Mictyris thailandensis, Davie, Peter J. F., Wisespongpand, Puntip & Shih, Hsi-Te, 2013
|
publication ID |
https://doi.org/ 10.11646/zootaxa.3686.1.3 |
|
publication LSID |
lsid:zoobank.org:pub:95D00754-558B-4382-87F5-3F05F5B5D841 |
|
DOI |
https://doi.org/10.5281/zenodo.5624021 |
|
persistent identifier |
https://treatment.plazi.org/id/03493333-FF8C-FFC6-45B9-8DBDFE221DA4 |
|
treatment provided by |
Plazi |
|
scientific name |
Mictyris thailandensis |
| status |
sp. nov. |
Mictyris thailandensis View in CoL sp. nov.
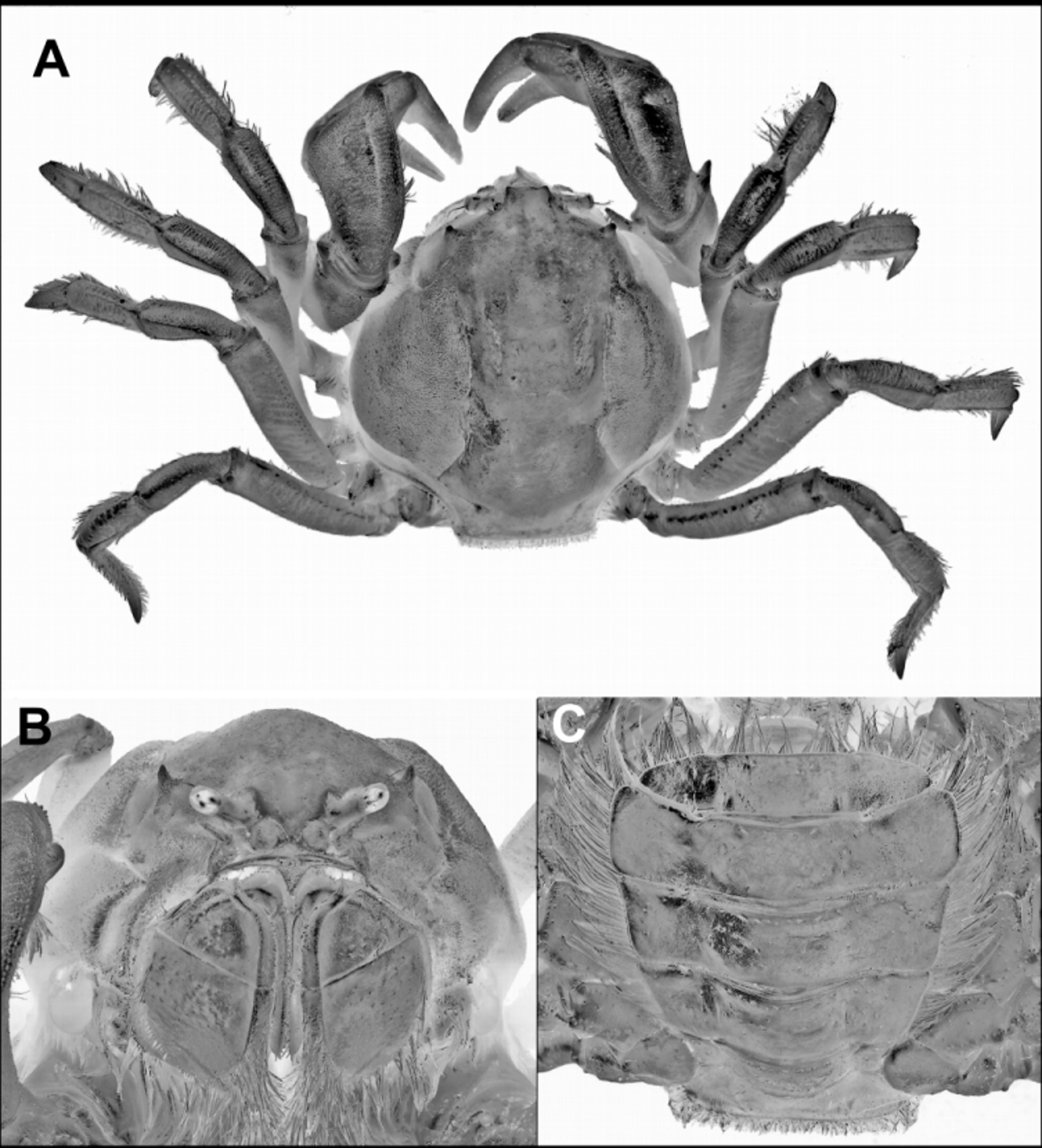
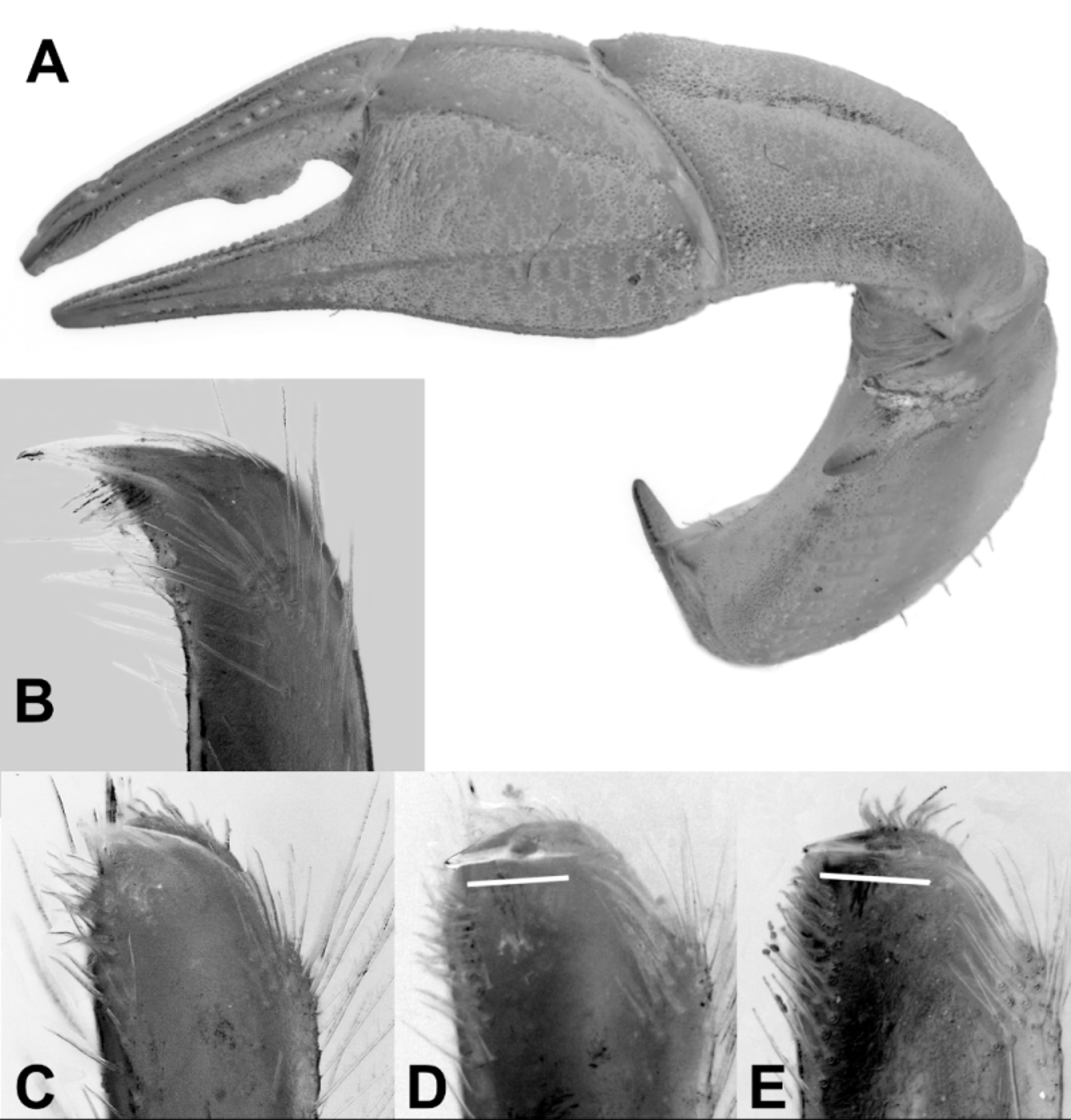
( Figs 1 View FIGURE 1 , 2A–C View FIGURE 2. A – C , 3A–F View FIGURE 3. A – F , 4 View FIGURE 4 )
Material examined. HOLOTYPE: deposited PMBC, male (14.6× 15.4 mm), Pakbara Beach, Satun Province, southern Andaman Sea coast, Thailand (6°51'21.4"N, 99°43'28.5"E), coll. P. Wisespongpand, 12.06.2012. PARATYPES: PMBC, 5 males (12.0×12.7; 12.2×13.1; 12.4×13.8; 12.8×13.3; 13.4× 14.3 mm), female (10.0× 10.7 mm), same data as holotype. QM-W29183, 3 males (12.7×13.7; 13.6×14.6; 14.4× 14.5 mm), DNA extraction, same data as holotype); QM-W29184, 4 males (9.5×10.4; 10.9×11.7; 12.0×12.6; 13.3× 14.4 mm), 4 females (9.3×10.5; 9.4×10.3; 11.2×12.3; 10.2 cw, shell damaged), same data as holotype. NCHUZOOL 13590, 5 males (11.5×12.7; 11.8×12.9; 12.2×13.1; 13.1×13.6; 13.6× 13.7 mm), female (10.0× 11.4 mm), same data as holotype. ZRC 2013.0763 3 males (8.4×9.3; 11.6×12.7; 12.4× 13.3 mm), 1 female (damaged, not measured), same data as holotype. NCHUZOOL 13587, 1 female (damaged, ~ 6.1×7.0 mm), DNA extraction; NCHUZOOL 13588, 1 female (damaged, 6.9× 8.1 mm), DNA extraction; NCHUZOOL 13589, 1 male (5.1× 5.8 mm), 2 females (6.3×7.4; 7.2× 7.8 mm), Bang Toei Pa Khlok, Thalang, Phuket, Thailand, 8°02'27"N, 98°24.42"E, Hsi-Te Shih, 30.05.2012. ZRC 2001.1070, 8 females (largest 12.0× 13.5 mm), 2 males (12.9× 14.1 mm), Ao Tang Khoen, Phuket, Thailand, near Cape Panwa, mudflat/sand beach/coral reef, P.K.L Ng et al., 17.02.2001. ZRC 2008.1344, 2 females, Kamphuan, Ranong Province, Thailand, P.K.L. Ng, 10.09.2000. ZRC 2001.2334, 1 male (damaged), Ranong, Thailand, Stn K4, P. Clark, 8.11.2001. ZRC 2001.2335, 1 juvenile female, Ko Pin Nam, Ranong, Thailand, P. Clark, 13.11.2001.
Etymology. Named for the type locality, Thailand.
Description. Body subglobular, slightly longer than broad (1.0–1.1 times), breadth about same as distance from hind margin to base of rostrum. Posterior border moderately projecting, square-cut, bluntly angular laterally. Carapace relatively smooth medially, but some sparse low granules anteriorly; sparse clusters of microscopic granules laterally on cardiac region; branchial regions evenly, densely, microscopically granular. Anterolateral spines prominent, directed anteriorly upward (not divergent), apically subacute; region immediately posterior to anterolateral spines swollen but without clearly defined ridge continuing posteriorly to edge of anterior branchials; short minutely granular ridge ventrally from anterolateral spines to form lateral edge of ill-defined lower orbital margin. Carapace regions well-defined; branchial regions moderately swollen (markedly so in large mature males), clearly separated from posterolateral margins above ambulatory legs by broad sulcus; subhepatic regions visible dorsally, separated anteriorly by groove running posteriorly from about level of anterolateral spine. Eyestalks short, corneas globose with 1 or 2 short distal setae. Rostrum deflexed, distally triangular, upper surface broadly sulcate; about as long as broad. External maxillipeds as for genus.
Chelipeds ( Fig. 1 View FIGURE 1 D). Anterior margin of ischium with large spine directed anteriorly. Merus: lower outer margin with stout subdistal spine, 1 or 2 smaller proximal spines; outer surface minutely granular, with faint transverse furrowing; posterior margin becoming more defined, granular distally. Carpus with finely granular transverse striations; deep longitudinal sulcus running full length parallel to inner edge; superior margin with row of short setae that become closer towards articulation with chela; small brush of long setae medially on inner face about one-third from distal end; inner ventral margin with short, blunt, anteriorly directed distal spine. Palm broad; dorsal margin about 0.27 times total length of palm including fixed finger, about 0.4 times length of dactyl; fingers long, slender, broadly deflexed; dactyl with broad, flat-topped, elevated tooth over proximal third of cutting margin; both dactyl, immovable finger, otherwise unarmed; cutting margin of fixed finger with submarginal groove lined with short setae; dactyl with similar subdorsal setiferous groove. Female chela markedly more slender; upper margin of palm shorter.
Ambulatory legs long, slender, somewhat flattened; minutely granular on anterior, ventral surfaces, unarmed except for small spine at anterodistal border of ischium; mostly lacking setae except for fringing setae on carpus, dactylus; upper face of meri with smooth well defined transverse sulci over most of length; dactylus of last leg recurved, apically pointed, trihedral in cross-section, with 3 prominent setal rows.
Male abdomen ( Fig. 1 View FIGURE 1 C) as broad flap typical for Mictyris ; fringed with fine setae becoming longer distally; all segments freely moveable, length of each segment similar; first somite deeply concave distally, edged with brush of short setae proximally; somites 2–5 laterally diverging, widest point near distal edge of fifth somite; sixth somite tapering, laterally rounded; telson about twice wider than long, articulating slightly below margin of sixth somite, deflexed to close sternal cavity. Female abdomen essentially similar to male.
G1 ( Fig. 2B, C View FIGURE 2. A – C ) slender, moderately setose; setae denser, longer distally; inner, lateral subdistal shoulder low, broadly convex, poorly defined; apex with conspicuous, sloping, thin chitinous crest pointed distolaterally; gonopore well-formed, placed medially.
Colour. Adult Mictyris thailandensis sp. nov. typically are plain coloured, with a light blue carapace paling to bluish cream on the swollen lateral branchial regions; chelipeds are white; walking legs yellowish fawn ( Fig. 3A, B View FIGURE 3. A – F ). Juvenile specimens are more uniform pale fawn ( Fig. 3E View FIGURE 3. A – F ), becoming darker and finely speckled as they increase in size, with more orange coloured legs and chelipeds ( Fig. 3D View FIGURE 3. A – F ), sub-adult crabs approach the adult colour with paler legs and white claws, but carapace is a darker grey blue ( Fig. 3C View FIGURE 3. A – F ).
Remarks. Mictyris thailandensis sp. nov. is most similar morphologically and genetically (see below) to M. brevidactylus and M. guinotae . Mature adults of all three species can be separated on colour differences. Mictyris brevidactylus , which is the geographically closest of the named species, with a known distribution extending to southern Vietnam, possesses striking broad red bands at the base of the walking legs, which immediately distinguish it ( Fig. 3 View FIGURE 3. A – F I). Mictyris guinotae ( Fig. 3 View FIGURE 3. A – F G, H) from Japan (Ryukyu Islands), is similar to M. thailandensis sp. nov. in being relatively plain coloured, but its walking legs and chelipeds are more typically a uniform bluishgrey, compared to yellow-fawn for the walking legs, and white for the chelipeds, in M. thailandensis sp. nov. ( Fig. 3A–C, F View FIGURE 3. A – F ). Munguia et al. (2013) recently showed that there were directional latitudinal changes in colour in the American fiddler crab, Uca (Leptuca) pugilator (Bosc, 1811) , but this was in response to a temperature gradient, and reflected the species seasonal physiological response to temperature change. While Mictyris species do vary in colour in relation to size and sexual maturity (e.g. see Davie et al. 2010), there is as yet no evidence of geographic clinal variation, and thus the differences here reported between M. thailandensis sp. nov. and other species can be assumed to be real.
The ongoing revision of the genus by the first author, has shown that the shape of the palm of the cheliped ( Fig. 2A View FIGURE 2. A – C ) can be important in distinguishing some species, and in particular the ratio of the length of the top margin of the palm to the length of the fingers. Mictyris thailandensis sp. nov. has a noticeably broader upper palm than either M. brevidactylus or M. guinotae , with the dorsal margin about 0.27 times the total length of the palm and fixed finger (0.21–0.24 respectively in the other two species); and about 0.4 times the length of the dactyl (cf. 0.32– 0.35 respectively).
Because most Mictyris species are morphologically similar, the shape of the tip of the male G1 is of major importance in separating them as there are always consistent differences, even though such differences may be small ( Davie et al. 2010). In Mictyris thailandensis sp. nov. ( Fig. 2B, C View FIGURE 2. A – C ), the chitinous apical crest is conspicuously curved, and sloping down towards a low, poorly discriminated subdistal shoulder. In M. guinotae the chitinous apical crest is pointed sternolaterally and slightly upwards, whereas in M. brevidactylus it is a little more deflexed and pointed slightly downwards. This difference is emphasised by the placement of the black bars parallel with the apical crests in Fig. 2 View FIGURE 2. A – C D and E. Also, in both M. guinotae and M. brevidactylus the subdistal shoulder is prominent and well-defined. The importance of these differences is reinforced by the results of genetic study, discussed later.
Mictyris “ longicarpus ” has been previously reported from Chon Buri, in the Gulf of Thailand ( Naiyanetr 1998), but this record is unlikely to be conspecific with M. thailandensis sp. nov. because they are from two different oceanic provinces, and other Mictyris species now appear to have relatively restricted ranges. Its identity must therefore remain in doubt, but it seems most likely that the Chon Buri record may simply represent a westward extension of M. brevidactylus from south-eastern Vietnam into the Gulf of Thailand.
Distribution. So far only known from the Andaman coast, Thailand, between Ranong Province and Pakbara Beach, Satun Province.
Habitat and ecology. Mictyris thailandensis sp. nov. occurs in the sandy-muds of sheltered estuarine and coastal intertidal flats. Pakbara Beach stretches about 5 km along the coast, but most crabs occur only at the northern end which is strongly influenced by a large estuary. Even more specifically, they have only been found in the upper littoral zone in a limited area beside the Pakbara pier ( Fig. 4 View FIGURE 4 A). The beach here is narrow, and quickly becomes dominated by silty-clay in the lower littoral zone. Since this habitat is next to the pier, the threat of negative impacts from humans is quite serious. This section of beach is heavily littered and polluted, and many small fishing and tourist boats rest on the beach where the crabs burrow ( Fig. 4 View FIGURE 4 A).
Pakbara Beach has a diurnal tidal regime, with the habitat of Mictyris typically exposed for 3–4 hrs. A preliminary study of environmental factors, including sediment cores where Mictyris occur show that the sand becomes black and anoxic at about 10 cm from the surface. Organic matter content ranged from 0.02–2.75% (average 1.09%). The sediment is predominantly fine sand (125–250 µ). There had been periods of rainfall on the main day of collection, with the ambient temperature ranging from 25–28°C. Surface tidal water salinity was 21‰, with a pH 7.51. Measurements taken from water in the holes where sediment cores were taken showed similar soil temperature and pH values (27–28°C; pH 7.34–7.89), but a markedly higher salinity (27–32‰).
Individuals tend to occur in relatively dense clumps, with between 8–43 burrows/m2 (an average of 19.1 burrows/m2) (personal observation).
The beach zone where Mictyris is found is shared with a variety of other crabs including: Uca hesperiae Crane, 1975 and U. annulipes (H. Milne Edwards, 1837) (Ocypodidae) , Dotilla myctiroides (H. Milne Edwards, 1852) (Dotillidae) , two species of Metaplax , M. elegans De Man, 1888, and M. crenulata (Gerstaecker, 1856) (Varunidae) , and a Scopimera species. Dotilla myctiroides forms large droving armies in the vicinity of Mictyris thailandensis sp. nov., but strangely do not form such armies in an adjacent area about 500 m away where Mictyris are absent.
Behaviour. Mictyris thailandensis sp. nov. does not march in the armies for which some other Mictyris species are noted. It spends most of its life cycle underground, similar to its Western Australian congener, M. occidentalis (see Unno & Semenuik 2008). A few individuals often emerge from the burrow for a short period after the lowest tide, especially on sunny days; however, they stand still on the beach and do not congregate into groups. When touched, they pull the claws and walking legs close against their body, and remain motionless for between 1–10 minutes ( Fig. 3A View FIGURE 3. A – F ).
Burrowing. As in other species of Mictyris , M. thailandensis sp. nov. burrows by corkscrewing into the sand ( Fig. 4 View FIGURE 4 B). They do not make permanent, maintained, burrows with a surface entrance, but dig vertically to a depth of 10–15 cm when feeding is finished. While a vertical hole is visible when examining the burrow structure at lowtide, it is likely that this is an emergence hole through the drier sand after the tide recedes, and that it is not present during high tide period. The “cork-screw” digging action when burrowing from the surface normally results in “back-filling” the hole, and normally leaves no surface evidence.
Feeding. As described for other Mictyris species, M. thailandensis sp. nov. feeds on meiofauna and detritus by processing sediment while tunnelling just below the surface — termed “subsurface feeding” (see Quinn 1980, 1986; Dittman 1994). Characteristic trails of pushed-up sand first begin to appear on the beach 15–30 min after low tide ( Fig. 4 View FIGURE 4 C, E). Careful removal of the surface mound reveals a vertical burrow made as the crab ascends from deeper in the sand, and the horizontal burrow that typically extends 10–15 cm just below the surface. Along the horizontal burrow the processed faecal sand is accumulated and pushed-up to form the surface mound behind the crab. Within 1 to 2 hrs the sand mounds of adjacent crabs are sufficiently numerous that they combine to form extensive “hummocks” covering the area. This subsurface feeding phase takes place over 2 to 3 hrs until the beach is covered by the incoming tide.
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
InfraOrder |
Brachyura |
|
Family |
|
|
Genus |