Marasmius gardneri Singer
|
publication ID |
https://doi.org/ 10.5252/cryptogamie-mycologie2022v43a5 |
|
DOI |
https://doi.org/10.5281/zenodo.7829386 |
|
persistent identifier |
https://treatment.plazi.org/id/9A684B45-300E-7129-FED5-7B34E33FE0A5 |
|
treatment provided by |
Felipe |
|
scientific name |
Marasmius gardneri Singer |
| status |
|
Marasmius gardneri Singer View in CoL View at ENA
( Figs 6B View FIG ; 8 View FIG )
Sydowia 12 (1-6): 114 ( Singer 1958). — Type: Brazil. Minas Gerais State, Gardner (Hooker Herbarium set at K), holotype.
Marasmius ferrugineus var. gardneri Singer View in CoL , Flora Neotropica 17: 223 ( Singer 1976).
EPITYPE. — Brazil. São Paulo State, São Paulo City, Parque Estadual da Cantareira, Núcleo Engordador, 16.II.2012, J.J.S. Oliveira & M. Capelari JO491 (epi-, designated here, SP[SP 445564]!).
ADDITIONAL EXAMINED MATERIAL. — Brazil. São Paulo State, São Paulo City, Parque Estadual da Cantareira, Núcleo Engordador, 31.X.2011, J.J.S. Oliveira & M. Capelari JO387 (SP[SP 445521]!); 19.XII.2011, J.J.S. Oliveira & M. Capelari JO438 (SP[SP 445532]!); J.J.S. Oliveira & M. Capelari JO454 (SP[SP 445542]!).
HABIT AND SUBSTRATE. — Marasmioid ( Figs 6B View FIG ; 8A View FIG 1), gregarious, on eudicotyledonous dried leaves and twigs in the forest litter.
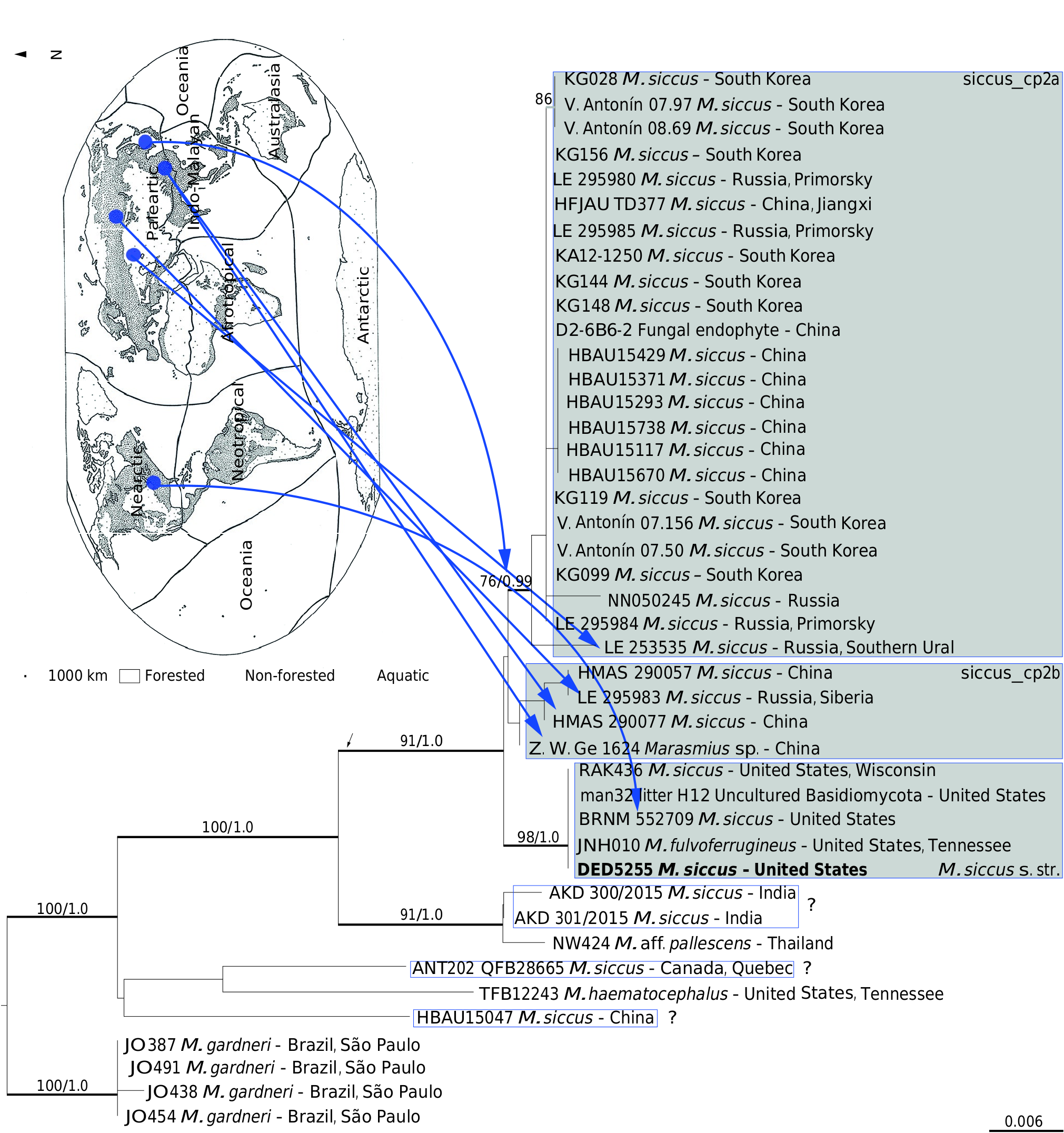
DISTRIBUTION. — This species was established with a nomen novum in Singer (1958) based on the type material of M. ferrugineus originally from Minas Gerais State ( Brazil) collected by Gardner. This type consisted of a mix of two close species, later split into two sets where the “Hooker Herbarium set” of basidiomata is the type of this species. Additional collections listed by Singer (1958, 1976) are from the Amazonas and Rio de Janeiro States ( Brazil).
DESCRIPTION
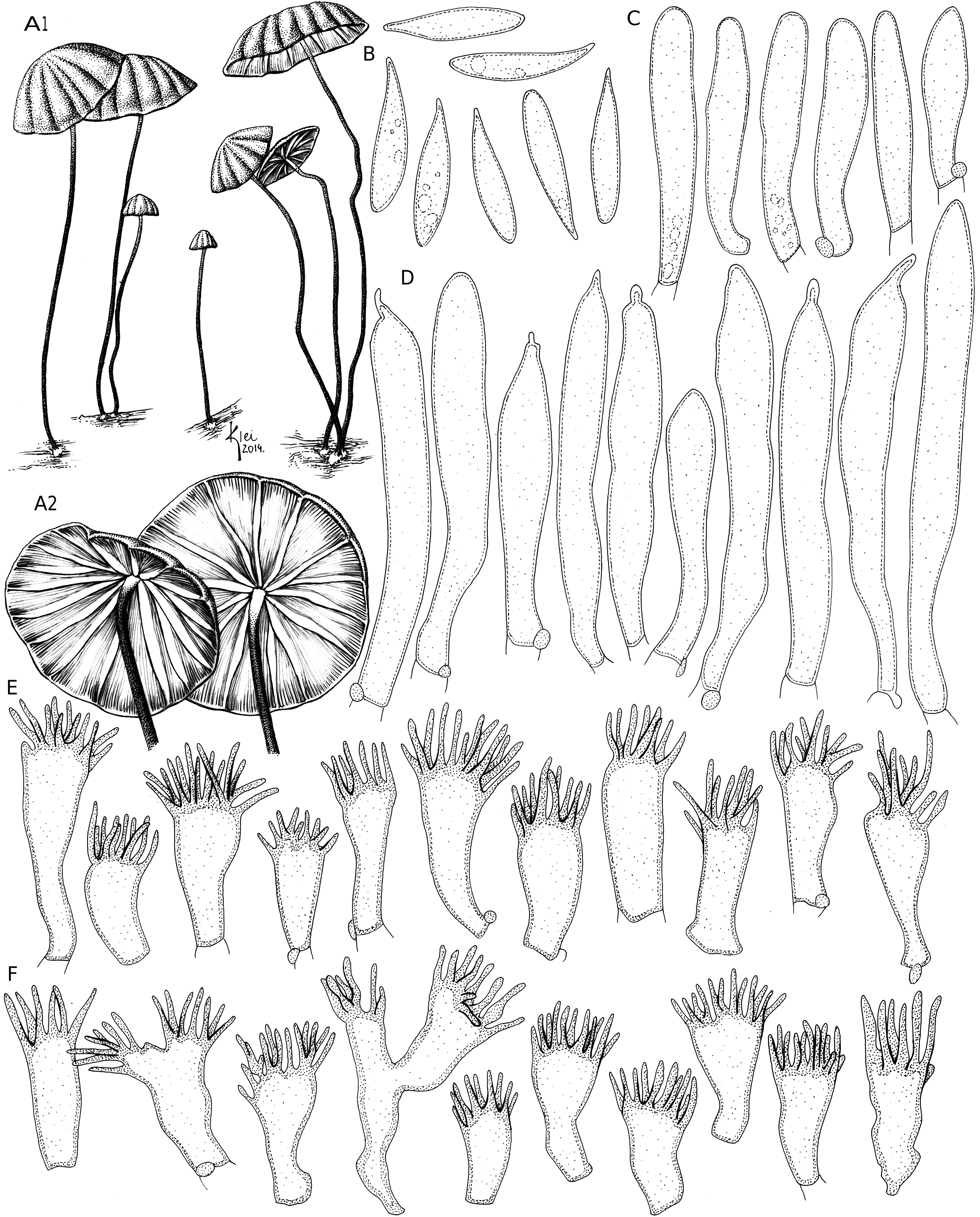
Pileus ( Figs 6B View FIG ; 8A View FIG 1)
Up to 2 mm diam. when immature, then 3-18 mm diam., conical to campanulate (because of a broad umbo), or hemispheric, becoming convex, sometimes applanate, smooth when young, often becoming slightly sulcate, or strongly sulcate when fully developed, center umbonate, later tending to flat and sometimes wrinkled, margin decurved to straight, edge mostly entire, or slightly crenate; center dark reddish brown (N 70-90 Y 90-99 M 80-90) to dark orangish brown (N 60 Y 99 M 70), then becoming more pale chestnut red or fulvous “tawny” (N 60 Y 60 M 60) or dark brown (N 60 Y 70-80 M 90-99) especially when young, or rarely pale orange (N 10 Y 99 M 30) or pale pinkish brown (N 60 Y 80 M 70), many times with darker sulci, some strongly ferruginous (N 80 Y 99 M 70), orangish brown to fulvous-ferruginous (N 40-50 Y 99 M 60 to N 40-50 Y 99 M 70) toward the margin, and orange to ferruginous brown when dried (N 80 Y 99 M 50-70); membranous, context white, thin (<1 mm); glabrous, dry, dull, subvelutinous, non-hygrophanous.
Lamellae ( Figs 6B View FIG ; 8A View FIG 2 View FIG )
Free to subfree, subdistant, L = 12-16, equal, mostly simple, or very slightly intervenose in very mature basidiomata, l = 0(-1), straight to slightly ventricose, opaque, smooth, white, or pale cream to whitish pink (N 00 Y 10 M 00-10), edges even, non-marginate, concolorous with the lamellae faces as well as the interlamellar hymenium.
Stipe ( Figs 6B View FIG ; 8A View FIG 1)
15-44 × 0.3-1 mm, central, filiform, or thicker, almost cylindrical thin, equal, with circular to slightly compressed caliber, simple, chitinous, hollow, apex whitish (N 20 Y 10 M 30) or pale brown (N 30 Y 40 M 30), becoming brown (N 90 Y 90 M 50-60), reaching dark brown (N 99 Y 99 M 80) at the base, glabrous, smooth, with a silky bright; with a more abundant, whitish, tomentose or cotton-like basal mycelium, also developing a mat on the substrate.
Odor
Not distinctive.
Basidiospores ( Fig. 8B View FIG )
(14.6-)15-19 × 3-5 µm (xrm = 16.6-17.6 × 3.9-4.1 µm; xmm = 16.7 [± 0.6] × 3.9 [± 0.1] µm; Qmr = 4.1-4.6, Qmm = 4.3 [± 0.3]; n / s = 30/3), oblong, clavate to fusoid, smooth, hyaline, thin-walled, inamyloid.
Basidia
Not observed.
Basidioles ( Fig. 8C View FIG )
21.3-33 × 5-6.3 µm, clavate, smooth, hyaline, thin-walled, inamyloid.
Pleurocystidia ( Fig. 8D View FIG )
32.5-62.5 × 4-7.5 µm, conspicuous, well-projecting above the basidioles, clavate to cylindrical, apex obtuse or sometimes fusoid to acuminate, or capitate, with a small apical vesicle, or mucronate, smooth, refractive, inamyloid, few fuscous, thin-walled.
Cheilocystidia ( Fig. 8E View FIG )
Similar to the Siccus-type broom cells of the pileipellis, but non-pigmented; main body 12.5-23.8 × 5.6-8.8(-10) µm, clavate to somewhat turbinate, or ventricose, regular in outline, thin-walled; setulae apical, erect, generally elongate, 2.5- 10.4 × 0.6-1.3 µm, cylindrical, filiform, rarely digitiform, regular in outline, simple, pale yellow, solid, apex obtuse to acute.
Lamellar trama
Dextrinoid, irregular, interwoven, hyphae cylindrical, 2-8.8 µm diam., regular in outline, branched, smooth, hyaline, thinwalled.
Pileus trama
Similar to the lamellar trama, hyphae 1.5-8.8 µm diam.
Pileipellis
Hymeniform, dextrinoid, composed of Siccus-type broom cells ( Fig. 8F View FIG ), abundant, pale orangish brown when grouped, hyaline when isolated; main body (8.8-)12.5-18.8 × 5-9.4 µm, clavate to turbinate, sometimes cylindrical, or branched, or somewhat irregular in outline, thin- to thick-walled, weakly dextrinoid; setulae apical, erect, 3-8.8 × 0.5-1.3 µm, cylindrical, filiform, thin, needle-like, simple, rarely branched, pale brown, apex acute or slightly obtuse.
Stipe trama
Dextrinoid, cortical hyphae parallel, cylindrical, 3.8-9.4 µm diam., regular in outline, pale brown, hyaline at the stipe apex, dark brown near the base, smooth, thick-walled; internal hyphae more hyaline, thin-walled, 2-16.3 µm diam.
Clamp connections
Present in almost all tissues, except in the cortical hyphae of the stipe.
REMARKS
The historical account for this species is not straightforward and it begins with Agaricus (Marasmius) ferrugineus ( Berkeley 1843) . Due to competing prior homonym, it was later combined by Berkeley & Curtis (1869) in Marasmius ferrugineus . Singer (1958), by arguing that this name was illegitimate in the basionym, proposed the nomen novum Marasmius gardneri in replacement, based on the type material of M. ferrugineus along with two additional collections: 1) ‘part’ of Spruce 139 (from Amazonas State, Brazil) segregated (mixed collection) from the type of M. poecilus Berk. ; and 2) Singer B 437 (from Angra dos Reis, Rio de Janeiro State). However, M. ferrugineus is deemed legitimate in Berkeley & Curtis (1869) and, therefore, M. gardneri became a nom. illegit. by the art. 52.1 of the International Code of Nomenclature for Algae, Fungi and Plants. According to Singer (1976), the type collection of M. ferrugineus was potentially a mix of two different species as the Gardner collection existed split into two sets ( Singer 1958), both collected at Minas Gerais and kept at K. The first set, kept in the Berkeley Herbarium, was indicated as the holotype of M. ferrugineus , and has relatively larger and narrower spores (15-21 × 2.8-4.3 m). The second set, kept in the Hooker Herbarium, has shorter and broader spores (13.5-18.3 × 3.2-5 m), and Singer (1976) named this collection M. ferrugineus var. gardneri Singer. This variety also differs from the type variety in the pileus often becoming somewhat darker (“cocoa”) on drying and in growing on small woody sticks, leaf petioles and veins ( Singer 1976).
The examined specimens fit best as M. ferrugineus var. gardneri . The dimensions of the pileus (3-18 mm diam.) and of the stipe (15-44 × 0.3-1 mm) are larger than those typically found in M. ferrugineus var. ferrugineus (3-11 mm diam. of the pileus and 11-30 × 0.3 mm of the stipe). Singer (1976) did not mention the dimensions of the pileus and stipe as distinctive for the specimens of var. gardneri , which implies that both varieties match relatively the macroscopic proportions. The protologue of Agaricus (Marasmius) ferrugineus (synonym of M. ferrugineus ) emphasizes the tiny proportion of the basidiomata with “pileus 1½-3 lines broad” (3.18-6.35 mm diam.) and stipe “ ½-¾ of an inch high, ⅛ of a line thick” (12.7-19.05 mm long and 0.265 mm thick). The basidiomata of the examined material are evidently larger and more robust (as in M. hypophaeus ) than a typical M. ferrugineus and, in combination with the shorter and broader basidiospores and by growing more on dried leaves or small twigs, should represent a different species.According to Singer (1958, 1976), Dennis also knew very well M. ferrugineus , especially the Berkeley Herbarium set which, agreeing with Singer, defended it should be the type of M. ferrugineus . With collections from Venezuala, Dennis (1961) found two spore’s dimension for “ M. ferrugineus ”: Dennis 1021 (19-21 × 3-4 µm) and Dennis 1021A (14-18 × 3-3.5 µm, this should be M. gardneri ). Revising the type specimen of M. ferrugineus ( Brazil, Minas Gerais, K [M] 92652), Antonín et al. (2012) found even longer and broader basidiospores 18-22 × 4.5- 6.0 µm and broader pleurocystidia (11-17 µm) which are not consistent with the specimens herein determined as M. gardneri . This is sister to M. ferrugineus from South Korea and China (Fig. 1).
Based on the combined divergences found between the two sets of Gardner collection together with the examined collections agreeing with the Hooker Herbarium set, we conclude that M. ferrugineus var. gardneri should be elevated to species level, legitimating the name M. gardneri typified on the ‘Hooker Herbarium set’. Moreover, if M. ferrugineus becomes a synonym of M. bambusinus (previous taxon), then we are quite sure we have two different species. Both Singer (1958) and Dennis (1951) considered M. paucifolius Murrill , a synonym of M. ferrugineus . Singer (1976) rather placed it in M. ferrugineus var. gardneri and, therefore, should claim its legitimacy for being prior than M. gardneri . However, we have scarce evidence to support M. paucifolius as synonym of M. ferrugineus var. gardneri and should be considered an independent species until more collections and analyses become available. If conspecific, the species would also occur in Puerto Rico.
Marasmius hypophaeus is very similar to M. gardneri but differs mainly in having distinctly marginate lamellae (rust brown) and larger basidiospores ([12-]14.5-21.5 × 3-5.5 µm), broader pleurocystidia (6-13 µm) and larger setulae (1-14[-20] × 0.7-2.2µm) on the broom cells of the pileipellis ( Singer 1976). Singer (1976) argued that Dennis (1951) described and illustrated a specimen named after M. ferrugineus orangish brown in both pileus and lamellar edge, then he suspected that M. ferrugineus sensu Dennis (1951) would be rather M. hypophaeus . Singer concluded that the pileus pigmentation of M. hypophaeus when fresh would be orangish brown (old bronze) rather than red (rufous blood red) as described in the protologue ( Berkeley & Curtis 1869) that possibly led Murrill (1915) to suppose it would be a synonym of M. haematocephalus . Next, Singer defended that M. hypophaeus is closer to M. ferrugineus than M. haematocephalus but differing from the former by the distinctly ferruginous brown lamellae edge instead of concolorous with the lamellae face.
Marasmius tenuisetulosus (Singer) Singer is similar to M. gardneri in many features, especially the basidiospores size (14.5-19 × 3-4 µm). However, the former differs by having larger (up to 28 mm diam.), orangish brown pileus which is radially striped, by having series of lamellulae, and by having thick-walled pleurocystidia ( Singer 1964, 1976). These pleurocystidia are long-acuminate and ventricose, tapered from the middle to the apex ( Singer 1964). Marasmius radiatus Desjardin is similar to M. gardneri especially in the orange or brownish orange pileus, in the basidiospores size (15.7-19.2 × 3.8-5.1 µm) and in the general aspects of the pleurocystidia. However, M. radiatus differs only in having a ferruginous pileus with pale radial stripes over the lamellae line when dried, and in having dimorphic, hyaline cheilocystidia: 1) Siccus-type broom cells; and 2) nonsetulose cystidia similar to the pleurocystidia ( Desjardin et al. 1992).
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |
|
|
Series |
Haematocephali |
Marasmius gardneri Singer
| S, Jadson José, Oliveira, ouza de, Capelari, Marina, Margaritescu, Simona & Moncalvo, Jean-Marc 2022 |
Marasmius ferrugineus var. gardneri
| Singer 1976: 223 |