Microspio granulata Blake & Kudenov, 1978
|
publication ID |
https://doi.org/ 10.11646/zootaxa.4019.1.15 |
|
publication LSID |
lsid:zoobank.org:pub:54E60C63-EC98-424A-B66E-A72CA79B65E8 |
|
DOI |
https://doi.org/10.5281/zenodo.5665414 |
|
persistent identifier |
https://treatment.plazi.org/id/971C0501-8921-FFB4-DFCD-94AAFC5865E0 |
|
treatment provided by |
Plazi |
|
scientific name |
Microspio granulata Blake & Kudenov, 1978 |
| status |
|
Microspio granulata Blake & Kudenov, 1978 View in CoL
( Figs 8–11 View FIGURE 8 View FIGURE 11 )
Microspio granulata Blake & Kudenov, 1978: 232 View in CoL , fig. 30.— Hutchings & Turvey 1984: 13 –14; Maciolek 1990: table 3.
Type material. Holotype: NMV F42947 View Materials , S Pacific Ocean, New South Wales, Botany Bay, Towra Point, NSWSF station 329, associated with Halophila, Apr 1973 , coll. Rand, af. Paratype: NMV F42950 View Materials , S Pacific Ocean, New South Wales, Botany Bay, Towra Point, station 211, coll. NSW fish., af.
Other material examined. AM W.44379, MI QLD 2380, af, formalin; AM W.44016, MI QLD 2340, broken, formalin; AM W.47430, MI QLD 2340, af, formalin; AM W.44018, MI QLD 2340, broken, formalin; AM W.44381, MI QLD 2376 (2), formalin; AM W.45022, MI QLD 2437 (2 af), formalin; AM W.44472, MI QLD 2395, broken, formalin; AM W.44480, MI QLD 2397, af, 96% ethanol; AM W.44484, MI QLD 2399, af, formalin; AM W.43956, MI QLD 2342, broken, formalin.
Comparative material examined. Holotype of Microspio minuta ( Hartmann-Schröder, 1962) , ZMH P- 14930, S Pacific Ocean, Chile, 4 km north of Taltal, eulitoral, from rhizoids of Macrocystis washed up on the beach, coll. G. Hartmann-Schröder, af. Holotype of Microspio microcera ( Dorsey, 1977) , LACM-AHF POLY 1137, North Pacific Ocean, USA, California, Channel Islands, San Clemente Island, Wilson Cove, 2 m, coralline algal mat (mostly Lithothrix aspergillum ) with sand and shell debris, coll. J. Dorsey, Jun 1973. Paratype of Microspio microcera ( Dorsey, 1977) , LACM-AHF POLY 1138 (4 af), same locality and information as holotype. Non-type material of Microspio microcera ( Dorsey, 1977) , LACM-AHF POLY 6592, same locality and information as holotype, additional information: 32°00’18”N 118°33’26”W.
Diagnosis. Prostomium anteriorly deeply incised, posterior part slightly elevated, extended into a caruncle terminating at chaetiger 2. Notopodial ramus of chaetiger 1 reduced, without notochaetae, small rounded lamella dorsally to the neuropodium present and in addition dorsal lobe present in vicinity to the lateral band of the nuchal organ. Branchiae from chaetiger 2, branchiae on chaetiger 2 as long as those on chaetiger 3 in adult specimens, only few posteriormost chaetigers without branchiae. Pronounced ciliation on all tcb´s and at the inner side of the branchiae. From chaetiger 9 two to five bi- or tridentate neuropodial hooded hooks, apical tooth hardly discernible; sabre chaetae present in middle and posterior chaetigers. Pygidium with 4 anal cirri.
Description. (based on specimens examined in the course of the present study) Specimens from Lizard Island considerably smaller than type material collected in New South Wales. Largest complete specimen with 42 chaetigers, 1 mm wide, about 10 mm long. Prostomium deeply incised, projecting over peristomium, without prominent occipital papilla (which is present in the type material) ( Figs 8 View FIGURE 8 A–C, 9A–C, 10A–C); posterior end extending to tcb on chaetiger 2, slightly elevated from about posterior pair of eyes (Figs 9A, B, 10C); two pairs of black eyes, arranged in trapezoid, anterior pair larger, crescent-shaped, widely spaced, posterior pair smaller, rounded, closely spaced ( Figs 8 View FIGURE 8 A–C, 9A, B); only anterior part of prostomium distinctly separated from peristomium (Fig. 10B).
Nuchal organ with short median and long lateral ciliary bands; median ciliary band laterally from the posterior part of prostomium, turning medial at its posterior tip, extending to end of chaetiger 2 ( Figs 8 View FIGURE 8 A, 9A, B 10A, B); lateral ciliary band starting near palps, from there first turning outwards then inwards again, by that surrounding tcb on chaetiger 2, extending to end of chaetiger 3 ( Figs 8 View FIGURE 8 A, 9A, B, 10A); long lateral ciliary band rather distinct, short median ciliary band only visible in well preserved specimens (SEM or methyl green staining required, but easily observed in large specimen from type material). Metameric dorsal ciliated organs present from chaetiger 3 to chaetiger 20 at maximum (usually to chaetigers 13–15), in between tcb´s of subsequent chaetigers ( Figs 8 View FIGURE 8 A, 9A, 10A); one pair of single comma-shaped ciliary bands per chaetiger, first ciliary bands short then getting longer and more straight (Fig. 10E), eventually covering entire distance between tcb´s of subsequent chaetigers. Pronounced ciliation on all tcb´s and at the inner margin of branchiae (Fig. 10A, C, E).
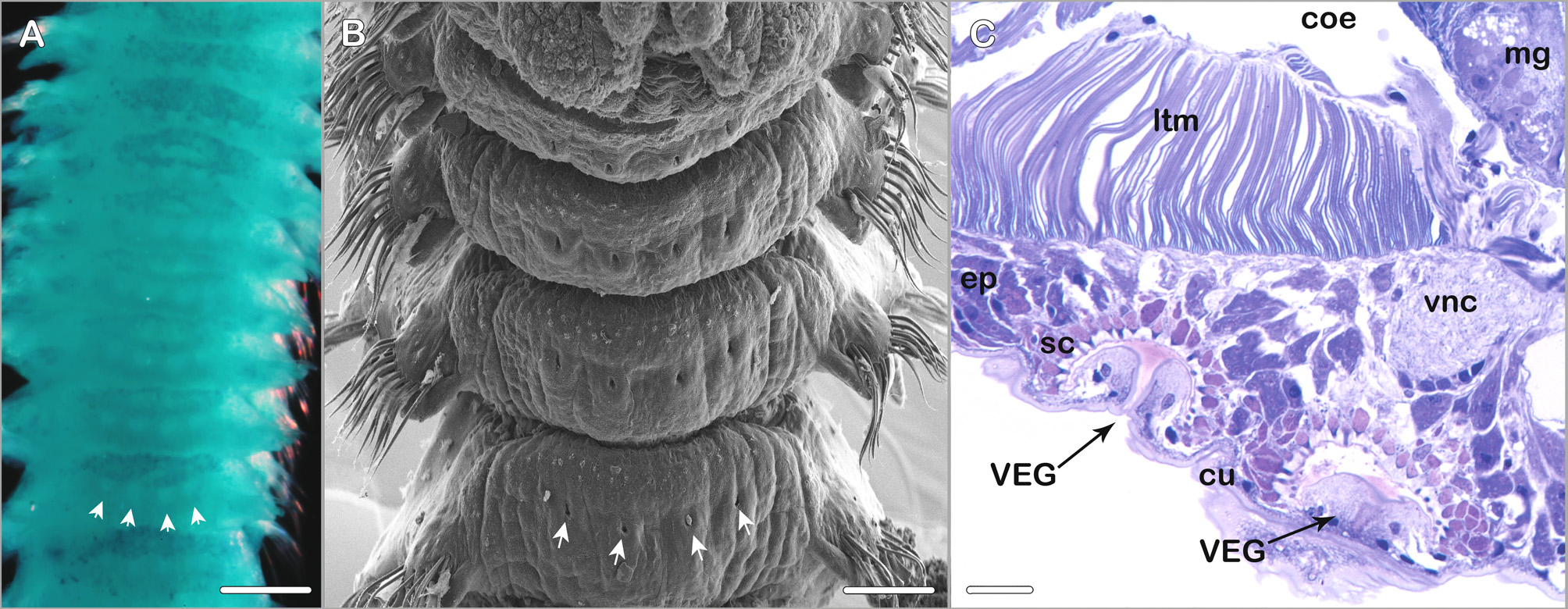
Ventral epidermal glands present from about chaetiger 3 to posterior middle body region; two pairs of glands per chaetiger: median pair slightly posteriorly to centerline on ventral side of respective chaetiger, second pair laterally to median pair directly at centerline of the chaetiger ( Fig. 11 View FIGURE 11 A–C) (best observed with SEM or after methyl green staining).
Branchiae from chaetiger 2, present throughout body except 5–9 posteriormost chaetigers ( Figs 8 View FIGURE 8 A, 9A, C, 10A, C, D, E); first pair of branchiae as long as those on following chaetiger ( Figs 8 View FIGURE 8 C, 9A, C, 10A), only in small specimens about half length of second pair of branchiae; branchiae on chaetigers 2(3)–5(6, 7) longest and most robust, nearly reaching midline dorsally; from about chaetigers 6–9 branchiae also long but more slender; in midbody region about half as long as longest anterior branchiae, continuously decreasing in length towards end of body, in last three to four branchiate chaetigers considerately decreasing in length (Fig. 10D); branchiae free, cirriform and distally rounded (Fig. 9F–J), with long cilia on inner margin.
FIGURE 9. Microspio granulata Blake & Kudenov, 1978 , AM W.44018, MI QLD 2340 (A, C); AM W.44016, MI QLD 2340 (B); AM W.43927, MI QLD 2340 (D–J). A. Anterior end, dorsal view, palps removed; B. Close-up of anterior end, dorsaloblique view with focus on nuchal organ and first chaetiger, chosen view improves observation of small rounded prechaetal lobe in neuropodium; C. Anterior end, lateral view, palps removed; D. Tridentate neuropodial hook from 9th chaetiger; E. Anterior notochaetae from 2nd chaetiger; F. Parapodium from 2nd chaetiger, anterior view; G. Same from 5th chaetiger; H. Same from 9th chaetiger; I. Same from 17th chaetiger; J. Same from 9th last chaetiger being last branchiate chaetiger. Scale bars: A–C = 500 µm, D, E = 10 µm, F–J = 50 µm.
FIGURE 10. Microspio granulata Blake & Kudenov, 1978 , AM W.44016, MI QLD 2340 (A–F); AM W.43927, MI QLD 2340 (G). A. Anterior end, dorsal view (labels refer to ciliary bands of the nuchal organ); B. Anterior end, antero-dorsal view with focus on indented anterior margin of the prostomium and on the first parapodium (label refers to the neuropodium, arrows point at lobes dorsally to neuropodial chaetae); C. Chaetigers 1–3, left side, lateral view (arrows point at lobes dorsally to neuropodial chaetae); D. Posterior chaetigers with second last and last branchiate parapodia (branchiae indicated by arrows), left side, lateral oblique view; E. Chaetigers 12–16, dorsal view; F. Pygidium, lateral oblique view; G. Tridentate hooded hooks from 9th last chaetiger. Abbreviations: lb = lateral band (nuchal organ), mb = median band (nuchal organ), ne = neuropodial postchaetal lamella. Scale bars: A–F = 100 µm, G = 10 µm.
First parapodium slightly shifted dorsally; neuropodium well developed; notopodium reduced without notochaetae (Figs 9A–C, 10 B, C); small rounded lamella present dorsally to neuropodium and additional dorsal lobe present in vicinity to lateral band of nuchal organ (Figs 9A, B, 10B, C) (see remarks). Notopodial postchaetal lamellae on anterior chaetigers subtriangular, slightly rounded at base (Fig. 9F, G); slightly decreasing in size along body (Figs 9H, I); in posterior chaetigers long triangular to almost cirriform (Fig. 9J). Neuropodial postchaetal lamellae low and rounded; in anteriormost chaetigers as long as chaetal row, in mid-body region longer than chaetal row; in posteriormost chaetigers shorter again (Figs 9F–J, 10C). Prechaetal lamellae absent.
Notopodial chaetae all capillaries; arranged in two distinct rows in anterior and middle chaetigers (Fig. 10C), in posterior chaetigers no longer arranged in distinct rows; anterior row of chaetae shorter and stouter than chaetae in posterior row (Fig. 10C), chaetae granulated with narrow sheath (Fig. 9E); otherwise chaetae smooth with very narrow sheaths; additional superior fascicle of long capillaries present. Neuropodia with rows of capillaries and hooded hooks as well as sabre chaetae in inferior position; capillaries of anterior neuropodia arranged in two rows, short, stout and distinctly granulated capillaries in anterior row, long, smooth, alimbate capillaries in posterior row; in middle chaetigers neuropodial capillaries of about same length; 3–5 tridentate hooded hooks replacing posterior row of capillaries from chaetiger 9; main fang with apical tooth and additional small uppermost tooth, uppermost tooth best seen in posterior parapodia (Figs 9D, 10G); hooks accompanied by anterior row of thin alimbate capillaries (Fig. 10D); few smooth, long capillaries in inferiormost position usually present before hooks start, hook-bearing chaetigers with one or two stout, distally granulated sabre chaetae with narrow sheath (Fig. 9I, J).
Pygidium with four cirriform anal cirri, in adult specimens of about equal size, in younger specimens dorsal pair sometimes slender or slightly longer; dorsolaterally attached pair pointing to dorsal direction; ventrolaterally attached pair pointing laterally; anus terminal (Fig. 10F).
Pigmentation. Formalin and ethanol fixed specimens with orange-brownish stripe across peristomium and prostomium ( Fig. 8 View FIGURE 8 A–C); in addition some pigment of the same colour anteriorly to tcb´s of chaetigers 3 and 4 ( Fig. 8 View FIGURE 8 B); in heavily pigmented specimens pigment present anteriorly and posteriorly to tcb´s of some anterior chaetigers ( Fig. 8 View FIGURE 8 A).
Methyl green staining pattern. Inconspicuous. Branchiae, anterior half of prostomium and peristomium, and postchaetal lamellae most intensely stained. Ventral epidermal glands visible as four white dots on a bluish background on the ventral side of anterior and middle body chaetigers ( Fig. 11 View FIGURE 11 A).
Remarks. Microspio granulata was described by Blake & Kudenov (1978) based on material from the New South Wales coast near Sydney. Examination of specimens collected in the Lizard Island area revealed minor differences to the original description. After examination of the type material it could be concluded that some observed deviations are partially explained by the different sizes of specimens (the Lizard Island specimens are considerably smaller than the type specimens). Other discrepancies might be attributed to imprecise observations. Information about additional characters is now provided in the amended species description.
Fully grown specimens exhibit a distinct occipital papilla which is not present in smaller specimens, a merely slight elevation can be observed instead in the smaller specimens from Lizard Island. The type specimens are more heavily pigmented than the specimens collected around Lizard Island ( Fig. 8 View FIGURE 8 ). The ciliation of branchiae and tcb´s is less pronounced in the type material compared to the Lizard Island material. Blake & Kudenov (1978) observed “... a transverse hood reminiscent of dorsal collars seen in genus Streblospio present posterior to prostomium, surrounded laterally and posteriorly by curved nuchal grooves”. It is likely that this observation of a hood by Blake & Kudenov (1978: fig. 30) was actually the tcb with long cilia on the second chaetiger and the ciliated bands of the nuchal organ in immediate vicinity. The nuchal organ of M. granulata is in good agreement with the general pattern typical for many Spio or Microspio species in consisting of a short median ciliated band and a longer curved or recurved lateral ciliated band. The metameric dorsal ciliated organs of M. granulata are described for the first time in the present study. The presence of ventral epidermal glands (VEGs) was not mentioned in the original species description but two pairs of VEGs per chaetiger are present in anterior and middle chaetigers ( Fig. 11 View FIGURE 11 ). Blake & Kudenov (1978) observed the presence of genital pouches from chaetiger 12, decreasing in size to chaetiger 29. Though this character was illustrated in the original species description it could not unambiguously be identified while examining material from Lizard Island or in the type material. Instead the type specimens exhibited lateral openings in form of vertical slits in the body wall from chaetiger 12. Gametes could be detected in both, the type specimens and in the recently collected Lizard Island specimens, in the latter lateral openings were absent. Genital pouches in terms of ventrolateral intersegmental pouches present in other spionid genera could not be observed in M. granulata . Blake & Kudenov (1978) state 8–9 neuropodial hooks to occur per fascicle from chaetiger 9 whereas 3–5 hooks were present in the Lizard Island specimens and 5–6 hooks were observed by us in the larger type specimens. Since complete specimens were among specimens recently collected on Lizard Island information on the pygidium could be added to the species description.
The probably most important discovery, as it is also important for e.g., species delimitation, concerns the first chaetiger. Blake & Kudenov (1978) described the first chaetiger as “reduced, with small digitiform notopodial lamellae shifted dorsally, lacking notosetae...”. We partially agree with this view: the first chaetiger is reduced, the neuropodial ramus is fully developed whereas the notochaetae are absent. However, the problem is that not only one lobe or lamella which could be attributed to the notopodial ramus is present dorsally to the neuropodium but two (Figs 10B, C). The lamella directly dorsally to the neuropodium is not easy to detect with light microscopy since it nestles in immediate vicinity of the neuropodial chaetae (Fig. 10C). The more dorsally positioned lobe is in comparison more distinct after methyl green staining. An associated ciliated tuft could be detected by means of SEM (Fig. 10B). The interpretation of this arrangement is not straightforward. The lamella directly above the neuropodium might be the notopodial lamella whereas the dorsalmost lobe could be interpreted as a branchial remnant (this view perhaps corroborated by the presence of the tuft of cilia being either part of the branchial ciliation or the transverse ciliated band usually starting next to the branchiae). This hypothesis would question the currently accepted generic diagnosis of Microspio that states that branchiae start on chaetiger 2. Detailed anatomic studies might shed light on this problem which currently is unresolved. However, the presence of the two lobes or lamellae helps differentiating between M. granulata and the morphologically very similar Microspio microcera ( Dorsey, 1977) from the Eastern Pacific Ocean. In M. microcera a single lobe dorsally to the neuropodium is easily detected after methyl green staining. The presence of this lobe is not mentioned in the original description ( Dorsey 1977) but has been illustrated by Maciolek (1990). After examination of the type material the following information on M. microcera can be added: the nuchal organ is as described for M. granulata ; metameric dorsal ciliated organs as one pair of single comma-shaped ciliary bands per chaetiger present from chaetiger 3 to about chaetiger 15; first branchiae on chaetiger 2, about half or two thirds the length of the second pair of branchiae on chaetiger 3, branchiae on chaetigers 3–5 most stout, in subsequent chaetigers thinner but slightly longer, branchial distribution otherwise very similar to M. granulata ; two pairs of ventral epidermal glands depictable as large white dots after methyl green staining until about chaetiger 11; sabre chaetae present in all hook-bearing chaetigers, inferior bundle of capillaries in anterior and middle chaetigers most probably present; longest paratype with 28 chaetigers 4.7 mm long and 0.6 mm wide, with eggs. In conclusion, M. microcera is morphologically very similar to M. granulata but can be distinguished by the presence of only one lobe dorsally to the neuropodium in the first chaetiger instead of two lobes; also, in M. microcera the first branchiae are always considerably shorter than the second pair of branchiae whereas they are of about the same length in M. granulata ; mature specimens of M. granulata exhibit an occipital papilla which is not distinct in smaller specimens of this species and is absent in M. microcera .
Another morphologically similar species is M. minuta ( Hartmann-Schröder, 1962) . Unfortunately, the type material is not in good condition and important characters like the shape of nuchal organs, the metameric dorsal ciliated organs, ventral epidermal glands or the first chaetiger cannot be observed. The general habitus of the species, however, is in very good agreement with M. microcera . Also details from the original species description (in German language) imply great morphological similarity between the two species: small size (holotype with 26 chaetigers 2.5 mm long), prostomium distinctly bifid, caruncle not conspicuous; branchiae from chaetiger 2 throughout the body, ciliated, cirriform, free from notopodial lamellae except at the base, first five pairs of branchiae distinctly longer than posterior branchiae, decreasing in length continously within a short distance; notopodial postchaetal lamellae ovate to tongue-shaped, neuropodial postchaetal lamellae broadly rounded; all parapodia with granulated capillaries with broad sheaths, notochaetae longer than neurochaetae, capillaries in posterior chaetigers shorter, more slender and less numerous; neuropodial bidentate hooded hooks from chaetiger 9, usually 2–3, maximally 4 per fascicle, accompanied by simple capillaries; pygidium with four cirri of equal length. Blake (1983) presents an emended description of M. minuta providing information on the presence of sabre chaetae in hook-bearing chaetigers, on the tridentate nature of neuropodial hooks, on the nuchal organs and the metameric dorsal ciliated organs, the two latter fitting at least partially the description of these features in M. microcera . The only reliable distinguishing character between M. minuta and M. microcera seems to be the complete reduction of the first notopodium in M. minuta , in contrast the notopodial lobe of the first chaetiger is still present in M. microcera . Considering the fact, that this notopodial lobe is not easy to detect in these small species and has been overlooked in the past it cannot be ruled out that M. microcera is a junior synonym of M. minuta . Both species occur in the Eastern Pacific Ocean, intertidally to 18 m water depth, either associated with coralline algal mats ( Dorsey 1977) or between rhizoids of Macrocystis washed up on the beach ( Hartmann-Schröder 1962). If new material becomes available for examination the validity of the two species has to be verified.
Other species of Microspio with neuropodial hooks from the 9th chaetiger differ from M. granulata in regard to the prostomial shape, the presence of notochaetae in chaetiger one or the branchial distribution along the body (see Maciolek 1990, Table 3, and description of M. atlantica Langerhans, 1880 ).
Habitat / Ecology. In the Lizard Island area the species occurred in the intertidal, in sand among seagrass and algae ( Halimeda ). At localities in South Australia and New South Wales the species also occurred in sand, Zostera sea grass beds, associated with Halophila (see collection data type material), or among mussels ( Hutchings & Murray 1984, Hutchings & Turvey 1984).
Distribution. Australia: Queensland, New South Wales, South Australia.
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.