Myxidium baueri Kovaleva and Gaevskaya, 1982
|
publication ID |
https://doi.org/ 10.11646/zootaxa.3647.4.4 |
|
publication LSID |
lsid:zoobank.org:pub:D5B8E3C7-36D1-42EE-8785-4C2BB99BC62F |
|
DOI |
https://doi.org/10.5281/zenodo.6150793 |
|
persistent identifier |
https://treatment.plazi.org/id/03E287B9-D552-736D-FF12-A4DDFECC2F78 |
|
treatment provided by |
Plazi |
|
scientific name |
Myxidium baueri Kovaleva and Gaevskaya, 1982 |
| status |
|
Myxidium baueri Kovaleva and Gaevskaya, 1982
Material studied
Host: Macruronus magellanicus Lönnberg, 1907
Type hosts: Macrourus holotrachys Günther, 1878 and Merluccius hubbsi Marini, 1933
Site of infection: gall bladder
Localities, dates and depths: (1) off Chiloe Island, Chile, 43ºS, 73ºW, June 2007, 300m; (2) west of Falkland Islands, 51º 00´to 52º 30´S, 62º 00´to 62º 30´W, October 2007, 200– 250m; (3) west of Falkland Islands, 51º 00´to 52º 30´S, 62º 00´to 62º 30´W, October 2009, 200– 250m.
Type locality: (1).
Prevalence: (1) 4% (1 of 23); (2) 13% (4 of 30); (3) 7% (3 of 42).
Host length range: 25–42 cm
Collection numbers: NHMUK 2012.3.19.3, 2012.3.19.4.
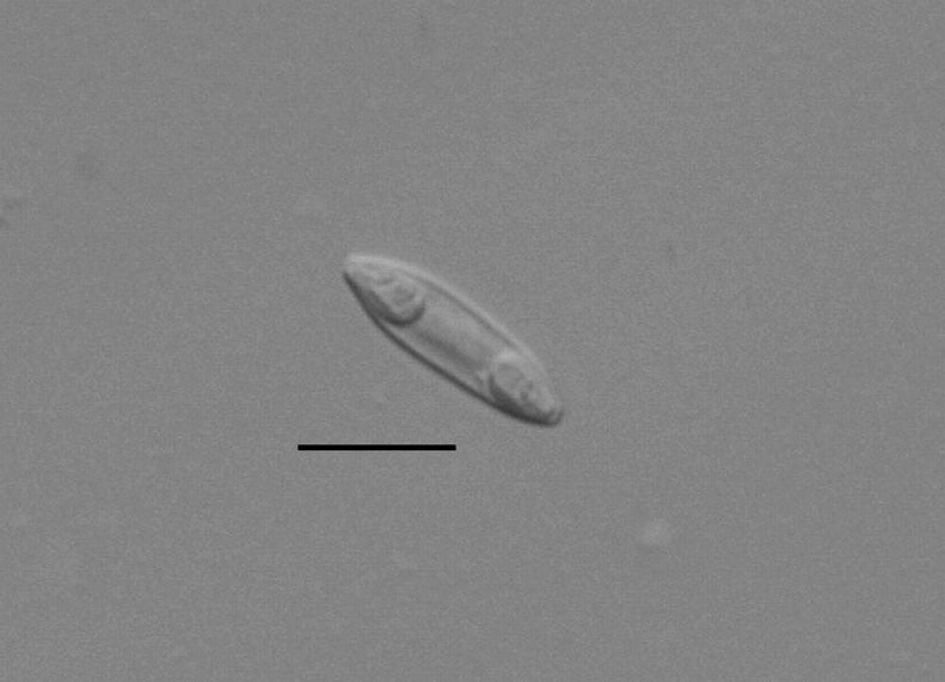
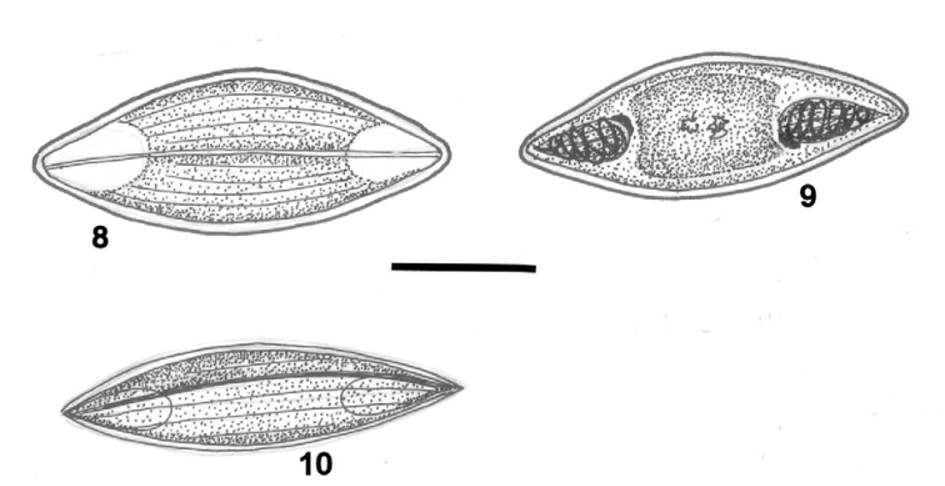
Morphological description. Vegetative stages not found.
Spore elongate with rounded ends in valvular view ( Figs. 8, 10 View FIGURE 8 – 10 ), flat elongate with attenuated ends in sutural view ( Fig. 9 View FIGURE 8 – 10 ). Sutural line straight and fine. Valve shells each with 4–5 surface striations. Sporoplasm located between the polar capsules, binucleate and deeply staining ( Fig. 11 View FIGURE 11 ). Polar capsules long, pyriform, rounded posteriorly and of equal size, at opposite ends of the spore and opening at the tips of the spores. Polar filament thick and with 4–6 coils. Dimensions, based on 25 fixed spores, as ranges with means ± SD in parentheses: spore length 14.4–20.8 (17.0 ± 2.0); spore width 5.4–9.6 (6.7 ± 1.2); spore thickness 3.2–5.2 (4.6 ± 0.6); polar capsule length 3.2–4.0 (3.6 ± 0.2); polar capsule width 1.6–3.2 (2.3 ± 0.5); polar capsule length: spore length = 1: 4.5–5.2; spore length: spore width = 1: 2.2–2.7.
Molecular result. PCR amplification of myxosporean 18S rRNA gene from one infected gall bladder from sample (3) above generated a product of approximately 1700 nucleotides in both of the samples processed from the individual fish in question. Forward and reverse sequences were obtained for each purified product. All sequences were identical. Seven polymorphic positions were observed. A final sequence of 1632 nucleotides was submitted to Genebank under Accession No. JX467674 View Materials .
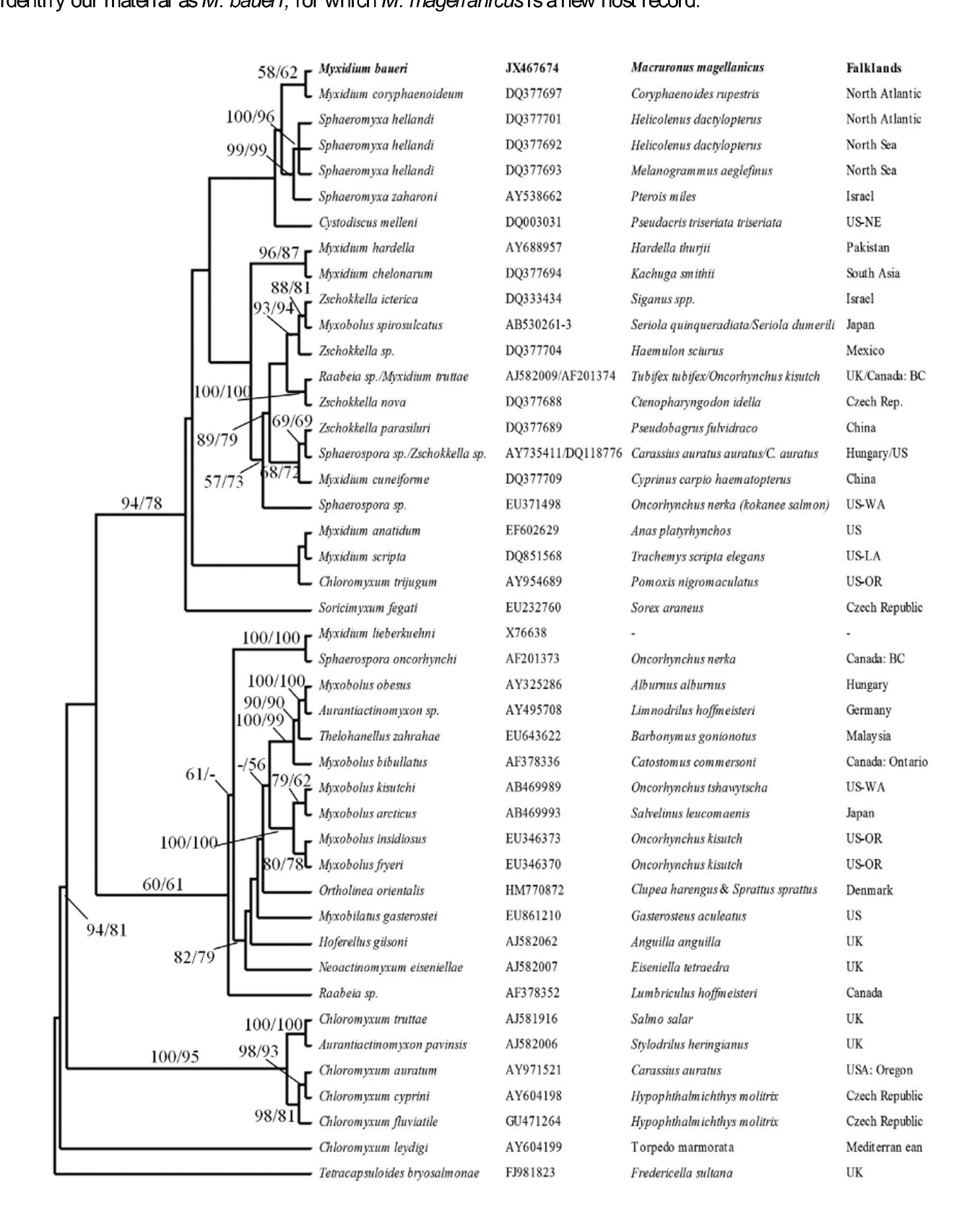
BLAST analysis revealed that the sequence was novel when compared to data currently deposited in Genbank. Based on the Max scores obtained following BLAST analysis, the sequence showed closest identity to Myxidium coryphaenoideum Noble, 1983 ( DQ377697 View Materials ), Cystodiscus melleni ( DQ003031 View Materials ) and Sphaeromyxa zaharoni Diamant et al., 2004 ( AY538662 View Materials ) with 89%, 87% and 86 % identity over 100%, 100% and 98% of nucleotide coverage respectively. A sequence of 874 nucleotides remained for phylogenetic analysis following removal of gaps and ambiguous bases. Tetracapsuloides bryosalmonae Canning et al., 1999 was used as the outgroup. MP and ML trees showed similar topologies, with M. baueri grouping with M. coryphaenoideum in both cases, though bootstrap support was relatively poor at 58 and 62 for MP and ML respectively ( Fig. 12 View FIGURE 12 ).
Discussion. The genus Myxidium includes more than 230 named species (Eiras et al., 2011), with more than 70 of these reported from marine fishes. The spores found in M. magellanicus most closely resemble those of Myxidium baueri Kovaleva & Gaevskaya 1982 , described from two deepwater fishes in the Southwest Atlantic, one of which, Merluccius hubbsi , is closely related to M. magellanicus , both species being merluccids. Kalavati et al. (1995) also reported M. baueri from Merluccius australis (Hutton, 1872) and M. hubbsi caught in the Southwest Atlantic. These authors noted certain differences between their own observations and the original description, most notably the difference between the numbers of spores in the trophozoite and the presence of distinctive knob-like extremities on the spores in their material. We did not find any vegetative stages and we did not observe the knoblike extremities on the spores of our material from M. magellanicus . Despite these differences and because of the otherwise similar morphology, the common locality and the relatedness of the hosts (Table 2), we tentatively identify our material as M. baueri , for which M. magellanicus is a new host record.
Unfortunately no sequence data are currently available for M. baueri in the public databases. Of the species of Myxidium for which molecular sequences are available, our material most closely resembles M. coryphaenoidium , collected from Coryphaenoides rupestris Gunnerus, 1765 caught in the North Atlantic (Fiala, 2006), though there is still a sufficient level of nucleotide differences to define it as a new species. Myxidium coryphaenoidium was originally described by Noble (1966) from “ Coryphaenoides sp.” caught in the Pacific off Mexico, and has since been reported from 21 species of deepwater marine fish worldwide, most of them belonging to the family Macrouridae , but also including two morid species and one member of the family Synaphobranchidae (Yoshino & Noble, 1973; Yoshino & Moser, 1974; Moser et al., 1976; Kovaleva & Gaevskaya, 1982; Threlfall & Khan, 1990). Such large host and geographical ranges raise the possibility that M. coryphaenoidium may not be a single cosmopolitan species but a complex of sibling species.
Moser et al. (1976) described two forms of spore in M. coryphaenoidium , which they called “long” and “stubby”, and the occurrence of which they interpreted as polymorphism within the one species rather than mixed infections of two species. Kovaleva & Gaevskaya (1982) considered their new species M. baueri to be identical to the “stubby” form of M. coryphaenoideum . They also described and figured shorter forms of spore in M. baueri , which they described as “anomalous”. We occasionally observed longer spores similar to those of the “long” form of M. coryphaenoideum in our material. This suggests that polymorphism may be a common feature of those species of Myxidium infecting deepwater marine fishes. It is clear that the taxonomy of the species identified as M. coryphaenoidium and M. baueri requires further investigation, using both morphological and molecular methods. Table 2 compares our material from M. magellanicus with the morphological descriptions of both M. baueri and M. coryphaenoidium .
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |