. 12.
1846 Exogyra virgula Goldfuss—Leymerie : pl. 9, fig. 6.
1851b Exogyra virgula Defr. —Quenstedt: p. 503, pl. 40, fig. 33.
1851 Exogyra angustata —Bronn & Roemer: p. 203, pl. 18, fig. 15a,b.
v 1862 Ostrea virgula Defr. —Thurmann & Etallon: p. 275, pl. 39, fig. 10.
1872 Ostrea virgula d´Orbigny—de Loriol, Royer & Tombeck: p. 397, pl. 23, fig. 8–14. 1882 Exogyra virgula Defr. —Alth: p. 297, pl. 27, fig. 21.
1884 Exogyra virgula Defr. —Quenstedt: p. 766, pl. 59, fig. 14, Text-Fig. 270. 1893 Exogyra virgula Defr. —Fiebelkorn: p. 397, pl. 18, fig. 1–3.
n 1893 Ostrea (Exogyra) virgula d´Orbigny (Defrance)—Greppin: p. 89, pl. 6, fig. 7, 8. 1910 Gryphaea angustata Lamarck—Douvillé : pl. 200.
1924 Exogyra virgula Defr. —Jourdy: p. 68, pl. 2, fig. V.i., pl. 3, fig. V 1–4, pl. 5, figs. 1, 8, pl. 6, fig. 4, pl. 8, figs.
9–10, pl. 9, figs. 2, 17–18, pl. 11, fig. 1.
1924 Exogyra alata n. sp. —Jourdy: p. 71, pl. 10, figs. 4 – 6 (for bilobate adult specimens). 1925 Exogyra virgula Defr. sp.—Dohm: p. 15.
1930 Exogyra striata Smith, W. 1817 —Cox: p. 298, pl. 12, fig. 8, 9.
1933 Exogyra virgula (Deshayes) —Arkell: p. 440.
1964 Exogyra virgula (Defrance) 1820 —Wellnhofer: p. 50, pl. 3, figs. 2–7, Text-Fig. 31 a–n. 1969 Exogyra virgula —Ziegler: pl. 2, figs. 1–3, 5,6, pl. 5, figs. 1–21, pl. 6, figs. 12–15. 1970 Exogyra virgula (Defrance 1825) —Dmoch: p. 81, pl. 7, figs. 5–6.
1971 Exogyra virgula (Defrance, 1820) —Pugaczewska: p. 287, pl. 1, fig. 8, pls. 28–30. 1971 Nanogyra striata (William Smith, 1817) —Stenzel in Moore: p. N1122, figs. 1a–m. 1982 Nanogyra virgula (Defrance), 1820 —Gautret: p. 35, pl. 5, figs. 1–6, 8–29, 32–38, 40–43, 46–47. 1983 Exogyra striata (W. Smith, 1817) —Birkelund et al.: p. 302.
1983 Nanogyra virgula —Birkelund et al.: p. 302 (with remark to the ICZN opinion 310). 1984 Nanogyra striata (Smith) —Buitrón: p. 95, pl. 2, figs. 7–10.
1984 Nanogyra cf. striata (W. Smith) —Gu Zhi-wei, Chen Jin-hua & Sha Jin-geng: p. 139, pl. 28, figs. 5, 6.
1984 Nanogyra striata (Smith 1817) —Pockrandt: p. 80, fig. 12a,b.
1986a Nanogyra virgula (Defrance) —Fürsich & Oschmann: p. 143, text-fig. 2a–c. 1986b Nanogyra virgula —Fürsich & Oschmann: p. 67, fig. 1, figs. 2a–f, figs. 6a–j. 1990 Nanogyra virgula Deshayes 1831 —Clausen & Wignall: p. 123, pl. 6, fig. A. 1996 Nanogyra striata (Smith) —Colleté, in Colleté, Fricot, Matrion, Tomasson & Treffot: p. 19, fig. 18. 1998 Nanogyra striata —Breton: pl. 1, fig. 14.
1998 Nanogyra virgula (Defrance, 1820) —Machalski: p. 622, fig. 9 A–H. 1998 Nanogyra virgula (Deshayes) —Radley et al.: p. 84, figs. 3d–e.
2001 Exogyra [ Nanogyra ] virgula (Defrance) —B.M. Cox: p. 2731.
2006 Nanogyra striata—Hicks: p. 36.
2006 Nanogyra striata (Smith 1817) —Richardt: p. 9, 18.
2007 Nanogyra virgula ( Defrance, 1821) —Delvene: p. 20, pl. 3, fig. 6. 2008 Nanogyra virgula ( Defrance, 1821) —Scholz, Schweigert & Dietl: p. 125, fig. 16.
Holotype (not seen). Gryphoea virgula Deshayes, 1831 : p. 90, pl. 5, figs. 12 – 13, by monotypy; non Defrance 1821, p. 26; validation as type by the ICZN, opinion 310 ( Hemming 1954, p. 355). France, exact type locality and position unknown, probably from a virgula marl unit of the Paris Basin, probably Upper Kimmeridgian, Upper Jurassic.
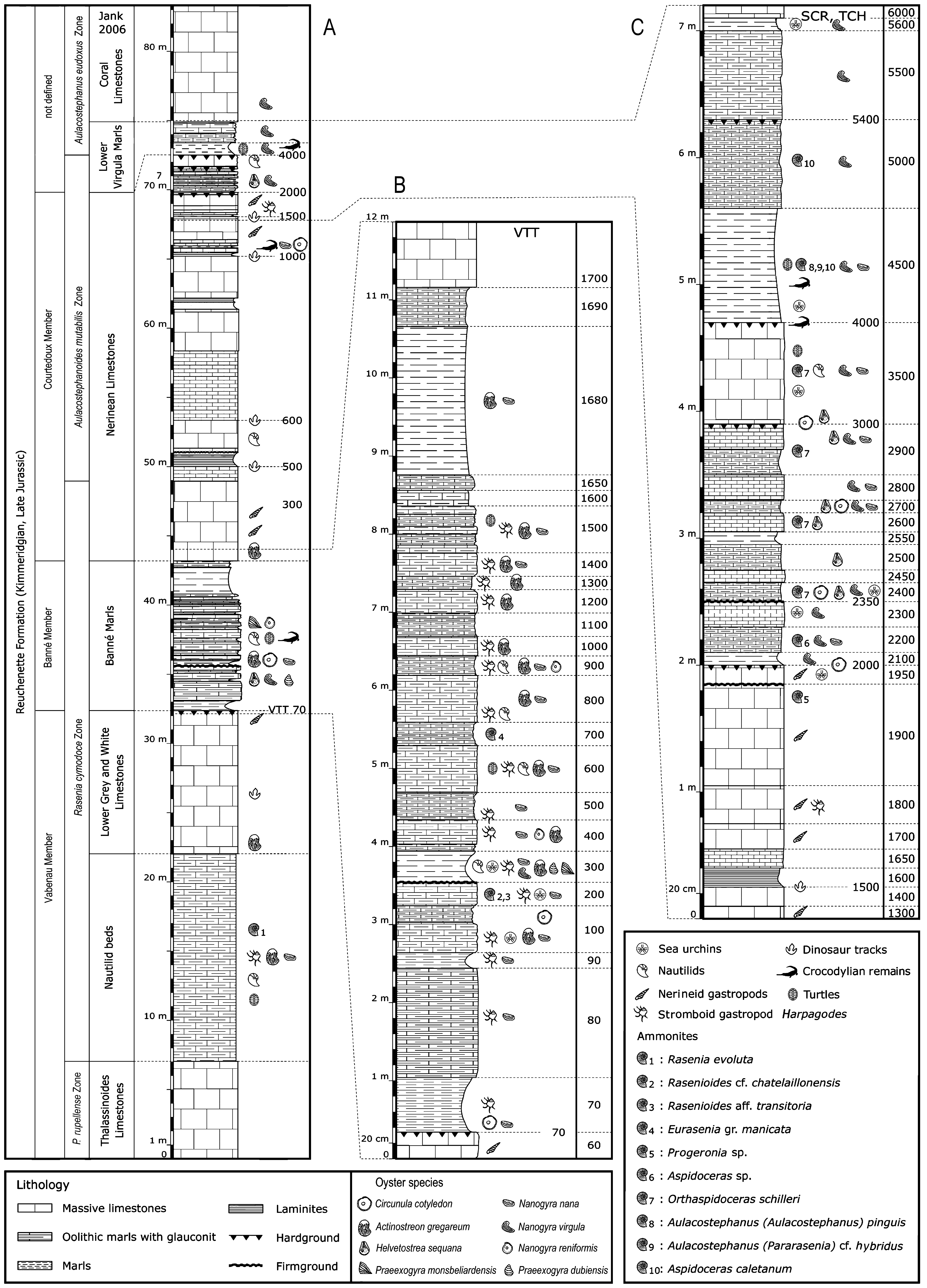
Material. Few specimens from the Banné Marls (cymodoce Zone, Lower Kimmeridgian) of Vâ Tche Tchâ ( VTT) near Courtedoux, Tunnel le Banné (TLB) at Porrentruy.
Numerous, mostly poorly preserved specimens from the mutabilis Zone (lallierianum Subzone, Upper Kimmeridgian) of Sur Combe Ronde (SCR), mostly from the “Lower Virgula Marl” (bed 4500, eudoxus Zone ) at Sur Combe Ronde (SCR) and Bois de Sylleux (BSY) near Courtedoux ( Switzerland) (for measurements see Appendix, Table 4 View TABLE 4 ).
Material from the “Lower Virgula Marl” of the Banné hill in Porrentruy (Thurmann-Collection, MJSN, S294/ 1 – 9, S294/11 – 12) (see Pl. 8.7–8 and Pl. 9.1–9).
Description. Shape, size—LV larger and much more inflated than flat RV; with a curved carina parallel to anterior margin; maximum convexity anterodorsal; outline variable but commonly comma-shaped (virguliform); larger (adult) specimens rarely with bialate (bilobate) ventral margin or, when attached to long objects, with an elongate xenomorphic outline (Pl. 8.5–6); umbo pointed, exogyroid (always opisthogyrate). RV of the convexconcave type (sensu Malchus 1990, p. 94). Coiling reaches ca. 225º (Pl. 9.10c–e: early shell is P1 and nepioconch; abrupt opisthogyrate twists of 90° between 1.5 and 4.3 mm shell height, 90° between 4.3 mm and 9 mm and ca. 45º between 9 mm and final height). Examined specimens 0.3–3 cm high (Pl. 9; Appendix, Table 4 View TABLE 4 ).
Sculpture—Attachment area usually few millimetres in diameter at tip of umbo (Pl. 9.1–5), but larger attachments with nearly the whole LV on hardgrounds or on other specimens and large objects also common. LV with fine antimarginal, dichotomous striae or thin riblets, crossed by inconspicuous commarginal growth lines and few irregularly spaced, growth ridges (Pl. 9.10a; Fig. 13.4); striation very narrowly spaced (Pl. 8.5) to rather coarse (Pl. 9.4b); striae restricted to outermost shell layers, eroded or chipped specimens from rock surfaces appearing smooth. RV nearly smooth, with commarginal growth lines, few growth crests (Pl. 9.10c–e; Fig. 13.3) and faint antimarginal striae (mainly on ventral half); anterodorsally bordered by a carina consisting of upturned crowded growth lamellae (Pl. 9.10e; Fig. 13.3); xenomorphic features rare and mostly weak (Pl. 8.6c; Pl. 9.9a).
Ligament area—Strongly twisted and prosodetic; juvenile ligament of LV narrow and deeply sunken, covered by the projected posterodorsal margin (overhang or ledge of Malchus 1990, p. 79). Visible part of the resilifer as a thin furrow, posterior bourrelet narrow, weakly elevated, anterior bourrelet twice as broad (Pl. 8.4a, 7a).
Internal shell characters—Umbonal cavity weak or absent; posterior adductor scar (PAM) in LV small, oval to kidney-shaped with truncated, convex dorsal end, situated posterocentral or slightly ventral of the midline (LV: Pl. 8.4a, 7a). PAM of RV high-oval, close to posterior margin (in the zenith of the curve) (Pl. 9.9b).
Commissural shelf well developed, in LV with small straight chomata (Pl. 8.4a, 7a, 7d), well developed dorsally, but may reach anteroventral margin (Pl. 8.4a). Posteroventral margin always without chomata (both valves); in LV posterior chomata (length 0.18–0.6 mm, width 0.08–0.1 mm) of the subligamental area larger than at anterodorsal margin (length 0.16–0.26 mm, width 0.05–0.1 mm); posterodorsal chomata more narrowly spaced and slightly curved, grooves of anterior catachomata short but with 0.1 mm relatively wide. Relict chomata present in both valves at dorsal margin (LV: Pl. 8.4a, RV: Pl. 9.10b).
Microstructure—Not investigated, but according to Siewert (1972, p. 18–19) regularly foliated, with few lamellar lenses close to umbo and towards shell margin. Dense radial striation of LV restricted to thin layer of the outer shell (as proven by strongly abraded shells from bed 2100). Prismatic outer shell layer of RV almost entirely destroyed.
Prodissoconch, juvenile—Two RVs (BSY009-917; SCR002-1367) showing calcitic internal moulds of prodissoconch and nepioconch (Pl. 9.10c–e) and, prodissoconch mould with missing umbo respectively (Pl. 9.11, Pl. 10.1a–b). Prodissoconch/nepioconch boundary of first shell hardly visible at present magnification; sharp margin (<2 mm) marking boundary between nepioconch and later juvenile shell stage; nepioconch with similar convexity and main growth direction as prodissoconch proper (species specificity of this observation not proven). Length of second, broken prodissoconch mould ca. 350 µm (Pl. 9.11 and 10.1a–b) (fide Malchus 2014, pers. comm.).
Paleoecology. In the Reuchenette Formation, the species occurs in abundance in marl beds 300 (cymodoce Zone), 2100 (mutabilis Zone), and in several lumachelles (4500, 6000, “Oyster Limestone”) above hardground bed 4000 (lower eudoxus Zone , caletanum Subzone ) (see Fig. 4 View FIGURE 4 C).
In Bed 300, the species co-occurs with N. (N.) nana , N. (P.) reniformis , Actinostreon gregareum , large Stegoconcha granulata (J. Sowerby, 1822) in life position (upper part of this horizon), and a rich echinid fauna including Hemicidaris mitra Agassiz , Pseudocidaris thurmanni (Agassiz) , Polydiadema sp., and Pygurus sp. Overall, individuals are small (<1 cm). Many specimens were attached to cylindrical objects (Pl. 8.6); similar attachment scars on co-occurring A. gregareum are identifiable as Goniolina geometrica imprints (Pl. 18.1–3). The paleoenvironment was probably a shallow, low energy, marine setting with dasycladacean meadows.
Bed 2100 also contains N. nana and Circunula cotyledon . The abundance of N. virgula defines the beginning of the Virgulian facies of the old literature. Shells are remarkably larger than material from the Lower Kimmeridgian (Appendix, Table 4 View TABLE 4 ). The outer shell layers of most of the left valves are eroded, which is probably due to in situ (or parautochthonous) reworking under a higher energy environment.
The first marly layers above hardground bed 4000 represent a nearly monospecific N. virgula lumachelle of 1 m thickness called “Lower Virgula Marl” ("Marnes à Virgula inférieur") referring to the lower lumachelles within the eudoxus Zone. The specimens are relatively small (mostly <1.5 cm). Specimens are usually attached to small shells (including other specimens of N. virgula ), tiny cerithiid gastropods or unidentified round, smooth objects. Bivalved specimens of N. virgula and of articulated infaunal Trigonia , Myophorella, Thracia , and of semi-infaunal Gervillella are common and suggest a calm, soft bottom paleoenvironment. The low diversity and small size of shells probably indicate unfavourable conditions for benthic life. N. virgula appears to be autochthonous and because of its abundance (high reproduction rate) to have been a r-strategist. The lumachelles of the “Upper Virgula Marl” ("Marnes à Virgula supérieur" sensu Contejean 1866; = “Oyster Limestone” of Jank 2006a – c), ca. 20 m higher in the section, were not investigated in detail for this study.
According to Ziegler (1969, text-figs. 9–10, pls. 3–4), N. (P.) virgula predominantly attached itself to unstructured, probably organic material such as algal leafs and thin oblong stems. Fürsich & Oschmann (1986a, p. 71, text-fig. 7) interpreted Kimmeridgian N. virgula lumachelles from the Aquitaine Basin ( France) as nearshore situated storm shell beds. They suggested a life style as secondary soft substrate dweller similar to Gryphaea . The present findings from bed 300 suggest a similar life style as inferred by Ziegler (1969), but present evidence from layer 4500 does not support a preference for soft substrate.
Occurrence. Oxfordian–Tithonian in Europe, Oxfordian in France, Kimmeridgian in France, England, Germany, Poland, Lower-Upper Kimmeridgian in Switzerland (Banné Marls, Virgula Marls); Upper Jurassic (Kimmeridgian?) of Ukraine; Tithonian of Germany ( Wellnhofer 1964, Ziegler 1969).
Remarks. Nomenclature— Gryphaea angustata Lamarck, 1801 and Chama striata Smith, 1817 are both senior synonyms of Nanogyra virgula ( Defrance, 1821: Gryphaea ). Of these, only Chama striata was accompanied by an adequate description, leading Cox (1930, p. 298, pl. 12, fig. 9) to re-establish the species as Exogyra striata (Smith, 1817) and to determine a lectotype.
Arkell (1951, p. 234), however, argued that the species name virgula had been commonly used during the past 150 years and was name-giving for the French "Marnes à Exogyra virgula ", the German “ Virgula -Mergel”, and the informal stratigraphic stage “Virgulien” of Thurmann (1852) (not 1833 as written in Arkell 1951). He therefore recommended declaring virgula a protected name which was supported by Cox (1951) and finally validated by ICZN opinion 310 (Francis Hemming, 1954, p. 355). Stenzel (1971, p. N1121) was apparently unaware of this formal act.
Evolution of N. virgula from N. nana — Jourdy (1924, p. 67) suggested that N. virgula evolved from N. nana via the transitional N. praevirgula ( Douvillé & Jourdy, 1874) , an idea that was followed by Thalmann (1966) and Ziegler (1969). However, the alleged transitional N. praevirgula ( Douvillé & Jourdy, 1874) , originally a nomen nudum, is doubtfully a valid species. According to the description and figures subsequently published by Jourdy (1924, p. 65, pl. 9, fig. 2, specimens 4–8 and 10–16), it appears more likely that the author was misled by phenotypic, slightly furrowed variants of N. nana caused by different attachment objects (e.g. Goniolina, Jourdy 1924 , pl. 9, fig. 2.12) and sizes. In addition, there are older, chomata-bearing species such as N. (P.) reniformis (Goldfuss) or the Callovian Nanogyra bigoti ( Jourdy, 1924) which are much more likely to have given rise to N. (P.) virgula .
Comparisons. Exogyra alata Jourdy, 1924 (p. 71, pl. 10, fig. 4–6)—This “species”, originally described from the Kimmeridgian of Saumont-la-Poterie (region Pays de Bray, Normandy, France), appears to be just an unusually shaped, large variant (up to a height of 4.7 cm and 7.2 cm length, Jourdy 1924, pl. 10, fig. 6) of N. (P.) virgula . [ Jourdy (1924) wrote Gaumont rather than Saumont which is here deemed to be a wrong interpretation of G for S on the handwritten (by Peron) label; an internet search provided no results for a locality named Gaumont-la-Poterie but for Saumont-la-Poterie, which is mentioned in context with the Kimméridgien]. Only few adult and megalomorphous specimens in large collections of N. virgula have a bialate ventral margin with a sulcus between an additional anteroventral projection and the normally developed posteroventral margin [ N. virgula , in de Loriol, Royer & Tombeck (1872, pl. 23, figs. 13–14)], but such phenotypic variations do not justify an independent species.
Nanogyra (Palaeogyra) catalaunica (Loriol in Loriol, Royer & Tombeck, 1872)—Taxonomic issues are discussed under N. (P.) reniformis . Nanogyra (P.) catalaunica shares well-developed chomata with N. (P.) virgula but differs by the presence of up to five prominent radial costae on the anterior side, a less curved shape, and a larger adult size up to 5 cm (see Jourdy 1924, p. 71, pl. 8, figs. XI-p, 4 specimens). The costae (ribs) and general morphology are very similar to the Cretaceous genus Ceratostreon Bayle, 1878 .
Nanogyra (P.) reniformis (Goldfuss, 1833) (b)—This long-ranging species occurs from the Bathonian to the Kimmeridgian in Europe. It is smaller than N. virgula , less inflated and auriform (ear-like) rather than commashaped. The LV also has a much larger attachment area and lacks antimarginal ornamentation on the LV. Chomata are better developed posterodorsally and disappear towards the ventral margin (in contrast to N. virgula ).
Nanogyra (P.) welschi ( Jourdy, 1924) —Taxonomic details are provided under N. (P.) reniformis . Nanogyra (P.) welschi is distinguishable by its oval shape, less inflation, a larger PAM, stronger developed chomata, and lack of antimarginal riblets ( Pugaczewska 1971, p. 294, fig. 5c,d, pl. 1, fig. 2, 6, pl. 33–34).
Nanogyra ? (N.) bigoti ( Jourdy, 1924) (p. 57, pl. 6, fig. 6)—This species was first described from the Callovian of Chemilli (Department Orne, France). It differs from N. virgula by having a smooth LV. The presence or absence of chomata is unknown, but the general appearance is closer to Nanogyra (Nanogyra) nana .
Nanogyra (N.) monoptera (J.A. Eudes-Deslongchamps & J.F.E. Eudes-Deslongchamps, 1859) (p. 32, pl. 5, figs. 1–4)—It was first described from the "couches à Leptaena " of the Lower Jurassic (Toarcian) from May-Sur- Orne, Calvados ( France). It differs from N. virgula by a strongly developed posterodorsal auricle in both valves, the more dorsally situated and larger PAM, and lack of chomata and of antimarginal ornamentation (see also Jourdy 1924, p. 52, pl. 2, fig. T, pl. 7, fig. 4; Malchus & Aberhan 1998, p. 630).
Nanogyra (Nanogyra) michalskii ( Lewinski, 1923) (p. 66, pl. 4, figs. 1–3)—This species from the Volgian (Tithonian) in Poland has a comparable shape to N. virgula but with strongly developed costae; it lacks chomata, which places it in N. ( Nanogyra ).
Praeexogyra acuminata (J. Sowerby, 1816) (p. 82, pl. 135, fig. 2)—A more detailed discussion is given under the comparisons of Praeexogyra dubiensis . This species resembles Nanogyra (P.) virgula in shape, size, and by the presence of fine antimarginal riblets on the RV. Main differences are the lack of a twisted umbo, and therefore not exogyroid ligamental area, and lack of both chomata and antimarginal riblets on the LV.
| VTT |
VTT Biotechnology, Culture Collection |
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
SubFamily |
Exogyrinae |
|
Genus |
|
|
SubGenus |
Nanogyra |
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
SubFamily |
Exogyrinae |
|
Genus |
|
|
SubGenus |
Nanogyra |
Chama striata
| Koppka, Jens 2015 |
Nanogyra (P.) welschi (
| Jourdy 1924 |
(N.) bigoti (
| Jourdy 1924 |
Nanogyra (Nanogyra) michalskii (
| Lewinski 1923 |
Ceratostreon
| Bayle 1878 |
Nanogyra (N.) monoptera
| J.A. Eudes-Deslongchamps & J.F.E. Eudes-Deslongchamps 1859 |
Nanogyra (P.) reniformis
| Goldfuss 1833 |
Nanogyra virgula (
| Defrance 1821 |
Nanogyra virgula (
| Defrance 1821 |
Praeexogyra acuminata
| J. Sowerby 1816 |